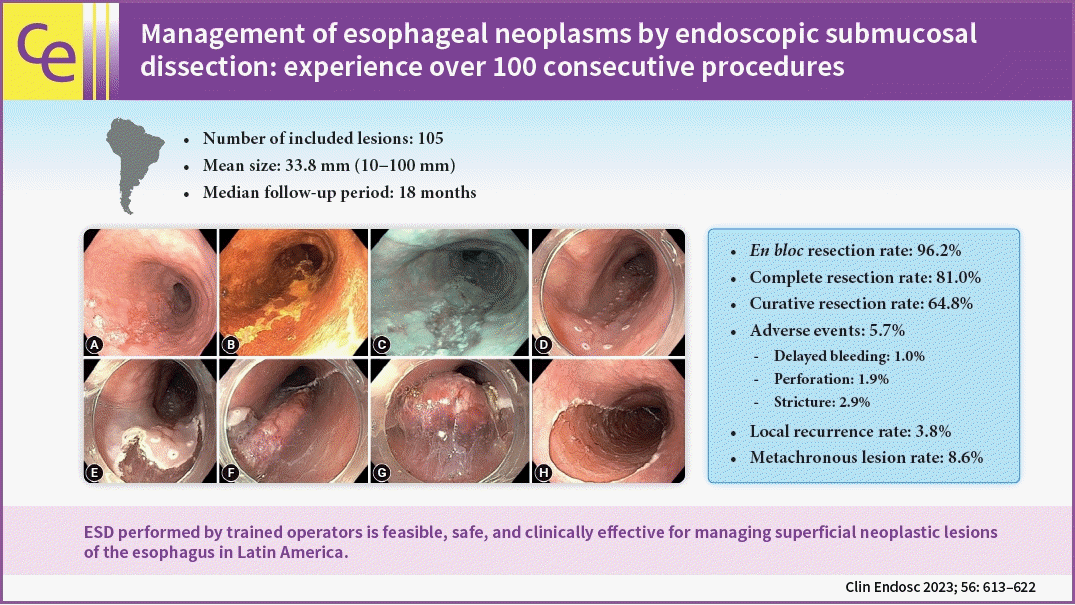
Management of esophageal neoplasms by endoscopic submucosal dissection: experience over 100 consecutive procedures
Article information
Abstract
Background/Aims
Endoscopic submucosal dissection (ESD) is currently considered the first-line treatment for the eradication of superficial neoplasms of the esophagus in Eastern countries. However, in the West, particularly in Latin America, the experience with esophageal ESD is still limited because of the high technical complexity required for its execution. This study aimed to present the results of the clinical application of ESD to manage superficial esophageal neoplasms in a Latin American center in over 100 consecutive cases.
Methods
This retrospective study included consecutive patients who underwent endoscopic ESD for superficial esophageal neoplasms between 2009 and 2022. The following clinical outcomes were assessed: en bloc, complete, and curative resection rates, local recurrence, adverse events, and procedure-related mortality.
Results
Esophageal ESD was performed mainly for squamous cell carcinoma (66.6%), high-grade intraepithelial neoplasia (17.1%), and adenocarcinoma (11.4%). En bloc and complete resection rates were 96.2% and 81.0%, respectively. The curative resection rate was 64.8%. Adverse events occurred in six cases (5.7%). Endoscopic follow-up was performed for an average period of 29.7 months.
Conclusions
ESD performed by trained operators is feasible, safe, and clinically effective for managing superficial neoplastic lesions of the esophagus in Latin America.
