Comparison on the Efficacy of Disinfectants Used in Automated Endoscope Reprocessors: PHMB-DBAC versus Orthophthalaldehyde
Article information
Abstract
Background/Aims
Since endoscopes are reusable apparatus classified as semicritical item, thorough reprocessing to achieve high-level disinfection is of utmost importance to prevent spread of infection. To improve disinfection efficacy and safety, disinfectants and endoscope reprocessors are continuously evolving. This study aimed to compare the efficacy of the combination of polyhexamethylenebiguanide hydrochloride-alkyldimethylbenzylammonium chloride (PHMB-DBAC) and orthophthalaldehyde (OPA) used respectively in ultrasonographic cleaning incorporated automated endoscope reprocessors: COOLENDO (APEX Korea) or OER-A (Olympus Optical).
Methods
A total of 86 flexible upper endoscopes were randomly reprocessed with either COOLENDO/PHMB-DBAC or OER-A/OPA. Culture samplings were done at two sites (endoscope tip and working channel) which were later incubated on blood agar plate. Bacterial colonies were counted and identified.
Results
The culture-positive rate at the endoscope tip and working channel was 0% and 2.33% for COOLENDO/PHMB-DBAC and 4.65% and 0% for OER-A/OPA. Staphylococcus hominis was cultured from one endoscope reprocessed with COOLENDO/PHMB-DBAC and Pseudomonas putida was isolated from two endoscopes reprocessed with OER-A/OPA.
Conclusions
The reprocessing efficacy of COOLENDO/PHMB-DBAC was non-inferior to that of OER-A/OPA (p=0.032; confidence interval, -0.042 to 0.042). During the study period, significant side effect of PHMB-DBAC was not observed.
INTRODUCTION
With the increase in the use of endoscopes for diagnostic and therapeutic purposes, the importance of reprocessing has become a top priority to minimize spread of infection through endoscopes. Since the late 1970s there have been sporadic reports of nosocomial infections linked to endoscopic procedures. Bacterial infections have been acquired during endoscopy, caused for example by Salmonella spp., Helicobacter pylori, and Pseudomonas spp.1-4 Transmission of viruses such as hepatitis B and C during endoscopy was not an exception5,6 and transmission of fungi via endoscopic procedures has also been documented.7-9
Since endoscopes are reusable apparatus, cleaning and disinfection are very important to minimize the spread of infection through endoscopes. Endoscopes are classified as semicritical item and thus, high-level disinfection (i.e., the removal of all bacteria except for resistant bacterial spores) is required.10 To standardize and improve disinfection efficacy, automated endoscope reprocessors (AERs) have been developed and are being widely used. The AERs perform automatic flushing, filling, and rinsing processes, with a preset timing for soaking according to the disinfectant or liquid chemical germicides being used. They guarantee proper disinfection of the internal channels and minimize exposure to disinfectants or fumes, thus causing less risk of skin sensitivity and allergic reactions to the medical staffs.
Disinfectants that have been widely used until now include glutaraldehyde, orthophthaldehyde (OPA), peracetic acid, superoxidized water, and chlorine dioxide. Among these agents, OPA is one of the most widely used and recently developed disinfectant11 that has been shown to have better microbiological efficacy compared to that of glutaraldehyde.10 However, there have been reports that exposure to OPA vapors from the endoscopes disinfected with OPA could cause irritation to the respiratory tract and eyes and also anaphylactic reaction.11 Therefore, to enhance safety and improve efficacy of endoscope reprocessing, many efforts are being put into making newer disinfectants and/or combining existing disinfectants. Polyhexamethylenebiguanide (PHMB) is a quaternary ammonium compound and polymeric cationic anti-microbial agent that has been used in ophthalmic solutions, peri-operative cleaning solutions, etc.12 The safety profile of PHMB is also excellent and has broad antimicrobial spectrum including Gram-positive and Gram-negative bacteria, intracellular bacteria, fungi, and viruses such as HIV and HSV. Alkyldimethylbenzylammonium chloride (DBAC) is also a quaternary ammonium compound with antimicrobial activity.
