Anesthesia care provider sedation versus conscious sedation for endoscopic ultrasound–guided tissue acquisition: a retrospective cohort study
Article information
Abstract
Background/Aims
We aimed to study the effects of sedation on endoscopic ultrasound–guided tissue acquisition.
Methods
We conducted a retrospective study evaluating the role of sedation in endoscopic ultrasound–guided tissue acquisition by comparing two groups: anesthesia care provider (ACP) sedation and endoscopist-directed conscious sedation (CS).
Results
Technical success was achieved in 219/233 (94.0%) in the ACP group and 114/136 (83.8%) in the CS group (p=0.0086). In multivariate analysis, the difference in technical success between the two groups was not significant (adjusted odds ratio [aOR], 0.5; 95% confidence interval [CI], 0.234–1.069; p=0.0738). A successful diagnostic yield was present in 146/196 (74.5%) in the ACP group and 66/106 (62.3%) in the CS group, respectively (p=0.0274). In multivariate analysis, the difference in diagnostic yield between the two groups was not significant (aOR, 0.643; 95% CI, 0.356–1.159; p=0.142). A total of 33 adverse events (AEs) were observed. The incidence of AEs was significantly lower in the CS group (5/33 CS vs. 28/33 ACP; OR, 0.281; 95% CI, 0.095–0.833; p=0.022).
Conclusions
CS provided equivalent technical success and diagnostic yield for malignancy in endoscopic ultrasound–guided tissue acquisition. Increased AEs were associated with anesthesia for the endoscopic ultrasound–guided tissue acquisition.
INTRODUCTION
Endoscopic ultrasound (EUS)–guided tissue acquisition (EUS-TA) using either fine needle aspiration (FNA) or fine needle biopsy (FNB) is an integral procedure for the evaluation of gastrointestinal (GI) and non-GI malignancies. Common indications for EUS-TA include pancreaticobiliary masses, luminal GI malignancies, lymphadenopathy for luminal GI and pulmonary cancers, subepithelial GI tract lesions and suspected metastases in the liver, adrenals, and peritoneal, pelvic, and pleural cavities.1 EUS-TA is highly sensitive in the diagnosis of solid pancreatic neoplasms (89% pooled sensitivity2) but this varies by lesion type and sensitivity is lower and more variable for other lesions such as malignant pancreatic cystic neoplasms (54%),3 lymphadenopathy (54%–100%), and subepithelial GI tract lesions (46%–93%).1 Other factors that can affect the diagnostic yield include size and location of the lesion,4 tissue acquisition technique,5 needle type6,7 and size,8 endoscopist’s experience,1 and the presence of rapid on-site evaluation (ROSE).9 Little is known about the effect of sedation in EUS-TA. The effect of sedation on the diagnostic yield of EUS has been previously studied, but the data are limited to solid pancreatic lesions.10 Therefore, our study aimed to evaluate the role of sedation on specimen adequacy and the diagnostic yield of malignancy in EUS-FNA/B of solid pancreatic and extra-pancreatic lesions.
METHODS
Inclusion criteria
This retrospective, single-center, cohort study included patients aged >18 years who were found to have suspicious solid lesions on imaging or endoscopy and underwent EUS-TA at Loma Linda University Medical Center from September 2018 to May 2021 for further evaluation.