INTRODUCTION
Esophageal cancer is currently considered the sixth leading cause of cancer death worldwide, with an increasing incidence rate in recent years in both histological subtypes, adenocarcinoma and squamous cell carcinoma. Notably, more tumors have been detected in the early stages, in part due to the increase in the use of image-enhanced endoscopy (IEE) and chromoendoscopy, as well as the implementation of screening programs for early neoplasms.1-3
The combination of IEE with great advances in therapeutic endoscopy, such as endoscopic submucosal dissection (ESD) and endoscopic mucosal resection (EMR), has enabled the detection and eradication of esophageal neoplasms in early stages with medium- and long-term results similar to those obtained with esophagectomy, while preserving the affected organ and sparing patients from post-surgical morbidity associated with esophagectomy.4-16
Esophageal ESD is a complex therapeutic procedure that has a long learning curve. Currently, ESD is routinely practiced in Japan and some Asian countries, and its role is rapidly expanding in the West. Nevertheless, in Latin America, esophageal ESD is still limited to a few tertiary centers, and there is a paucity of scientific evidence from this region that supports the use of ESD for the treatment of early esophageal cancer. The expansion of ESD in the Latin America is crucial to increase the en bloc resection rate of esophageal tumors regardless of their size, allowing precise histological assessment and reliable staging.17-29 The aim of our research was to present the results of a large series of patients with early esophageal neoplasms managed by ESD by a single trained operator and to compare the clinical outcomes of the patients with those obtained at Japanese endoscopic centers.
METHODS
Patients
This was a retrospective, observational study. Data were extracted from a prospectively generated database, including consecutive patients from 2009 to 2022 who underwent ESD for superficial esophageal neoplasms.
The inclusion criteria were patients referred for endoscopic resection (ER) with early neoplasms assessed by IEE, including high-resolution white light endoscopy (WLE), Fuji intelligent chromoendoscopy, late narrow band imaging (NBI), blue laser imaging, and linked color imaging. In addition, chromoendoscopy with stains such as Lugol for squamous cell cancer and acetic acid for Barrett’s related lesions and adenocarcinoma were utilized in the preoperative assessment. In selected cases, endoscopic ultrasonography (EUS) and computed tomography were performed for preoperative staging. Patients with advanced tumors or clinical conditions considered unsuitable for general anesthesia and endoscopic surgery were excluded. Figure 1 shows an outline of the selection process for our patients before ESD.
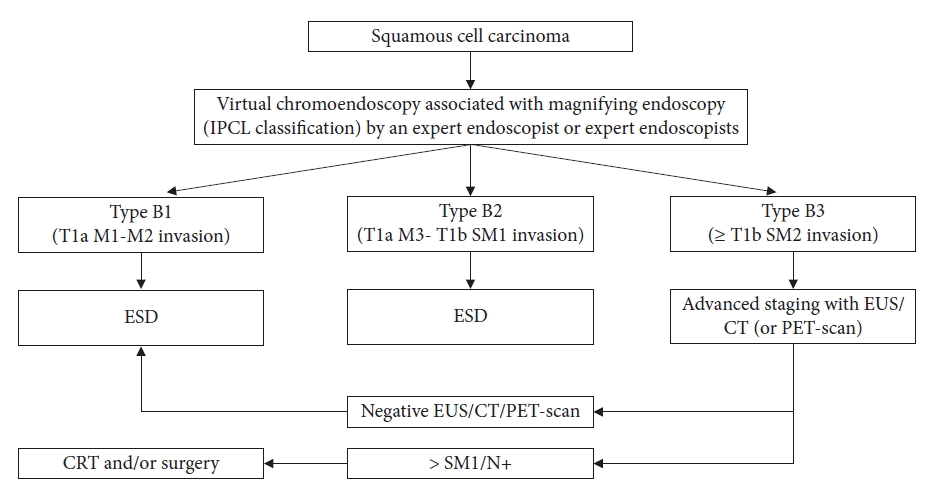
Outline of the selection process. IPCL, intrapapillary capillary loop; M1, intramucosal M1; M2, intramucosal M2; M3, intramucosal M3; SM1, superficial submucosa; SM2, deep submucosa; ESD, endoscopic submucosal dissection; EUS, endoscopic ultrasonography; CT, computed tomography; PET, positron emission tomography; CRT, chemoradiotherapy. N+ is locoregional lymphatic metastasis.