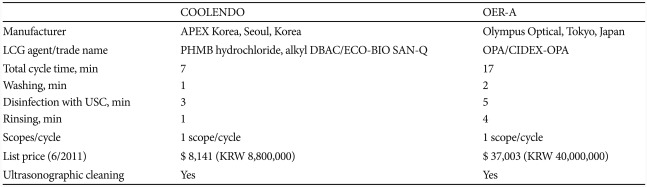
In addition to the evolution of disinfectants, more advanced and upgraded AERs are also being developed to augment cost-effectiveness of endoscope reprocessing, in part by incorporating ultrasonographic cleaning to enhance the efficacy and shorten the time required for reprocessing procedure. OER-A (Olympus Optical, Tokyo, Japan) is one of the widely used endoscope reprocessor that has integrated ultrasonographic cleaning into the AERs and uses OPA or peracetic acid as disinfectant. Recently, a novel endoscope processor, COOLENDO (APEX Korea, Seoul, Korea) has been released to market in Korea (Table 1). This device uses the combined solution of PHMB-DBAC as disinfectant and also applies ultrasonographic cleaning. Compared with OER-A, endoscope reprocessing using COOLEND takes shorter time and is cost effective. Until now, PHMB has not been applied for endoscope reprocessing and COOLENDO is the first AER that has utilized this agent as a disinfectant. The aim of this study is to compare the efficacy of PHMB-DBAC and OPA used as disinfectants in ultrasonographic cleaning incorporated AERs, COOLENDO, and OER-A, respectively.
MATERIALS AND METHODS
Patients and methods
This study was carried out from August 2010 to January 2011 at Korea University Anam Hospital Endoscopy Unit. A total of 16 flexible upper endoscopes (GIF-H260, GIF-Q260; Olympus Optical) that had been used for diagnostic purposes, regardless of the underlying diseases of the patients, were selected to be randomly assigned for endoscope reprocessings. The endoscopes that had been used to perform therapeutic procedures were excluded from the current study. The mean service age of the endoscopes was 57 months. Endoscopic reprocessing was carried out by medical staffs with more than 3 years' experience in this procedure and in accordance with the guideline recommended by Korean Society of Gastrointestinal Endoscopy.13 In short, as soon as endoscopic examination was finished, the outer surface of the endoscope insertion tube was wiped with enzyme detergent moistened gauze, and the working channel was flushed by suctioning the detergent solution while the tip of the endoscope was placed in this agent. The endoscope was then moved to a separate room for disinfection where manual cleaning was first performed by brushing all accessible operating channels and scrubbing the whole outer surface of the endoscope with enzyme detergent moistened gauze again. After rinsing the endoscopes with water, it was then randomly placed in either COOLENDO or OER-A for disinfection.
Endoscope reprocessing procedures of both of these AERs were similar: water washing, sterilization with disinfectant combined with ultrasonographic cleaning, and rinsing. Total time required to carry out endoscope reprocessing was 7 minutes for COOLENDO with each main procedural time as follows: washing (1 minute), sterilization and ultrasonographic cleaning (3 minutes), and rinsing (1 minute). With OER-A, it took 17 minutes with each procedural time as follows: washing (2 minutes), sterilization and ultrasonographic cleaning (5 minutes), rinsing (4 minutes). For sterilization, COOLENDO and OER-A uses ECO-BIO SAN-Q (Choongwae Humantech, Seoul, Korea) and Cydex OPA (Johnson and Johnson, Tokyo, Japan) as disinfectants, respectively (Table 1). ECO-BIO SAN-Q is a disinfectant whose major components are PHMB and DBAC. Cydex OPA is a widely used disinfectant whose major component is OPA.