Procedure technique and sedation type
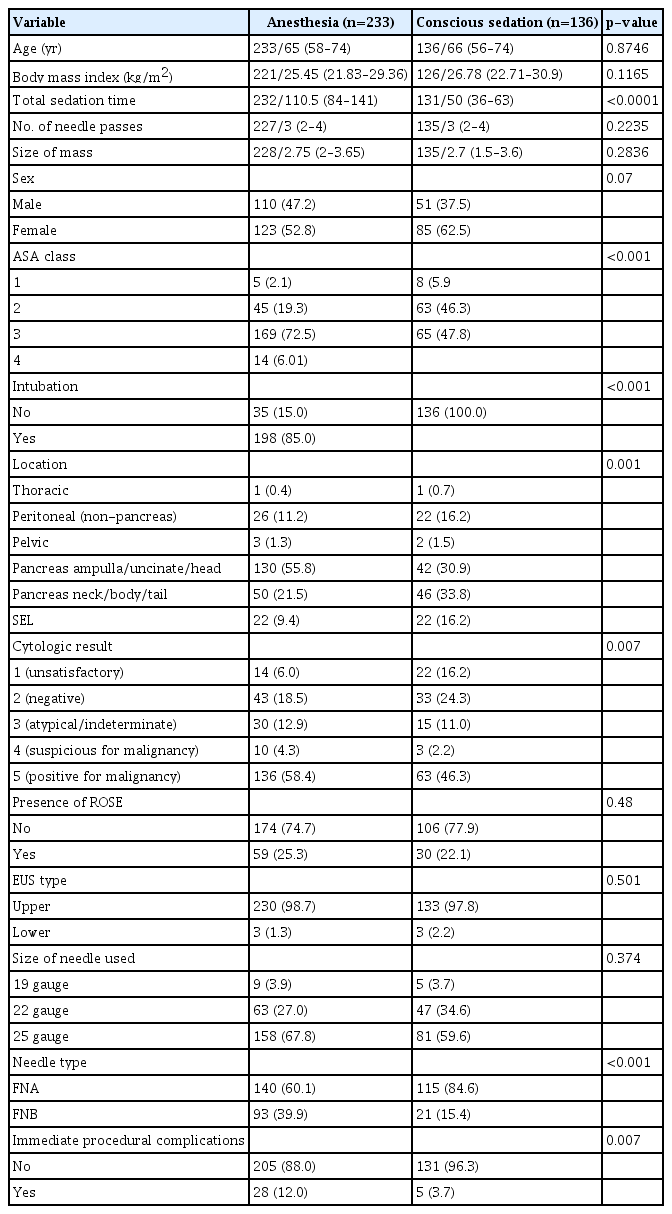
EUS procedures were performed by seven endoscopists with varied experience, all of whom had performed at least 225 EUS procedures. EUS was performed using a curvilinear echoendoscope (GF-UCT140-AL5 or GF-UCT180; Olympus Medical Systems) with patients under conscious sedation (CS), monitored anesthesia care (MAC), or general anesthesia (GA). An endoscopist performed CS. The medications used for CS included midazolam, fentanyl, meperidine, and diphenhydramine. Anesthesia was administered by an anesthesiologist or certified registered nurse anesthetist, collectively termed anesthesia care provider (ACP). Medications utilized by anesthesia included propofol, ketamine, fentanyl, and lidocaine, and were determined by ACP. During the procedure, all patients underwent monitoring per institutional protocol and routine post-procedure recovery and observation. The decision to perform ACP sedation or CS factored in patient comorbidities, procedure complexity, physician preference, and anesthesia availability.
Data collection and definitions
Data were collected from endoscopy software (EndoSoft LLC), electronic medical records (Epic Systems Corporation), and anesthesia documentation records. Variables such as age, sex, lesion location and size, body mass index, American Society of Anesthesiologists (ASA) class, needle size and type, number of needle passes, presence of ROSE, sedation type, intubation, and procedure-related complications were extracted. Cytological results were grouped into five diagnostic categories according to established cytology classifications11: (1) unsatisfactory specimen, (2) negative for malignancy, (3) atypical/indeterminate, (4) suspicious for malignancy, and (5) positive for malignancy. Specimens categorized as 2 to 5 were deemed technically successful. Specimens categorized as unsatisfactory were judged as technically unsuccessful. Benign cases were included in the evaluation of technical success and adverse events (AEs).
We further obtained follow-up data for categories 2 to 4 to determine whether adjunctive testing correlated with the cytologic diagnosis. True negative (i.e., confirmed benign lesions on follow-up) were excluded from the primary endpoint analysis to allow for diagnostic yield calculation according to categorization by established cytology classifications11 that focus on neoplastic lesions. We further dichotomized the five categories into successful and unsuccessful specimens, using a priori definitions. We grouped “suspicious for malignancy” and “positive for malignancy” into the successful group. Unsatisfactory, false negative, or atypical/indeterminate results were considered unsuccessful. The diagnostic yield was defined as the proportion of patients who underwent successful EUS-FNA based on the above-mentioned classification.