The following data were collected: age, sex, histological type of the esophageal neoplastic lesion, preoperative biopsy, location, size, PARIS endoscopic classification, duration of the procedure, specimen histological report, adverse events, and hospital permanence. The en bloc resection rate, complete resection rate with free margins (R0), and curative resection rate were calculated according to current European guidelines.2 The curative criteria for squamous cell carcinomas are as follows: very low risk of lymph node metastasis: (1) en bloc and complete resection; (2) tumors limited to the epithelium (pT1a-EP) or lamina propria (pT1a-LMP); (3) absence of lymphovascular invasion; and (4) well-to-moderate grade of differentiation. Low risk of lymph node metastasis: (1) en bloc and complete resection; (2) tumors with muscularis mucosa (pT1a-MM) or superficial submucosal (pT1b-SM1, ≤200 µm) invasion; (3) well-to-moderate grade of differentiation; and (4) absence of lymphovascular invasion. Likewise, the curability criteria for adenocarcinomas were considered as follows: very low risk of lymph node metastasis: (1) en bloc and complete resection; (2) tumors limited to the epithelium (pT1a-EP), lamina propria (pT1a-LMP), or muscularis mucosa (pT1a-MM); (3) absence of lymphovascular invasion; and (4) well-to-moderate grade of differentiation. The low risk of lymph node metastasis was as follows: (1) en bloc and complete resection; (2) tumors with superficial submucosal invasion (pT1b-SM1, ≤500 µm); (3) absence of lymphovascular invasion; and (4) well-to-moderate grade of differentiation.
In addition, the local recurrence rate and metachronous lesions were analyzed in the patients who returned for endoscopic follow-up. Endoscopic follow-up was scheduled three months after ESD and once a year thereafter. Local recurrence was defined as the appearance of a new esophageal neoplastic lesion at the same site as the previous resection during endoscopic follow-up. A metachronous lesion was defined as the appearance of a new esophageal lesion at a site different from the previous resection after a minimum of 6 months of follow-up. If a second esophageal tumor was detected within 6 months of ER, it was considered a missed lesion rather than a metachronous tumor.
ESD procedures
All the patients who underwent esophageal ESD were admitted to the hospital. The procedures were performed by a single endoscopist (VA) under general anesthesia. The operator was trained by experienced endoscopists (YM and TT) in a high-volume referral center in Japan and has currently performed close to 300 ESD procedures in the gastrointestinal tract. After a detailed endoscopic assessment with high-definition WLE, virtual chromoendoscopy, and 0.8% Lugol staining (or 1% acetic acid chromoscopy for Barrett’s related neoplasia), lesions were classified according to the Paris classification.3 ESD procedures were carried out with a therapeutic endoscope and a working channel of 3.2 mm (EG-450 RD; Fujifilm), Flush knife (DK-2618JN; FTS) BT 1.5 (Fujifilm) connected to the electrosurgical unit (ERBE VIO 200S, 200D, or 300D), and a 4 mm long cap (Elastic Touch; Top) attached to the tip of the endoscope to ensure optimal vision of the dissection field. Each procedure consisted of the following six steps: (1) lesion marking with diathermy using soft coagulation mode, effect 6, 100 watts, (2) submucosal injection to lift the lesion with 0.4% sodium hyaluronate in a teardrop form (Adaptis Fresh; Legrand Laboratory),4 (3) mucosal incision with endocut I, effect 2, cut length 3, and cut interval 2, (4) submucosal layer dissection using forced coagulation mode, effect 3, 50 watts, (5) pre-hemostasis of the blood vessels using the soft coagulation mode, effect 6, 100 watts. Blood vessels were sealed with a knife or coagulation forceps (Coagrasper; Olympus) depending on the vessel size, and (6) antibiotic prophylaxis with intravenous cephalosporin (or clindamycin if history of allergy) was administered to all patients, despite the absence of standardized consensus supporting the routine use of antibiotic prophylaxis in patients undergoing esophageal ESD, in contrast to colorectal ESD.5
In the postoperative period, all patients received proton pump inhibitors for four weeks and sucralfate for two weeks. A 4-week prednisone-based protocol was administered to patients with semi-circumferential resection over 75% of their circumference. The protocol consisted of 30 mg of oral prednisone starting on the third postoperative day, and the dose was tapered over 4 weeks period: 30 mg/day week 1, 20 mg/day week 2, 10 mg/day week 3, and 5 mg/day week 4.6
Statistical analysis
Data were tabulated using Microsoft Excel for Windows 2010 (Microsoft Corp., Redmond, WA, USA), and statistical analyses were performed using IBM SPSS ver. 24.0 (IBM Corp.). A descriptive analysis of the data was performed with frequency and proportion for categorical and average variables, and standard deviation (SD), median, and mean±SD for continuous variables.
