Dual Therapy Trial Using Esomeprazole and Amoxicillin as Third-line Rescue Therapy for Helicobacter pylori Infection
Article information
Abstract
Background/Aims
The purpose of this study was to evaluate the efficacy and tolerability of dual therapy consisting of esomeprazole and amoxicillin as a rescue therapy for Helicobacter pylori infection.
Methods
From December 2009 to August 2010, 21 patients who experienced two consecutive eradication failures were included. They received esomeprazole (40 mg, b.i.d.) and amoxicillin (1,000 mg, b.i.d.) for 14 days as a third eradication regimen. Compliance and side effects were determined from an interview. H. pylori status was evaluated using the 13C urea breath test at least 6 weeks after treatment.
Results
The mean age of the patients was 59 years and included 52% males. Indications for treatment were functional dyspepsia (61.9%), peptic ulcer disease (28.6%), and gastric adenoma (9.5%). H. pylori was eradicated in 14 of 21 (66.7%) patients. Minor side effects were reported in three of the 21 patients (14.3%). These side effects consisted mainly of nausea and epigastric discomfort.
Conclusions
A 2-week course of dual therapy failed to show satisfactory results in third-line H. pylori eradication, but it was very safe and tolerable. Therefore, dual therapy constitutes an encouraging empirical strategy for the elderly and infirm patients with multiple previous eradication failures.
INTRODUCTION
Since 1983 when Helicobacter pylori was first cultured, H. pylori infection is known as the main cause of chronic antral gastritis, peptic ulcer disease, low grade gastric mucosa-associated lymphoid tissue lymphoma (MALT lymphoma) and gastric cancer.1 H. pylori eradication therapy is quite effective for the protection of peptic ulcer disease and the treatment of low grade gastric MALT lymphoma.
A triple therapy using proton pump inhibitor (PPI) and 2 antibiotics or a quadruple therapy which includes bismuth to the triple therapy are currently recognized as the most effective eradication therapies and are widely used.2
The eradication rates of these standard regimens, however, are decreasing due to the increased antibiotic resistance, as with the eradication rates reported in South Korea decreasing to 75-85%, which is of great concern.3,4
Triple therapies including quinolone or rifabutin could be used when the first and second eradication therapies were failed.5,6 These therapies, however, have various limitations such as their cost-effectiveness or resistance rates, and there is no definite standard regimen for a 3rd line rescue therapy.
Recent studies reported that a dual therapy consisting of high-dose PPI and amoxicillin provided significant eradication rates in patients who did not respond to the prior H. pylori treatments, suggesting that this new dual therapy could be promising as a rescue therapy.7,8
We evaluated the eradication rate, side effects and compliance of the esomeprazole and amoxicillin dual therapy as a 3rd line therapy in patients of H. pylori infection who did not respond to a 1st and 2nd line eradication therapy, in order to determine its availability as a rescue therapy.
MATERIALS AND METHODS
Patient population
This trial was performed in patients who experienced two consecutive eradication failures, among those who visited Seoul National University Bundang Hospital and were diagnosed as H. pylori infection between September and December of 2010. The patients who received the dual therapy as a 3rd line therapy and were available for the follow up were included in this retrospective analysis. Patients with a history of esophagus or gastric surgery or receiving a treatment for chronic disease other than hypertension or diabetes mellitus were excluded from the study.
Study design
H. pylori test
13C urea breath tests (UBT) were performed each after the first and second eradication therapy, in order to confirm the H. pylori status, by measuring 13CO2 before and 20 minutes after the administration of 13C urea. A patient was determined positive when the level had changed 2.5% or more. The third eradication regimen was provided when the UBT result was confirmed positive after the second therapy. UBT was repeated 6 weeks later to confirm whether H. pylori were eradicated.
Treatment
The dual therapy consisting of esomeprazole and amoxicillin was prescribed to patients who were determined positive in UBT after the first and second eradication therapies. Prescription record was analyzed to identify the drugs prescribed for the first and second therapies, and patients were interviewed to confirm their compliance to these drugs. Patients received esomeprazole (40 mg, b.i.d.) and amoxicillin (1,000 mg, b.i.d.) for 14 days as a third eradication regimen, which were to be taken at least 30 minutes before the breakfast and dinner when the stomach was empty. Compliance and side effects were determined by interview and self-reported symptoms during the visit 6 weeks after completing the eradication regimen. Compliance was deemed good when a patient took more than 85% of the prescribed drugs. The enrolled patients were informed about the possible side effects associated with the esomeprazole/amoxicillin dual therapy and the possibility of eradication failure. The patients provided written informed consent before starting the treatment.