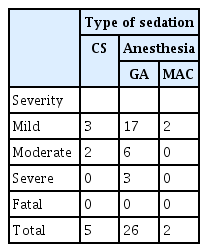
Procedure-related complications were categorized according to established classification,12 where mild severity included complications such as post-procedure medical consultation (i.e., nausea), moderate severity includes unplanned admission or transfusion, and severe severity implies prolonged hospital stay (that is >10 nights) or the need for surgery for AEs and fatalities implies death related to complications of the procedure.
Statistical analysis
Baseline patient characteristics were compared by type of sedation (ACP sedation versus CS) using the chi-square test for categorical variables and Wilcoxon rank sum test for continuous variables.
Univariable and multivariable logistic regression analyses were used to examine the effects of sedation type (ACP vs. CS) on three outcomes: (1) technical success, (2) diagnostic yield of neoplasia, and (3) AEs. In multivariable models, our main predictors were types of sedation, and other key covariables included the size of the mass, location, needle size and type, number of needle passes, and presence of ROSE. These variables were determined a priori and were included in the models as potentially relevant. Odds ratios (ORs) with 95% confidence intervals (CIs) are reported.
All p-values were two-sided, and statistical significance was set at p<0.05. All statistical analyses were performed using SAS Software (ver. 9.4; SAS Institute Inc.).
Ethical statement
This study was approved by the institutional review board at the Loma Linda University Medical Center (approval number: 5210176). Written informed consent was waived by the board.
RESULTS
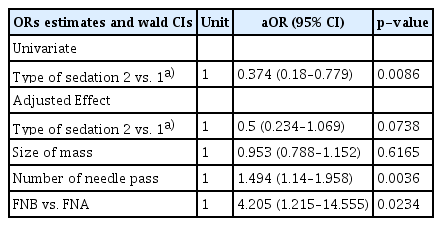
The initial inclusion criteria were met by 369 patients. A total of 136 patients received CS and 233 received ACP sedation (ACPS). Table 1 summarizes the two cohorts. The ACP sedation cohort patients had significantly higher ASA class and more pancreatic lesions, use of FNB needle, and immediate procedural complications. Technical success was achieved in 114/136 (83.8%) in the CS group and 219/233 (94.0%) in the ACP group (p=0.0086). However, after adjusting for mass size, number of needle passes, and needle type, there was no statistically significant difference in technical success between the sedation groups (adjusted OR [aOR], 0.5; 95% CI, 0.234–1.069; p=0.0738) (Table 2).
Sixty-seven patients with confirmed or suspected benign lesions were excluded from the diagnostic yield analysis (Fig. 1). Among the remaining 302 patients, 212 had a successful cytological diagnosis and 90 had unsuccessful cytological results due to unsatisfactory, false negative, or indeterminate results.

Patient flow diagram of included and excluded patients. EUS-TA, endoscopic ultrasound–guided tissue acquisition; CS, conscious sedation; ACP, anesthesia care provider; FNA, fine needle aspiration; FNB, fine needle biopsy.
A total of 134 patients with cytological results were negative, indeterminate, or suspicious. Of these, 75 had follow-up with adjunct testing and 59 had no follow-up. Among these 75 patients, 9 initially had negative cytologic results, which were positive for malignancy on adjunct testing and were therefore recategorized as unsuccessful.