Ethical statements
The authors declare that the study consisted of a retrospective assessment of Western experience with more than 100 consecutive procedures in esophageal ESD and was conducted in accordance with the Declaration of Helsinki. This study was limited to the analysis and description of the statistical calculations of one of the largest cases of esophageal ESD in the West. This study was conducted in an endoscopic referral center in Brazil with informed consent obtained from the patients, and the research received initial institutional review board (IRB) approval in 2019 and updated IRB approval in 2021 both attached by the Hospital das Clinicas, Federal University of Minas Gerais (IRB No: 77/2019).
RESULTS
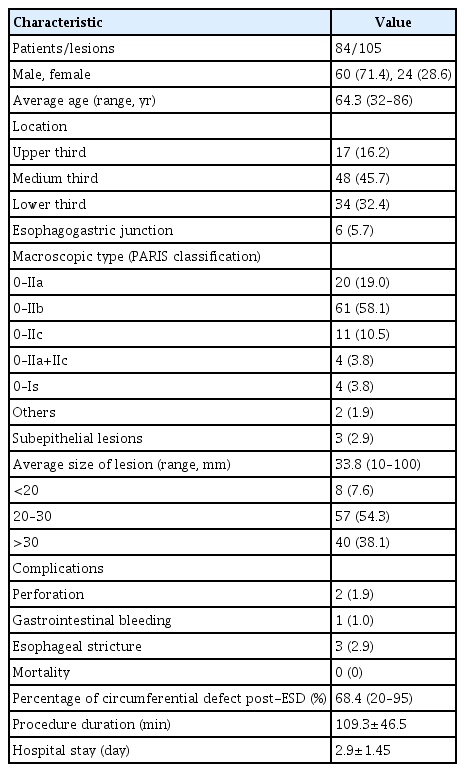
During the study period, 108 esophageal ESDs were performed on 87 patients. Three procedures were discontinued due to the presence of a non-lifting sign and the impossibility of safely carrying out submucosal dissection. Of the 84 patients included in the analysis, 24 were women (28.6%) and 60 were men (71.4%). The average age was 64.3 years (SD, ±10.9 years). The mean size of the lesions was 33.8 mm (SD, ±16.2 mm). The mean duration of the procedure was 109.3 minutes (SD, ±49.0 minutes). The distribution of the lesions was as follows: upper third, 17 tumors (16.2%); medium third, 48 (45.7%); lower third, 34 (32.4%); and esophagogastric junction, 6 (5.7%). Table 1 shows the clinicopathological characteristics of the patients.
Of the 84 patients, nine underwent more than one ESD because of synchronous esophageal neoplasms. In three patients, the second ESD was performed in the same endoscopy session, while in five cases, the second ESD was performed in a different endoscopy session due to clinical conditions of the patient and comorbidities or because of the larger size of the lesion with a prolonged procedure. Of the 105 resected lesions, 70 (66.6%) were squamous cell carcinomas, 18 (17.1%) were high-grade intraepithelial neoplasia, 12 (11.4%) were esophageal adenocarcinomas, three (2.9%) were granular cell tumors, and two (1.9%) were low-grade intraepithelial neoplasia. It should be noted that the 20 intraepithelial neoplasias (dysplastic lesions) found in our casuistry were equally distributed with respect to the epithelium of origin, with ten cases (50.0%) developing on squamous epithelium and the remaining 10 on Barrett's epithelium. Regarding macroscopic morphology, 61 (58.1%) lesions were classified as 0–IIb, 20 (19.0%) lesions were 0–IIa, 11 (10.5%) lesions were 0–IIc, 4 (3.8%) lesions were 0–IIa+IIc, 4 (3.8%) lesions were 0–Is, 3 (2.9%) lesions were elevated with a subepithelial appearance, 1 (0.9%) lesion was 0–IIa+IIb, and 1 (0.9%) lesion was 0-Is+IIb.
