Acute necrotizing esophagitis (AEN) is a rare disease characterized by black discoloration on endoscopy. It is often referred to as a āblack esophagusā. The cause of this disease remains unknown, but ischemia is most likely involved.1 AEN typically occurs in elderly males with multiple comorbidities presenting with signs of upper gastrointestinal bleeding. AEN often occurs in patients with underlying diseases, such as diabetes, infections, renal failure, postoperative conditions, and malignancy.2-5 Generally, AEN has a favorable prognosis.6 Surgical intervention is required only in severely ill patients with extensive esophageal ulceration, necrosis, or potential perforation of the esophagus. However, if complicated, esophageal perforation can be fatal in frail patients with underlying diseases.1,7 Here, we report a severe case of AEN following endoscopic retrograde cholangiopancreatography (ERCP). The patient provided informed consent for the publication of this case report.
An 88-year-old woman was admitted to our hospital with abdominal pain and fever. She had a history of hypertension, dyslipidemia, and gallstones, but no history of diabetes or cardiovascular, renal, or malignant diseases. The patient had no history of alcohol consumption. The laboratory findings on admission were as follows: white blood cell count, 13,600/Ī¼L; hemoglobin level, 12.3 g/dL; platelet count, 177,000/Ī¼L; serum albumin level, 4.5 g/dL; aspartate aminotransferase, 662 IU/L; alanine aminotransferase, 264 IU/L; alkaline phosphatase, 634 IU/L; gamma-glutamyl transpeptidase, 328 IU/L; total bilirubin 1.8 mg/dL; and C-reactive protein level, 0.09 mg/dL. The coagulation profile, serum creatinine levels, and blood urea nitrogen-to-creatinine ratio were all within normal ranges. Abdominal computed tomography (CT) revealed a dilated common bile duct and calcified bile duct stone. The patient was diagnosed with moderate acute cholangitis secondary to choledocholithiasis. She had fever, but her vital signs were stable, and she had no concurrent disseminated intravascular coagulation.
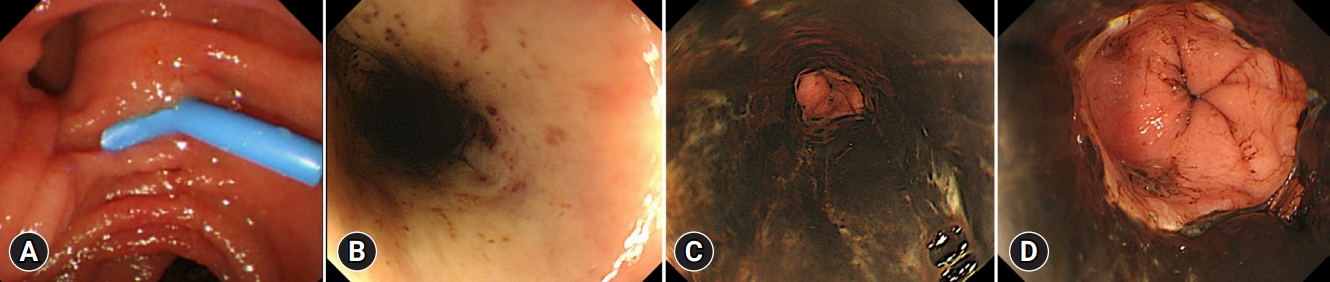
As the patient was elderly and had complications due to cholangitis, we decided to perform only biliary drainage first and perform the stone removal later. Vital signs were carefully monitored using electrocardiography and oxygen saturation assessments during ERCP. The patient received oxygen therapy via a nasal cannula, and carbon dioxide was insufflated during ERCP. The duodenal mucosa appeared normal on endoscopy (Fig. 1A). Endoscopic biliary drainage was performed using a 7-Fr plastic stent (Flexima Biliary Stent System Biliary Drainage Tube Stent; Boston Scientific). The total procedure time was 20 minutes. Her vital signs were stable during ERCP, and radiographs obtained immediately after the procedure showed no abnormalities. The patient did not vomit during the procedure.