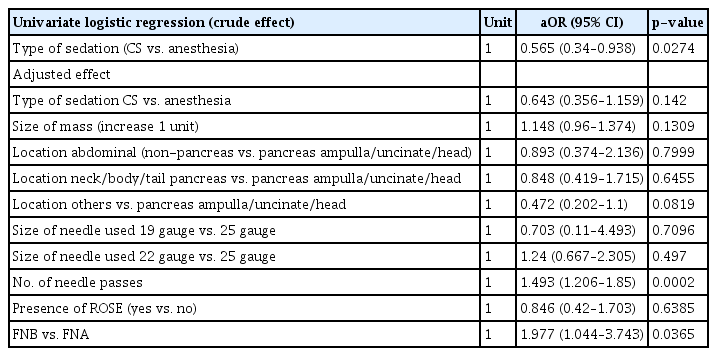
Successful FNA/B was present in 146/196 (74.5%) patients in the anesthesia group and 66/106 (62.3%) in the CS group (p=0.0274). On multivariate logistic regression, the difference in diagnostic yield between the two sedation groups was not significant (aOR, 0.643; 95% CI, 0.356–1.159; p=0.142) (Table 3) after factoring in the size of the mass, location of the mass, size and type of the needle used, presence of ROSE, and number of needle passes.
In evaluating the factors associated with success, the number of needle passes and needle type were both statistically significant (Tables 2, 3). Conversely, the size of the mass, the location of the lesion, needle size, and the presence of ROSE were not significant covariables (Tables 2, 3).
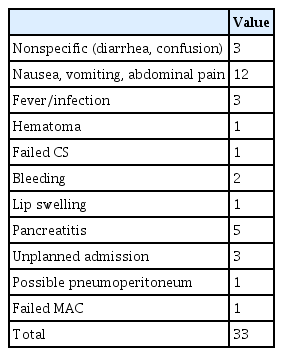
A total of 33 AEs were reported (Tables 4, 5).12 The majority of patients had mild complications (22/33, 66.7%). No fatal complications were reported in either group of patients. A total of 28/33 AEs occurred in the ACP group of which 26/28 (92.9%) occurred in those who received GA. The incidence of AEs was significantly lower in the CS group than in the anesthesia group (OR, 0.281; 95% CI, 0.095–0.833; p=0.022). On multivariate analysis, the presence of intubation nullified the difference in AE rates (OR, 0.471; 95% CI, 0.081–2.721; p=0.3998).
DISCUSSION
We performed a retrospective study comparing sedation type with specimen adequacy and the diagnostic yield of EUS-TA. Our results demonstrated superior technical success (94% for the ACPS group vs. 83.8% for the CS group) and diagnostic yield for malignant lesions (74.5% for the ACPS group versus 62.3% for the CS group) of EUS-TA using anesthesia-provided sedation, although the difference in both technical success and diagnostic yield was not significant on multivariate analyses. Although variable lesion types were included in our study, our overall technical success rates are similar to those in the available literature.13 Nevertheless, our conclusions are contradictory with a prior study that evaluated the diagnostic yield of EUS-FNA of solid pancreatic masses and showed that diagnostic yield was superior in the GA group.10 Our study expanded on the Ootaki et al.10 study in that it included extra-pancreatic lesions and evaluated specimen adequacy in addition to diagnostic yield. Unlike their findings, our findings suggest similar rates of diagnostic yield for both types of sedation in diagnostic EUS. This difference could partially be due to our liberal use of diphenhydramine as an adjunct to sedation, which was not the case in the Ootaki et al.10 study. Diphenhydramine is known to provide a synergistic sedative effect and plausibly allows adequate sedation for effective tissue acquisition. Although our findings are limited by the retrospective single-center nature of our study, we believe that data suggesting equally effective EUS-TA using CS has important implications, especially in low-resource settings.
As mentioned, an important difference in our study was the definition of tissue acquisition success—several studies define success based on specimen adequacy which is defined as the percentage of lesions sampled in which the obtained material is representative of the target site and adequate for cytologic evaluation.14 Although we evaluated this endpoint, we also focused on the diagnostic yield, defined as percentage of lesions sampled for which a tissue diagnosis was obtained, as our primary study endpoint and focused on non-benign lesions to allow for characterization according to established cytology classifications that focus on neoplastic lesions.11 We also believe this to be a more meaningful clinical endpoint given that the majority of tissue acquisition is more malignant lesions.