An overall en bloc resection rate of 96.2% (101/105) was achieved. In seven cases, ESD was completed with en bloc snaring of the dissected lesion due to technical difficulties. Complete tumor resection with histologically free margins was obtained in 85 of the 105 lesions (81.0%). In 68 lesions (64.8%), the procedure was considered to be curative. In the carcinoma group, the tumor invasion depth was distributed as follows: 55 lesions with intramucosal tumor (adenocarcinoma, 4; squamous cell carcinoma, 51), 8 lesions with superficial submucosa (SM1) invasion (adenocarcinoma, 3; squamous cell carcinoma, 5), and 19 tumors with deep submucosal (SM2) invasion (adenocarcinoma, 5; squamous cell carcinoma, 14). The histopathological characteristics of the included patients are shown in Table 2.
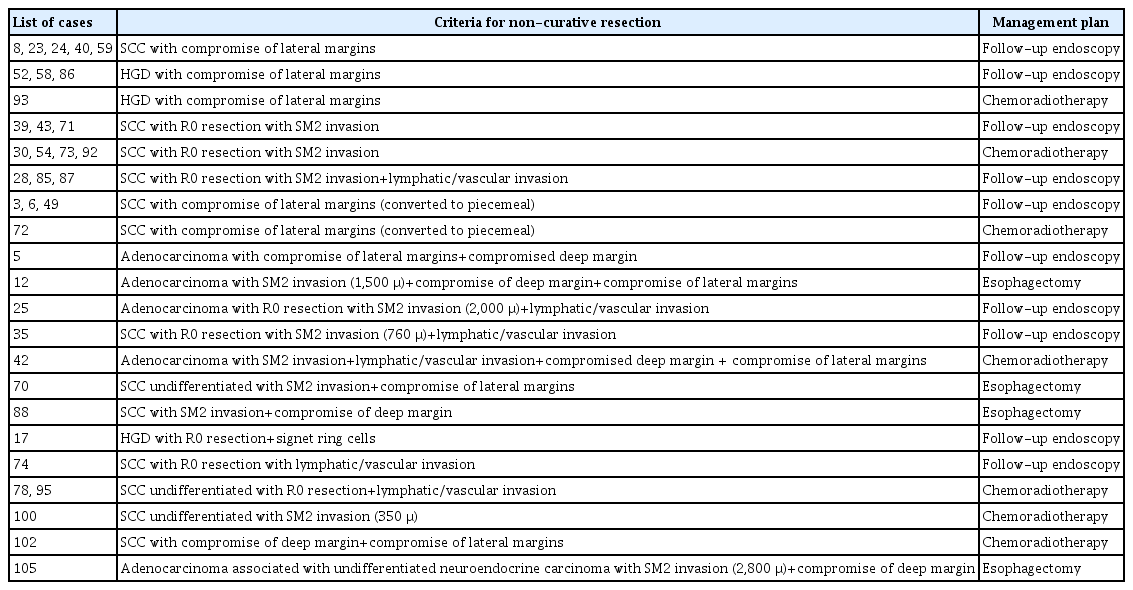
There were no mortality cases during the 30-day postoperative period or mortality related to the procedure. Among the 37 lesions (35 patients) considered to be non-curative (35.3%), 21 (22/37 lesions; 59.4%) were managed with endoscopic follow-up only, as they were patients with positive lateral margins or with multiple comorbidities and high surgical risk, unsuitable for additional interventions. The remaining 14 patients with non-curative ESD were discussed on a multidisciplinary tumor board, and in ten patients (11/37 lesions; 29.7%), a decision was made for adjuvant chemoradiotherapy, and four patients (4/37 lesions; 10.8%) underwent esophagectomy. The main indications for adjuvant chemoradiotherapy were lymphovascular invasion, SM2 invasion, and compromised deep margins. Table 3 shows the list of patients with the criteria for non-curative ER and subsequent management plans.
