Endoscopic vacuum therapy for treatment of spontaneous and iatrogenic upper gastrointestinal defects
Article information
Abstract
Background/Aims
Endoscopic vacuum therapy (EVT) can heal a variety of defects within the gastrointestinal (GI) tract via applying negative pressure, which reduces the defect size, aspirates the infected fluid, and promotes granulation tissue. Here we present our experience with EVT as it relates to both spontaneous and iatrogenic upper GI tract perforations, leaks, and fistulas.
Methods
This retrospective study was conducted at four large hospital centers. All patients who underwent EVT between June 2018 and March 2021 were included. Data on multiple variables were collected, including demographics, defect size and location, number and intervals of EVT exchanges, technical success, and hospital length of stay. Student t-test and the chi-squared test were used to analyze the data.
Results
Twenty patients underwent EVT. The most common defect cause was spontaneous esophageal perforation (50%). The most common defect location was the distal esophagus (55%). The success rate was 80%. Seven patients were treated with EVT as the primary closure method. The mean number of exchanges was five with a mean interval of 4.3 days between exchanges. The mean length of hospital stay was 55.8 days.
Conclusions
EVT is a safe and effective initial management option for esophageal leaks and perforations.
INTRODUCTION
Negative pressure or vacuum therapy has been widely applied in the surgical field since 1993, when it was initially used for complex bone fractures to promote wound cleansing and granulation tissue proliferation.1 It has since been used in nearly all surgical disciplines for postoperative wound closure. Vacuum therapy was initially described in 2004 for intraluminal gastrointestinal (GI) applications by surgeons familiar with its technical aspects and effectiveness.2 The endoscopic vacuum therapy (EVT) procedure involves affixing a piece of polyurethane sponge used in conventional wound vacuum applications to the tip of a nasogastric (NG) feeding tube. The assembled EVT device is then positioned under endoscopic guidance, either within or across the luminal defect, to fully occlude the defect. The NG tube was then attached to a commercially available vacuum device and set to the desired negative pressure, typically 150 to 175 mmHg.2
The first case series of EVT use for anastomotic leaks after rectal resection was published in 2008.3 In the same year, a case report of two patients demonstrated EVT success for upper GI anastomotic leaks.4 Given vacuum therapy’s early adoption within the surgical field, EVT was utilized in the post-surgical treatment of anastomotic leaks within the rectum and upper GI tract, with reported success rates exceeding 90%.5 Some centers subsequently adopted EVT as a prophylactic measure to prevent anastomotic leaks.6 The theoretical benefits of EVT include its ability to create negative suction with increased tissue perfusion, the aspiration of exudate and pus, the promotion of granulation tissue, and secondary wound closure.7 Retrospective case-control studies have shown superior results of endoscopic vacuum versus stent therapy in terms of defect closure success, mortality rates, median treatment duration, and complication rates.8
Most literature to date on EVT revolves around post-surgical anastomotic leaks, with limited evidence in other applications, such as iatrogenic perforation or nonsurgical fistula management. Given the limited evidence for EVT in applications outside anastomotic wound closure, here we present our experience with EVT in three large academic hospitals and one community hospital in a variety of iatrogenic, acquired, and post-surgical scenarios.
METHODS
This was a retrospective case series of patients who underwent EVT between June 2018 and March 2021. The enrollment criteria included having undergone at least one procedure with endoscopic vacuum placement. The collected variables included age, race, sex, medical comorbidities, indications for EVT placement, luminal defect size, presence of sepsis, previous endoscopic or surgical management of the intraluminal defect prior to EVT, time from defect identification to EVT placement, EVT placement location, number of EVT exchanges, time interval (days) between EVT exchanges, hospital length of stay, mortality, and follow-up duration.
Data were obtained through a medical record review that was independently performed by two experts. The primary outcome was the rate of complete defect closure with EVT. Follow-up duration was calculated from the time of initial presentation to the treating institution to the time of the last clinical follow-up as documented in the medical records. Time to healing was defined as the time between the first and last EVT procedures in patients with partial or complete defect closure. Subgroup analyses were performed to determine differences in characteristics between patients in whom complete versus incomplete closure was achieved.
Pre-EVT endoscopy was performed to confirm luminal defect location and size. After the decision is made to proceed with EVT, an open-pore polyurethane sponge is manufactured at the bedside based on defect size, and intra-cavitary or intraluminal placement is utilized (Fig. 1). The sponge was sutured to the distal end of the NG tube and positioned under endoscopic guidance. After EVT placement, suction was applied via an electronic vacuum device with a negative pressure of 125 to 175 mmHg in a continuous fashion. The endoscope was removed and the sponge left in place for 3 to 5 days, at which time it was removed and the defect endoscopically re-evaluated. The EVT was replaced and exchanged at a recurring interval of 3 to 5 days until the defect/cavity had completely resolved. Intraluminal contrast was then administered to confirm defect closure via fluoroscopy.