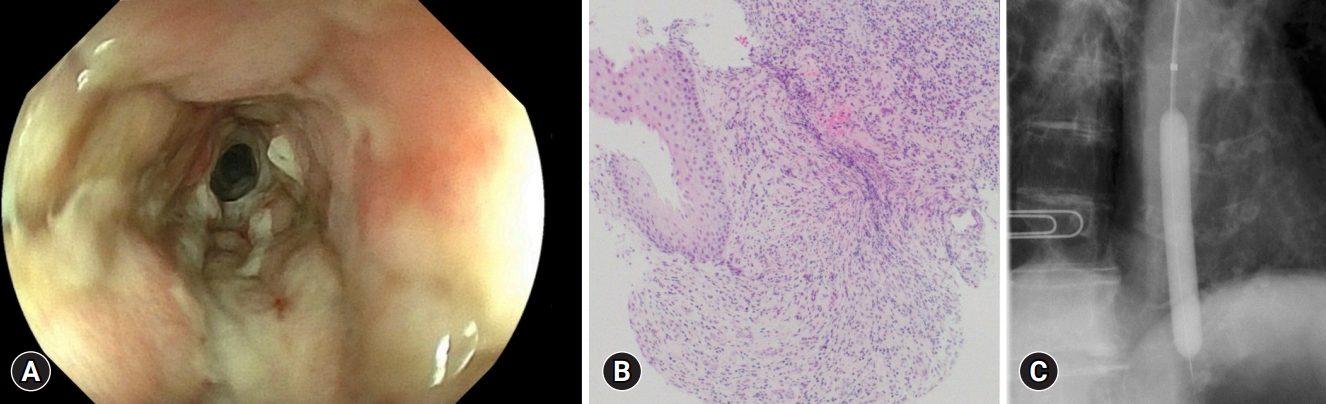
The following day, the cholangitis improved; however, coffee ground vomitus was noted. Emergency upper gastrointestinal endoscopy revealed a black esophageal mucosa with ulcers, mainly in the distal esophagus (Fig. 1BāD). Multiple ulcers were observed in the duodenum. CT revealed no findings suggestive of AEN or post-ERCP pancreatitis. We also performed cardio-ankle vascular index (CAVI) tests. The CAVI values were 9.5/9.6 (right/left), which suggested that the patient had arteriosclerosis. The patient was administered intravenous acid suppression using proton pump inhibitors. However, a follow-up endoscopy revealed stricture formation in the distal esophagus (Fig. 2A). The pathology of the esophageal tissue biopsy revealed degenerated esophageal epithelium and granulation tissue extending into the submucosa (Fig. 2B). Endoscopic balloon dilation was performed using a 10-mm balloon catheter (CRE Balloon Dilatation Catheters; Boston Scientific) (Fig. 2C). After endoscopic balloon dilation, the patient was able to ingest adequate food and was discharged 45 days after ERCP. However, two weeks after discharge, she was readmitted because of vomiting associated with esophageal restenosis. Endoscopic balloon dilation was performed again using a 10-mm balloon catheter (Fig. 3A, B); however, after the endoscopic procedure, the patient complained of severe abdominal pain. Abdominal CT revealed massive free air in the abdominal cavity (Fig. 3C), and the patient was diagnosed with esophageal perforation due to balloon dilation. She received conservative treatment; however, her condition did not improve. Thus, a subtotal esophagectomy was performed. The resected esophageal specimen revealed circumferential erosion and ulcers were observed in the distal esophagus (Fig. 3D, E). Three months after surgery, the patient was transferred to another hospital for rehabilitation.
The AEN was first described by Goldenberg et al.8 in 1990. AEN, also known as black esophagus, is a rare esophageal disease commonly observed in elderly and frail patients with underlying diseases.7,9 The causes of AEN include impaired blood flow, exposure of the esophagus to gastric acid, mechanical damage, infection, and gastrointestinal stricture; however, the definitive cause remains unknown. AEN is diagnosed based on characteristic endoscopic findings and pathological examination of esophageal biopsy tissue.1,7 AEN lesions primarily occur in the distal esophagus. The distal third of the esophagus has fewer blood vessels than the rest of the esophagus and is thus more susceptible to ischemic injury.1,7,10 However, tissue damage may occur diffusely throughout the esophagus.
In the present case, the patient was an elderly woman with no major underlying diseases other than hypertension or lipid abnormalities. She was well-nourished and had no drinking habits. She had acute but not severe cholangitis on admission that was not complicated by disseminated intravascular coagulation. Despite successful biliary drainage via ERCP, the patient developed AEN the following day. Histological examination of the esophagus revealed no evidence of fungal infection. However, the CAVI test indicated arteriosclerosis. Thus, the combination of age-related arteriosclerosis and mechanical damage to the esophageal mucosa caused by ERCP may have reduced the blood flow to the esophagus, resulting in the development of AEN.
Appropriate medical management of AEN includes fasting until clinical status improves, aggressive use of intravenous proton pump inhibitors, and a change to oral agents when oral intake can be resumed.1 Many patients with AEN show improvement with conservative treatment; however, death due to AEN occasionally occurs. In most patients, the endoscopic findings and general condition improve with supportive care from 7 days to 1 month after diagnosis.1,2,6,9 However, in patients with severe underlying diseases, mortality is high; Gurvits1 reported a mortality rate of 31.8%. In addition, stenosis occurs in >10% of patients as a sequela of AEN,1 and endoscopic balloon dilatation may be necessary.3 Esophageal perforation also occurs in 7% of patients,9 but esophageal perforation after endoscopic dilatation of severe esophageal strictures, as in our case, has not been reported. Esophageal perforation is the most serious complication of AEN and may require surgical treatment. Esophageal perforation can be fatal if conservative treatment does not improve the patientās condition.
Although rare, unexpected fatal adverse events, such as AEN, can occur after ERCP. Furthermore, while endoscopic balloon dilatation for esophageal stricture following AEN is a useful procedure, it should be noted that repeated endoscopic balloon dilatation for severe stricture carries the risk of perforation.