Statistical analysis
SPSS version 17.0 (SPSS Inc., Chicago, IL, USA) was used for statistical analysis, with frequencies expressed by percentage (%). Chi-square test or Fisher's exact test were performed, and p-values of less than 0.05 were considered to indicate statistical significance.
RESULTS
Patients
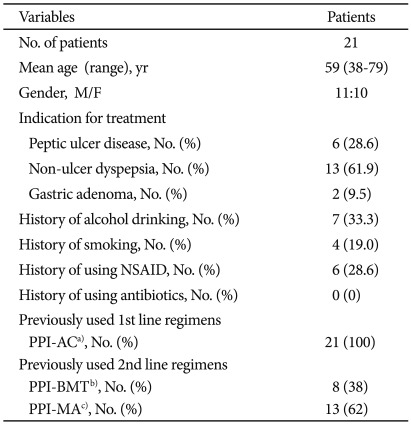
Overall 21 patients were enrolled in this study, of which 11 were male and 10 were female (sex ratio, 1.1:1). The mean age of the patients were 59 years (range, 38 to 79). Some of the patients had the history of alcohol consumption (n=7, 33.3%), smoking (n=4, 19.0%), or nonsteroidal anti-inflammatory drug (NSAID) use (n=6, 28.6%). Six patients (28.6%) had the history of peptic ulcer disease, 13 patients (61.9%) functional dyspepsia, and 2 patients (9.5%) gastric adenoma. Every patient received a PPI-based triple therapy for the first eradication regimen. For the second therapy, 8 patients (38%) received a bismuth-based quadruple therapy and 13 patients (62%) received a moxifloxacin-based triple therapy (Table 1).
H. pylori eradication rate and side effects
Fourteen patients of the overall 21 patients were confirmed negative from the UBT performed 6 weeks after completing the 2-week esomeprazole and amoxicillin period, indicating 66.7% (95% confidence interval, 45-87%) of eradication rate.
All 21 patients took more than 85% of the prescribed drugs. Three patients (14.3%) reported side effects, and the most common symptoms were mild nausea and epigastric discomfort. No patient discontinued the trial due to a test drug-related side effect (Table 2).
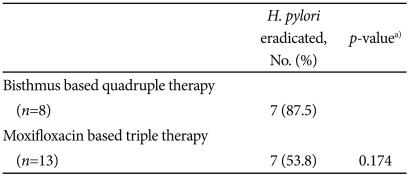
The subgroup of patients who received bismuth-based quadruple therapy showed higher eradication rate than patients who received moxifloxacin-based triple therapy (87.5% vs. 53.8%), but the difference was not statistically significant (Table 3).
There was no significant difference in the eradication rates by gender, underlying disease, smoking status, or NSAID use.
DISCUSSION
According to the report by Yim et al.,9 the prevalence of H. pylori infection in South Korea is still considerably high at 59.6%, although the figure has been dropping. While the number of H. pylori eradication therapy cases has increased a lot thanks to the universal screening esophagogastroduodenoscopy and greater interest in gastrointestinal disease, the success rate of eradication is continuously decreasing, which is of great concern.9
Even the currently recognized and widely used standard triple and quadruple eradication therapies show eradication failure rate as much as 20%.10 More and more patients, therefore, are found still H. pylori positive even after the second eradication therapy, but we still don't have a definite guideline for a 3rd line or more rescue therapy.
As the antibiotic resistance and the number of patients who do not respond to the 1st and 2nd line eradication therapy increases, drugs such as levofloxacin, moxifloxacin, and rifabutin are becoming subjects of studies as a 3rd line therapy. Levofloxacin and moxifloxacin, which are both broad spectrum fluoroquinolones, draw much attention for being highly active, easy to take, and less likely to induce side effects.11,12 It seems prudent, though, to consider carefully before using these drugs widely as a treatment for H. pylori infection, since resistance to fluoroquinolones is rising rapidly due to the increased use of antibiotics.13
Rifabutin is studied mostly as a rescue therapy for patients who failed the first or second eradication therapy, with eradication rates reported around 60-79%.14,15 There is no resistant H. pylori strain reported yet against rifabutin,16 which is why it might be effective as a rescue therapy for patients who did not respond to the existing eradication therapy. Rifabutin, however, is very expensive and possible to induce serious side effects including bone marrow suppression,17 and in some areas where prevalence of tuberculosis is high, such as in South Korea, it is highly possible to induce drug-resistant tuberculosis.