Data are expressed as mean±standard error of the mean or as median and range. A statistical analysis was performed using Student t-test as appropriate. p-values of <0.05 were considered significant.
Ethical statement
Approval was obtained from the Institutional Review Board of Baylor College of Medicine (No. H-52709).
RESULTS
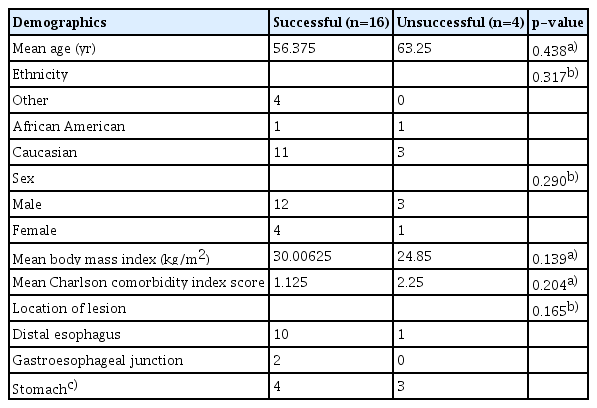
Twenty patients underwent EVT between June 2018 and March 2021. Of them, 15 (75.0%) were male. In our group, 14 (70.0%) were Caucasian, three (15.0%) were Hispanic, two (10.0%) were African American, and one (5.0%) was Asian. The mean body mass index was 29 kg/m2, while the mean Charlson comorbidity index was 1.4 (Table 1).
The defect cause was spontaneous esophageal perforation in 10 patients (50.0%), iatrogenic in six (30.0%), and fistula formation in four (20.0%). Spontaneous esophageal perforations were in the setting of food impaction (n=7, 70.0%), foreign body ingestion (n=1, 10.0%), and Boerhaave syndrome (n=2, 20.0%). Iatrogenic perforations occurred in the setting of surgery (n=5, 83.3%) or post-esophageal dilation (n=1, 16.7%). All fistula formations occurred during surgery. The most common defect location was the distal esophagus (n=11, 55.0%), followed by the gastroesophageal junction (GEJ) (n=2, 10.0%), sleeve gastrectomy site (n=2, 10.0%), gastric fundus (n=2, 10.0%), pylorus (n=1, 5.0%), gastrojejunal anastomosis (n=1, 5.0%), and esophagogastric anastomosis (n=1, 5.0%). The closure success rates were as follows: distal esophagus, 91%; GEJ, 100%; sleeve gastrectomy site, 100%; gastric fundus, 100%; pylorus, 0%; gastrojejunal anastomosis, 100%; and esophagogastric anastomosis, 0%.
The average defect size was 21.2 mm. The average time between presentation and EVT initiation was 9.8 days. The mean healing was 24.7 days. Seven patients (35.0%) were treated with EVT alone, while the remaining 13 patients (65.0%) received adjunctive therapy with an esophageal stent, over-the-scope clip, double pigtail stent, percutaneous drainage, endoscopic suturing, or argon plasma coagulation. Adjunctive therapy was used to close small residual defects at the conclusion of EVT therapy in seven patients (53.8%). Adjunctive therapy was used in conjunction with, after EVT failure, and prior to EVT in 3 (23.1%), 2 (15.4%), and 1 (7.7%) patient, respectively.
All 20 patients had an infection at the time of presentation and were treated with antibiotics. The patients received an extremely wide variety of antibiotics depending on the chronicity of the injury and culture results. Most patients initially started on broad-spectrum antibiotics covering gram-positive, gram-negative, and anaerobic organisms that was later narrowed. Seventeen patients also received antifungal treatment. The mean antibiotic therapy was 49.9 days.
The outcomes and mean exchange intervals for each patient are shown in Figure 2. Technical success was achieved in all patients; there were no procedural complications. Twelve patients experienced complete defect closure, four experienced partial closure, and four did not achieve defect closure. Of the four patients in whom treatment failed, three underwent surgical repair and one was lost to follow-up. The overall mortality rate was 0%. The average length of hospital stay was 55.8 days, while the average follow-up duration was approximately 4 months.