The mean duration of hospital stay after the procedure was 2.9 days (SD, ±1.5 days). Six patients (6 lesions) experienced adverse events (5.7%); one case of post-ESD gastrointestinal bleeding, two cases of intraoperative esophageal perforation, and three cases of postoperative esophageal stricture (ES). The overall ES rate was 2.9% (3/105 lesions). All adverse events were successfully controlled endoscopically, including two perforations by clip closure, a case of gastrointestinal bleeding by clip hemostasis associated with thermal coagulation and three 3 cases of ES by sessions of endoscopic balloon dilation with a mean of two sessions of endoscopic dilation up to 15 mm in diameter. Figure 2 shows an illustrative case of esophageal ESD.
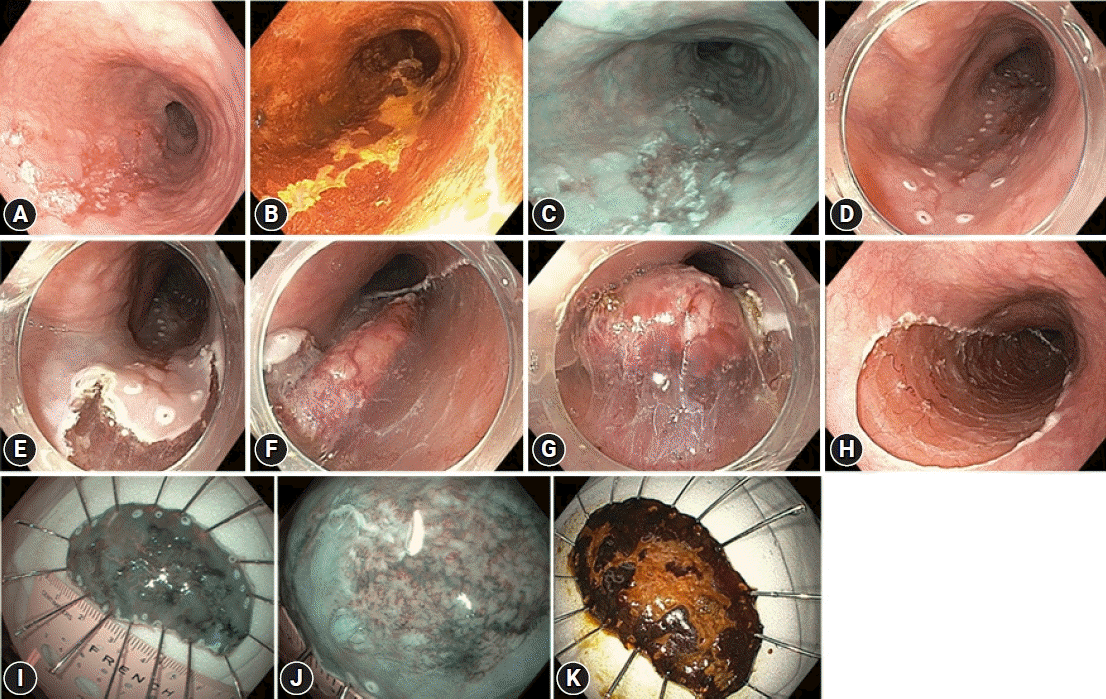
An illustrative case of esophageal endoscopic submucosal dissection (ESD). (A) A slightly depressed, erythematous lesion (type 0-IIc) was observed in the distal esophagus in white-light view. (B) Lugol chromoendoscopy demonstrating lesion extension. The biopsy findings were consistent with those of squamous cell cancer. (C) Narrow band imaging (NBI) view revealing a slightly depressed neoplasm with clear margins and a good indication for endoscopic resection. (D) Markings were placed. (E) After submucosal injection of sodium hyaluronate, oral incision was started. (F) After circumferential incision and submucosal dissection, a flap was created toward the gravity side. (G) ESD was performed in the oral to anal direction. A clear view of the submucosal space was noted for trimming. (H) ESD was successfully accomplished with a final defect occupying 50% of the circumference and 6 cm in longitudinal extension. (I) A 60-mm specimen was fixed for histological assessment. The NBI view showing all the markings inside the specimen. (J) Closed-view NBI demonstrating a mixed B1 microvascular pattern with minimal avascular areas. (K) Lugol chromoendoscopy of the specimen revealing a tumor with free margins. Histology revealed squamous cell carcinoma with lamina propria invasion (M2), free margins, and no lymph/vascular invasion. ESD was considered curative, and endoscopic follow-up was recommended.