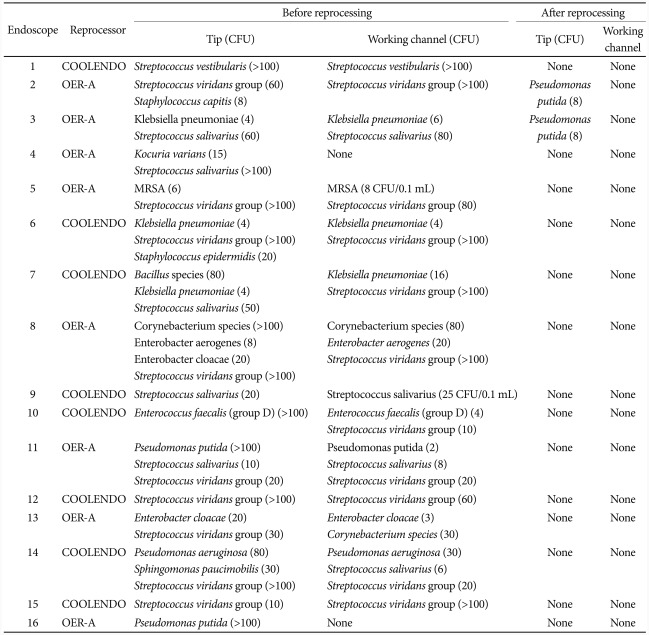
To see whether microorganisms were actually present and cultured from the endoscopes before the reprocessing procedure, samples were collected right after the completion of first 16 endoscopic examinations (Table 2). After confirming that microorganisms were truly present in the majority of the cases before cleaning and disinfection, samples were collected only after the termination of endoscope reprocessing. Two samples were collected from each endoscope, one from the endoscope tip and the other from working channel. The first sample was taken from the tip of the insertion tube using 0.9% NaCl soaked cotton swab, which was streaked onto blood agar plate. The second sample was obtained by flushing down 30 mL of 0.9% NaCl through the working channel to be collected in a sterile tube, and the effluent was later filtered through a 0.22 µm nitrocellulose membrane filter (Millipore, Billerica, MA, USA) which was plated onto blood agar plate.14-17 The blood agar plate was incubated in CO2 incubator at 42℃ for 48 hours for isolation of Campylobacter spp., and at 35.5℃ for 48 hours for isolation of all the other bacteria. The number of colonies on each plate was counted and all cultured bacteria were identified (VITEK 2; BioMérieux, Marcy L'Ètoile, France). This study was approved by Institutional Review Board of Korea University Anam Hospital.
Statistical analysis
Endoscope reprocessing was first performed with 40 endoscopes as a pilot study in order to calculate the sample size. Based on the result of the pilot study, sample size for non-inferiority analysis was calculated using PASS 2008 (NCSS LLC, Kaysville, UT, USA). With 90% power at a significance level of 5%, the minimal sample size required to detect a significant difference was calculated to be 86 endoscope reprocessings (43 endoscopes for each group), the data of which were used for final analysis. Statistical analysis was performed using the SPSS version 13.0 (SPSS Inc., Chicago, IL, USA). p-value of less than 0.05 was considered statistically significant.
RESULTS
Pilot study
A total of 40 endoscopes were reprocessed in a pilot study to calculate the sample size needed to carry out non-inferiority test comparing COOLENDO/PHMB-DBAC (n=20) and OER-A/OPA (n=20) (Table 3). The results of isolated microorganisms cultured from the samples collected before and after reprocessing the first 16 endoscopes are shown in Table 2. Before reprocessing, microorganisms were isolated from all samples taken from the tip and in the majority of samples collected from the working channel. After reprocessing, Pseudomonas putida was isolated from the tip of two endoscopes reprocessed with OER-A/OPA and no organism was recovered from those reprocessed with COOLENDO/PHMB-DBAC. After completing the reprocessing procedure with 16 endoscopes, additional 24 endoscopes were reprocessed and this time, samples were collected only after finishing reprocessing procedure. No additional microorganisms were cultured from the samples, and the results of isolated microorganisms and colony count from 40 endoscopes after the reprocessing procedure are summarized in Table 3. In the end, no microorganisms were cultured from the samples obtained from endoscopes reprocessed with COOLENDO/PHMB-DBAC but P. putida was isolated from two endoscopes reprocessed with OER-A/OPA.