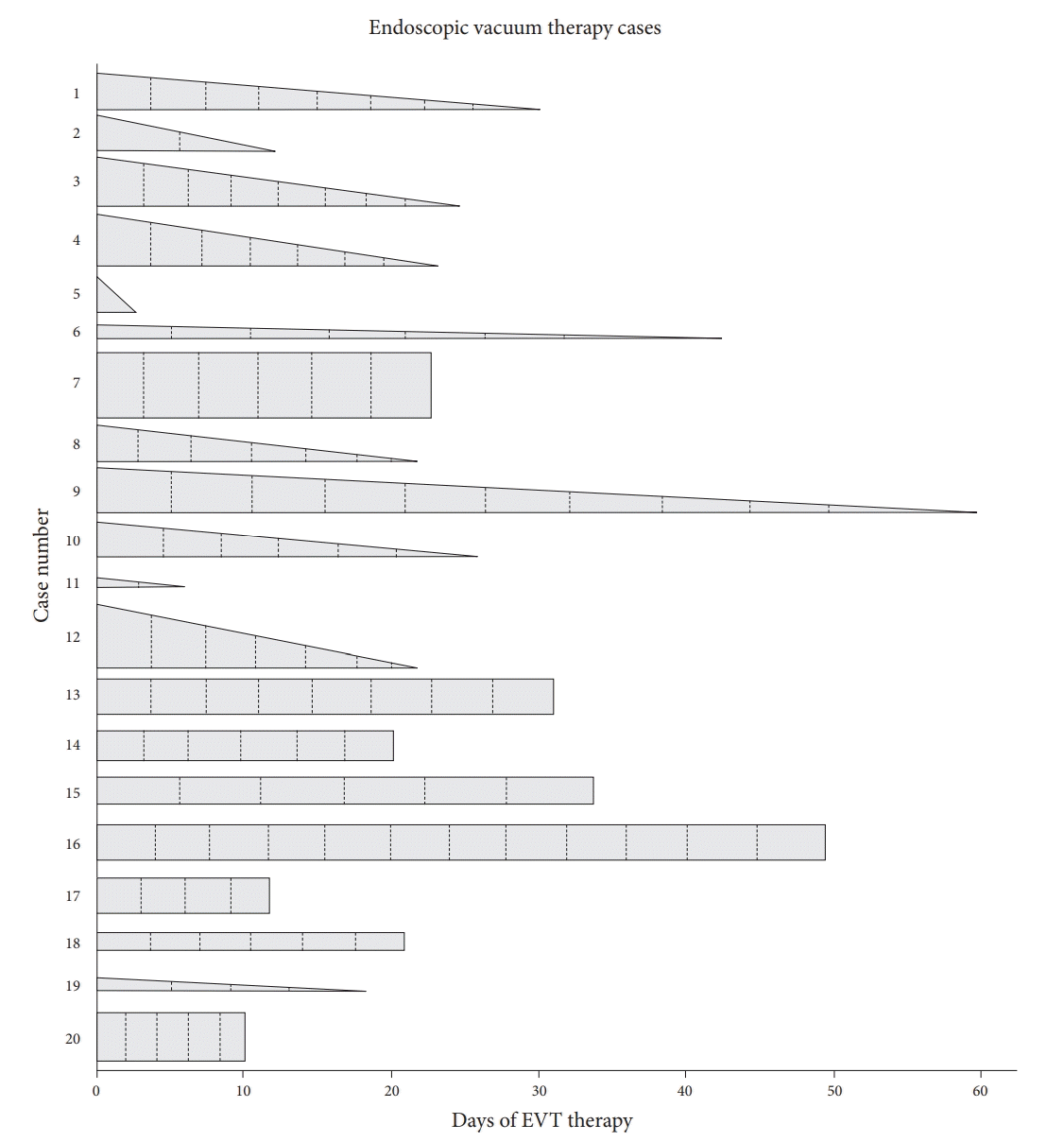
Illustration of endoscopic vacuum therapy (EVT) case series. Relative width of base of each bar represents defect size. Cases with complete closure achieved are represented by triangles, and those without complete closure with rectangles. Dotted lines represent mean time of exchange.