Endoscopic follow-up for a median period of 18 months (range, 3–156 months; mean: 29.7 months) was performed, disclosing four cases of local recurrence (3.9%) and nine cases of metachronous lesions (8.9%). Among the group of recurrent lesions, one occurred in the first 3 months and the other three originated after 20 months of endoscopic follow-up and thereafter. Of the four patients with local recurrence, three were referred for chemoradiotherapy, and one patient who was unfit for surgery was kept on for close endoscopic surveillance. With regard to metachronous lesions, three cases were detected within the first 6 to 12 months of follow-up, and the other cases were detected after at least 19 months of follow-up. In four patients, the metachronous lesion was managed by EMR, two patients with advanced metachronous lesions underwent esophagectomy, one patient underwent an additional ESD, one patient was referred for chemoradiotherapy, and in one patient, a new ESD was contraindicated due to severe pulmonary disease; thus, only endoscopic surveillance was recommended.
DISCUSSION
This case series adds further evidence to the efficacy and safety of esophageal ESD in the treatment of superficial esophageal neoplasms, but only in a Latin American population and by a single trained operator. The main strength of our study is that it reports 105 consecutive esophageal ESD procedures in the West, to our knowledge, the largest published experience from Latin America, presenting clinical outcomes similar to those obtained in world reference Japanese endoscopic centers. Moreover, this study highlights the importance and relevant impact of Japanese centers of excellence offering ESD training to international trainees to disseminate ESD all over the world.
One of the main issues prior to the therapeutic approach for superficial carcinomas of the esophagus is the assessment of tumor invasion depth, which influences the management plan.7-11 In addition to EUS, recent studies from Asia have shown that endoscopic assessment using improved imaging technologies by an expert operator can obtain reliable results. Mizumoto et al.12 performed a retrospective study in 174 patients with early esophageal squamous cell carcinomas and compared the effectiveness of EUS and magnifying endoscopy with NBI (ME-NBI) to estimate tumor invasion depth. The authors demonstrated significantly higher sensitivity and accuracy for ME-NBI compared to EUS in distinguishing squamous cell carcinoma limited to the epithelium or lamina propria from those that invade the muscularis mucosae (MM) or superficial submucosa and more deeply invasive lesions before ESD (sensitivity, p=0.048; accuracy, p=0.017). These findings show that endoscopic analysis of lesions with IEE tools could be used as an effective alternative in endoscopic centers where EUS expertise is unavailable. In our study, in the first period, we frequently used EUS (either miniprobe or radial echoendoscopes) to assess tumor depth invasion. Recently, we prioritized the use of IEE, particularly with virtual chromoendoscopy and magnification, to estimate the depth of invasion and to select patients for ER. Our current protocol for the selection of patients for ESD relies mainly on endoscopic assessment with virtual chromoendoscopy (blue laser imaging or NBI) and magnifying endoscopy, considering the microvessel morphology (intrapapillary capillary loops) according to the current classification proposed by the Japan Esophageal Society.13 If a lesion has Japan Esophageal Society type B3 or features suspicious for deep SM invasion, such as nodules, ulceration, or depression, we consider additional staging procedures such as EUS and computed tomography.
In the early periods of endoscopic therapy, superficial esophageal carcinomas were treated with esophagectomy with considerable post-surgical morbidity. The development and improvement of ER techniques, such as EMR and, more recently, ESD, has revolutionized this management strategy. At present, different studies have shown great benefits of ESD over surgery in the eradication of early esophageal carcinomas.14,15 Min et al.16 found in a comparative study of 240 patients (ESD, 120; esophagectomy, 120) during a follow-up period of 5 years; overall survival, disease-specific survival, recurrence-free survival, and metachronous recurrence-free survival rates of 93.9% vs. 91.2%, 100% vs. 97.4%, 92.8% vs. 95.3%, and 90.3% vs. 100% for ESD and esophagectomy groups, respectively (p=0.004). The authors also noted adverse event rates of 55.5% and 18.5% for the esophagectomy and ESD group, respectively (p<0.0001). The same type of observation can be demonstrated in this study, indicating that with proper training and after experiencing the learning curve period, ESD can be consolidated as a safe and efficient therapy for early esophageal tumor management, not only in Asia but also in different Western countries.