The dual therapy consisting of high-dose PPI and amoxicillin was first introduced in mid-1990s as a first-line regimen against H. pylori,18,19 which was discontinued later due to its lower eradication rate than triple therapies.20 When combined with PPI, however, amoxicillin concentration is increased within gastric mucosa, thanks to the inhibition of gastric acid by PPI, and the minimal inhibitory concentrations (MIC) against H. pylori becomes greatly reduced, which makes it appropriate for eradication regimen.21,22 Also, amoxicillin shows high eradication rate when used as a third-line therapy, since it induces less antibiotic resistance even after used as the first and second-line regimen.23
Recently, Miehlke et al.7 used high-dose PPI/amoxicillin dual therapy as a 3rd line rescue therapy for patients with resistance to metronidazole and clarithromycin, and confirmed the eradication rate as high as 75.6% with minimal side effects, suggesting that this regimen could be a good alternative for a rescue therapy.
The most important factors in H. pylori eradication are drug sensitivity and compliance.24,25 PPI/amoxicillin regimen has strong advantage with this regard, since it induces less side effects than other 3rd line regimen, and it requires patients to take less number of drugs, contributing to the improved patient compliance. Especially older patients are influenced more by regimen and side effects in their compliance to medications. Patients in this study were comprised mostly of older patients with mean age of 59, ranging up to 79 years. It was conceivable, accordingly, that this simple PPI dual therapy would be more effective in this patient population than other complicated, side effect-inducing triple therapies. We tried 3rd line regimen using esomeprazole and amoxicillin, which showed 100% of compliance in all 21 patients. Three out of 21 patients reported only minor side effects, such as nausea and epigastric discomfort, and these side effects did not affect the medication.
The overall eradication rate of this 2-week esomeprazole and amoxicillin regimen was 66.7%, which was less than expected for a 3rd line eradication therapy. Unlike previous dual therapy studies used 3 or 4 times higher dose of PPI than standard dose,7,8 this study used 80 mg of esomeprazole which is twice the standard dose. In South Korea, it is very difficult to use 4 times higher dose of PPI than standard dose, due to the limited medical insurance coverage. The study also has limitation that the sample size is not large enough to evaluate the effectiveness of PPI/amoxicillin dual therapy. Further prospective studies are needed, therefore, using various dose of PPI in larger patient populations.
Most conventional PPI/amoxicillin dual therapies used omeprazole for PPI, supposedly because there were only limited PPIs at the time. This study used esomeprazole, one of the most commonly used PPIs at the moment. A meta-analysis study on eradication rate differences based on the eradication effect of omeprazole did not show any significant eradication rate difference between PPIs,26 which was similar in studies of South Korea.27 It is suggested, therefore, that the eradication rate reported in this study was not influenced much by the use of esomeprazole instead of omeprazole.
When classified according to the 2nd line regimen, the sub-group of bismuth-based quadruple therapy was found to show higher eradication rate than the subgroup of moxifloxacin-based triple therapy (87.5% vs. 53.8%). Although the difference was not statistically significant, given that amoxicillin was included in moxifloxacin-based triple therapy, repeated use of amoxicillin might be associated with the reduced eradication rate. It would be important, therefore, to confirm what prior regimen was used for a patient, before considering PPI/amoxicillin dual therapy as a 3rd line regimen. H. pylori culture and drug sensitivity test is required, in this regard, for additional analysis, but the drug sensitivity test was not performed in this study.
Antibiotic sensitivity is an important factor in H. pylori eradication. A drug sensitivity test is recommended before selecting a 3rd line regimen, because a patient might have already used various antibiotics if the 2nd line therapy was failed.2,28 The patients involved in this study also had already used 3 or more antibiotics through 1st and 2nd therapy. This study, however, did not perform drug sensitivity test, which could limit the result of this study.
Nevertheless, there is much debate on the necessity of drug sensitivity test by H. pylori culture. H. pylori culture is quite difficult, expensive, and time-consuming to perform, and it requires endoscopy to collect samples, further limiting routine culture test.29 In addition, some studies that performed drug sensitivity test against H. pylori before selecting a 3rd line regimen showed rather disappointing eradication rate of less than 60%, raising question to its practicality.30,31
In conclusion, the result of this study proved that a 2-week esomeprazole/amoxicillin dual therapy produces rather limited but moderate 67% eradication rate. This dual therapy showed improved patient compliance and minor side effects, making it a promising empirical 3rd line rescue therapy for patients unsuitable for multiple drug use or with high risk of side effects. This finding warrants further study in a larger sample, since this study is limited by its small sample size. Given that the frequency of H. pylori eradication therapy would increase in the future, continuous study on H. pylori infection rescue therapy is required.
Notes
The authors have no financial conflicts of interest.