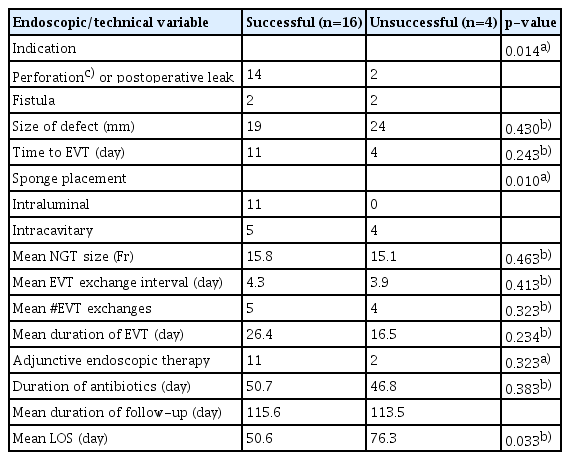
Patient demographics and EVT-related data were compared between patients in whom defect closure was successful versus unsuccessful. Patients in whom defect closure was partially successful were included in the successful group. This was done because the three patients in whom defect closure was partially successful had only diminutive defects after EVT that were successfully closed with adjunctive therapies. The baseline patient characteristics did not differ significantly between patients with successful versus unsuccessful closure. However, patients in the successful group more commonly had an indication of perforation or postoperative leak (88%), while those in the unsuccessful group had an equal number of perforation/postoperative leaks and fistulas (p=0.014). Of the four patients with unsuccessful defect closure, one had a perforation of the gastroesophageal anastomosis, one had a pyloric defect after pyloroplasty, and two had spontaneous esophageal perforation due to food impaction. They also differed in terms of sponge placement area: 69% in the successful group underwent intraluminal sponge placement, while 100% in the unsuccessful group underwent intracavitary sponge placement (p=0.010). The mean length of hospital stay was also significantly higher in the group that did not achieve closure success (51 vs. 76 days, p=0.033). The other EVT characteristics showed no significant differences (Table 2).
DISCUSSION
EVT is an emerging option for managing esophageal leaks and perforations. Several case reports and case series have detailed the successful management of esophageal defects with EVT.3,6-10 Although initially used to treat post-surgical anastomotic leaks, evidence shows that EVT could be used as a prophylactic measure to prevent anastomotic leaks and as a first-line therapy for esophageal defects.11,12 This case series details our experience using EVT for a variety of esophageal defects.
Our success rate of 80% is similar to that reported in the literature.5 The overall success rate of EVT varies based on GI tract location, but it is reportedly as high as 100% in the small bowel, 95% in the esophagus, and 60% in the colon and rectum.5,13 Initial studies showed promising evidence that EVT may be more effective than other commonly used modalities for treating esophageal perforation. A review by Mennigen et al.8 showed that EVT has a similar success rate to over-the-scope clip for iatrogenic perforations but had a higher success rate for other types of perforations. Another study of 45 patients showed that EVT may be more effective than covered stents for the treatment of anastomotic leaks in the esophagus.14 A retrospective analysis of 39 patients showed that EVT was more effective than self-expanding metal and plastic stents for managing intrathoracic esophageal leaks.6 One retrospective analysis of 119 patients showed that intraluminal sponge placement, rather than intracavitary placement, was an independent risk factor for EVT failure.15 In our study, success was achieved in only five of nine (55.6%) patients who underwent intracavitary placement versus 100% of the patients who underwent intraluminal sponge placement. This difference could be explained by the chronic nature of the defects associated with intracavitary placement (fistula formation) versus intraluminal placement (perforation). The small sample size also likely contributed to this difference.
EVT is a relatively safe procedure with a low complication rate.16 Reported complications include sponge dislocation, migration, and stenosis. The complication rate was low at 0–40%. The mortality rate was as low as 0% in the majority of reported case series.17,18 Mortality is usually due to the failure of organs other than EVT.9,18 Compared with other modalities, EVT may have lower complication rates. However, in one prospective study of five patients, two developed esophageal stenosis after EVT, one of whom died after developing an aortoesophageal fistula after a dilation procedure.16 The rates of esophageal stricture and stenosis may be higher with stents than EVT.6 Although EVT seems relatively safe, it is important to consider adjunctive therapies in patients who develop complications or in those in whom EVT is no longer feasible.
Our mean healing of 24.7 days was similar to that reported in the literature—11 to 29 days with sponge exchanges every 2 to 4 days.15,16 A concern with EVT is cost and resource utilization due to frequent sponge exchanges. Ward et al. reported that the total cost per patient was $4,528 in the endoscopy suite.19 The average length of hospital stay in our case series was 55.8 days, similar to that reported in other studies. In one report of patients who underwent primary EVT with those who underwent rescue therapy, the length of hospital stay differed significantly between the two groups (53 vs. 72 days).7 This suggests a benefit in using EVT as a first-line therapy rather than as a salvage therapy when other modalities fail.
Although this case series represents one of the largest studies on EVT in a wide variety of applications, its limitations include its small sample size, retrospective design, and lack of a standardized EVT protocol.
Our study shows that EVT is a safe and effective treatment for a wide variety of gastrointestinal defects, with high technical and clinical success rates and no complications. EVT should be considered an initial management option for upper GI perforations and leaks, and it can be used in conjunction with other previously accepted therapies.
Notes
Conflicts of Interest
The authors have no potential conflicts of interest.
Funding
None.
Author Contributions
Conceptualization: RES, RJS; Data curation: KP, RES; Formal analysis: KP, JSJ; Investigation: AT, GAK, WMA; Project administration: RJS; Supervision: RJS; Writing–original draft: KP; Writing–review & editing: all authors.