Main study
The sample size calculation indicated that a total of 86 endoscope reprocessings were needed to perform non-inferiority test (Table 3). Since 40 endoscopes had already been reprocessed during the pilot study, additional 46 endoscopes were randomly assigned again to be reprocessed with either COOLENDO/PHMB-DBAC (n=23) or OER-A/OPA (n=23). No microorganisms were subsequently isolated from endoscopes reprocessed with OER-A/OPA after P. putida was recovered from the two endoscopes in the pilot study. However, as for the endoscopes reprocessed with COOLENDO/PHMB-DBAC, Staphylococcus hominis (5 colony forming unit [CFU]) was isolated from the working channel of an endoscope. Therefore, the culture-positive rate was 2.33% (1/43) for COOLENDO/PHMB-DBAC and 4.65% (2/43) for OER-A/OPA (Table 3) and thus, the reprocessing efficacy of COOLENDO/PHMB-DBAC was non-inferior to that of OER-A/OPA (p=0.032; confidence interval, -0.042 to 0.042).
Adverse events
No adverse events attributable to PHMB could be observed among healthcare workers in our endoscopy unit during the study period. Among previously reported adverse events of OPA such as temporary skin discoloration, staining of clothing and surrounding surfaces due to direct contact with this agent,18 only temporary skin discoloration was observed among endoscopists who did not wear gloves during the procedure. In our endoscopy unit, all the endoscopes are reprocessed by AERs always wearing appropriate gloves, fluid-resistant gowns and goggles in the adequately ventilated disinfection room. Most likely as a result of these preventive measures, many of the previously reported adverse events that occur by exposure to OPA vapors such as mucosal irritation, respiratory symptoms, IgE-mediated hypersensitivity reactions, eye irritation, and anaphylactic reactions19,20 were not observed during the study period. Furthermore, no immediate adverse events relevant to the disinfectants used in our study (PHMB-DBAC and OPA) were observed among the patients following endoscopic examinations.
DISCUSSION
Our results show that reprocessing efficacy of COOLENDO/PHMB-DBAC was not inferior to that of OER-A/OPA. On the whole, microorganisms were was isolated from one of the endoscopes reprocessed with COOLENDO/PHMB-DBAC (2.33%) and from two endoscopes reprocessed with OER-A/OPA (4.65%).
COOLENDO uses ECO-BIO SAN-Q as disinfectant which is a mixture of PHMB and DBAC. PHMB is the major component of ECO-BIO SAN-Q used in COOLENDO that has broad spectrum antimicrobial activity against microbes including Gram-positive and Gram-negative bacteria, plaque-forming and biofilm-building bacteria, some spore-forming bacteria, intracellular bacteria such as chlamydiae and mycoplasma, and fungi including Candida spp. as well as Aspergillus spp.21-23 PHMB also has been shown to inactivate HIV-1 and HSV in vitro.24,25 PHMB is a widely used agent that has been mainly used for eye and wound antisepsis. In spite of extensive use of PHMB as preservative in cosmetics and personal care products, the frequency of sensitization remains low and only 3 cases of anaphylactic reactions have been reported in the literature so far.26-28 PHMB is classified as 'practically nontoxic' and no uptake of PHMB from intact skin has been demonstrated.12 Until now, PHMB has not been applied for endoscope reprocessing and COOLENDO is the first AER that utilizes this agent as a disinfectant. In the current study, no adverse effects were observed either to the medical staffs reprocessing the endoscopes, endoscopists or the patients, and the results of our study show that this agent is an effective disinfectant. DBAC is a cationic surface-acting agent belonging to the quaternary ammonium group that has intermediate to low disinfection activity. In ECO-BIO SAN-Q, DBAC is combined to PHMB in order to broaden the spectrum of microbiocidal activity and to increase wetting properties.29 Antimicrobial activity is amplified when the n-alkyl chain of quaternary ammonium compound length is increased by the combination of PHMB and DBAC.29
The reprocessing efficacy of both AERs used in our study is much better than that of the previous studies in which, the culture positive rate after reprocessing ranged from 10.7% to 37.2%.14,16,30 One of the reasons for this difference could be due to the fact that the AERs used in our study were equipped with ultrasonographic cleaning system compared with the previous AERs. Ultrasonographic cleaning uses high frequency sound waves to agitate the aqueous solution or organic compound and can remove material from hard-to-clean areas.31
Application of ultrasonographic cleaning not only improved reprocessing efficacy but also shortened the time required for reprocessing. This was more pronounced with COOLENDO/PHMB-DBAC which only took 7 minutes to complete reprocessing, whereas OER-A/OPA needed 17 minutes to finish the procedure. Increasing the time efficiency for reprocessing could enhance the compliance of high-turnover endoscopy units to conform to the recommended reprocessing guidelines.