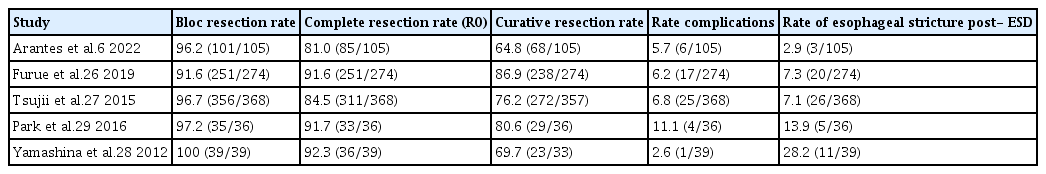
The limited experience with esophageal ESD in the treatment of superficial esophageal neoplasms outside Asia is mainly due to its high technical complexity and long learning curve.17 Few studies representing Western experience with ESD for superficial esophageal carcinomas have been published thus far.18-20 Our group reported our initial experience with esophageal ESD in 2013. The en bloc, complete, and curative resection rates obtained in this first period were 92.0% (23/25 lesions), 84.0% (21/25 lesions), and 80.0% (20/25 lesions), respectively, with a complication rate of 12.0% (3/25 lesions), two subcutaneous emphysemas, and one perforation.21 If we compare these numbers to the data accumulated over the entire cohort, we observed that the en bloc resection rate increased to 96.2%, and the adverse event rate dropped to 6%. Nevertheless, the rates of complete and curative resections decreased to 81.0% and 64.8%, respectively. We hypothesized that after accomplishing the learning curve period and with accumulated experience, we have admitted for ESD more challenging cases and larger tumor lesions with higher rates of submucosal invasion or lymphovascular compromise, which ultimately resulted in a non-curative intervention despite complete tumor removal. The compilation of our cumulative data shows that in the West, rates of clinical efficacy and complications associated with esophageal ESD can be achieved in a similar proportion to those reported in Asian studies,22-25 with a significant improvement over time after further accumulating experience with the method.26-29 Table 4 presents a comparative analysis of our results in terms of esophageal ESD clinical effectiveness in different published case series.6,26-29
The current study had some limitations. The study population was heterogeneous and included premalignant dysplastic lesions originating from both squamous epithelium and Barrett’s-related adenocarcinoma, and subepithelial tumors (granular cell tumors). Nevertheless, in all cases, the same ESD approach was used, enabling the appreciation of the clinical utility of ESD in a wide range of superficial neoplasms. The number of cases included in our casuistry can be considered relatively small when compared to previous studies conducted in Asia; however, to our knowledge, this is one of the largest case series from Western centers, particularly from Latin America. In addition, since this is a single-operator experience report of patients who received focused training in Japanese centers, the data presented in this manuscript may not be representative of endoscopy units without operators with extensive training in ESD and cannot be generalized.
In conclusion, esophageal ESD for the optimal eradication of superficial neoplasms is a reality in the West and Latin America, with high clinical efficiency and low complication rates in expert hands similar to those obtained in Japanese referral endoscopic centers, with the potential to become the first-line treatment for this type of neoplastic lesion.
Notes
Conflicts of Interest
The authors have no potential conflicts of interest.
Funding
None.
Acknowledgments
We gratefully acknowledge the staff at the Department of Gastroenterology, Kobe University and International Clinical Cancer Research Center, Kobe, Japan for their participation and for making this study possible.
Author Contributions
Conceptualization: all authors; Data curation; all authors; Formal análisis: all authors; Investigation: all authors; Methodology: all authors; Project administration: all authors; Resources: all authors; Software: all authors; Supervision: all authors; Validation: all authors; Visualization: all authors; Writing–original draft: all authors; Writing–review & editing: all authors.