In our study, the number of needle passes and the needle type were important confounders. The number of needle passes was statistically similar between the two sedation groups (3.29 GA vs 3.14 CS, p=0.2235). The optimal number of passes required to achieve optimal sensitivity remains unclear, but a recent study suggested that a maximum of four passes detected malignancy with 92% sensitivity for solid pancreatic masses.15 There was a detected difference in the use of needle type, which likely accounts for the loss of statistical significance in the difference in both technical success and diagnostic yield after factoring in this variable, as FNA needle use was more prevalent in the CS group. It is well established that FNB needles are superior to FNA needles for tissue acquisition.6,7,16 Other factors, including lesion size and location, needle size, and presence of ROSE, did not affect diagnostic yield in our study.
Our study demonstrated that the incidence of AEs was significantly lower in the CS group than in the anesthesia group (OR, 0.281; 95% CI, 0.095–0.833; p=0.022). This is consistent with a previous study using a large United States national database (n=1.38 million procedures) that compared the safety of sedation administered by anesthesiologists and gastroenterologists. In that study, the odds of AEs were higher for anesthesiologists compared with endoscopist-directed sedation for EGD (1.33; 95% CI, 1.18–1.50) but not for colonoscopy.17 Although known to be risk factors for AEs, ASA class and sedation time did not affect AE rates in our study. However, intubation was the main predictor of AEs. In their study, Vargo et al.17 missed key procedural data points, including intubation, and thus could not identify whether this was an independent risk factor for increased AEs. Our findings contradict those of a previously published randomized controlled trial evaluating GA versus MAC, and the incidence of sedation-related AEs during endoscopic retrograde cholangiopancreatography in known high-risk patients that sedation with GA was associated with a significantly lower incidence of sedation-related AEs compared to those with MAC. In that cohort, the use of MAC was associated with more frequent procedure interruption and conversion to GA, higher cardiopulmonary AEs, post-endoscopic retrograde cholangiopancreatography pancreatitis, infection, and pain.18 In the present study, given that the majority of patients were not considered high-risk as they were primarily ASA class I-III, the risks associated with endotracheal intubation and GA likely outweighed the benefit in this group, thus contributing to more AEs and identifying intubation as a main predictor for these AEs. Although there are guidelines outlining the qualifications required to provide sedation,19 there is little consensus or guidelines explicitly identifying who the preferred provider should be, whether it is an anesthesia provider or endoscopist.20 Given the increased use of anesthesia professionals for endoscopic procedures,21 further study is required to better understand which patients and procedure types require anesthesia and strategies to minimize the risks of AEs for endoscopic procedures performed by ACPs.
Our study had several important limitations. This was a retrospective single-center study conducted at a tertiary medical center, which limits the generalizability and leaves the possibility of unaccounted confounding variables. Furthermore, we were unable to corroborate cytological findings with adjunct studies in all patients; nevertheless, this was employed merely as a quality control to ensure better characterization of diagnostic yield. We believe that this is a partial limitation of the diagnostic yield in our study but had no effect on specimen adequacy or AEs. In addition, our intubation rate was considerably high (85%) for anesthesia provider cases, which likely reflects institutional anesthesia conservative practices and confounds the AE rate difference observed between sedation groups. It is plausible that less GA and deeper sedation with MAC would nullify the differences in AEs rates between the two groups. Lastly, although sedation times were collected for both groups, anesthesia records reported ‘room in time’ as sedation start and ‘post anesthesia care unit patient drop off’ as sedation end and thus accurate comparisons between both groups could not be made.
Despite these limitations, our study suggests that ACPS is equivalent to CS for specimen adequacy and diagnostic yield for malignancy during EUS-TA for suspicious solid lesions. Further studies are needed to validate these findings.
Notes
Conflicts of Interest
The authors have no potential conflicts of interest.
Funding
None.
Author Contributions
Conceptualization: WS; Data curation: SS, YG; Formal analysis: JP; Investigation: WS; Methodology: JJK, WS; Project administration: WS; Software: JP; Supervision: WS; Validation: JP; Writing–original draft: SS, YG; Writing–review & editing: all authors.