In the first part of the pilot study with 16 endoscopes, various kinds of bacteria including P. aeruginosa and MRSA were detected from the pre-reprocessing cultures of tip and working channel. This shows that fatal infection through the endoscopic procedure could occur if the reprocessing is insufficiently and not properly done. After reprocessing with AERs, the microorganism isolated from endoscopes reprocessed with OER-A/OPA was P. putida and that isolated from the endoscopes reprocessed with COOLENDO/PHMB-DBAC was S. hominis. P. putida is found in most soil and water habitats where there is oxygen. P. putida, which belongs to the fluorescent group of Pseudomonas spp. has been recognized as a rare pathogen of bacteremia. Most reported cases of bacteremia with P. putida have been infections in immunocompromised patients or outbreak infections due to transfusion of contaminated blood or fluid.32 S. hominis is a coagulase-negative member of the bacterial genus Staphylococcus. S. hominis has a very low virulence and it occurs very commonly as a harmless commensal on human and animal skin. However, like many other coagulase-negative staphylococci, S. hominis may occasionally cause infection in immunocompromised patients,33 and thus the presence of these organisms after reprocessing should not be overlooked.
This study has several limitations. First, owing to the fact that PCR for viral DNA was not performed, the efficacy against the disinfected virus could not been determined. Since transmission of hepatitis B and C virus has also been previously reported,5,6 testing for the efficacy of ECO-BIO SAN-Q against the aforementioned viruses should also be considered. However, viruses are known to be more easily killed at a lower threshold compared to the bactria (i.e., with intermediate to low-level disinfection).34,35 In addition, the aforementioned viral transmissions occurred after manual cleaning which might not have been adequately performed. Therefore, the use of standardized AERs incorporating ultrasonographic cleaning and adequate disinfectant can be expected to prevent viral transmissions. Second, our study did not perform culture for mycobacterium. Because the incidence of tuberculosis is still not negligible in Korea, evaluation for mycobacteria would be an essential and important step to confirm the efficacy of endoscope reprocessing. However, bactericidal efficacy evaluation by Korean Institute of Tuberculosis demonstrated that >99.9% of this bacterium was killed after contact with PHMB for 3 minutes and thus, it can be speculated that the efficacy would also be comparable in the clinical setting. Third, the sample size was only large enough to perform non-inferiority test and larger sample size would be necessary to perform equivalence trial and superiority trial. However, speculating from the result of the present non-inferiority trial, it could be inferred and expected that COOLEND/PHMB-DBAC would at least be equivalent to OER-A/OPA, albeit not superior.
In this study, we could see that COOLENDO that used PHMB-DBAC as disinfectant is non-inferior to OER-A which utilized OPA as disinfectant. The microorganisms cultured from both AERs were those with low virulence and its microbial burden was also low. COOLENDO is faster than OER-A in terminating the reprocessing, thus being more advantageous with regards to the time factor. In addition, PHMB-DBAC was also safe in terms of side-effects. Concerns about possible transmission of microorganisms during endoscopic procedures has been questioned for decades. With the increase in the demand to perform endoscopy for diagnostic and therapeutic purposes, proper endoscope reprocessing cannot be overemphasized, even more so with increase in the number of immunocompromised patients with acquired immune deficiency syndrome, organ transplant or those undergoing chemotherapy who need endoscopy. Many efforts are being put into enhancing the efficacy, safety, and cost-effectiveness, and COOLENDO/PHMB-DBAC seems to be a good alternative meeting all these needs.
Notes
This work was financially supported by APEX Korea.