Endoscopic Management of Afferent Loop Syndrome after a Pylorus Preserving Pancreatoduodenecotomy Presenting with Obstructive Jaundice and Ascending Cholangitis
Article information
Abstract
Afferent loop syndrome is a rare complication of gastrojejunostomy. Patients usually present with abdominal distention and bilious avomiting. Afferent loop syndrome in patients who have undergone a pylorus preserving pancreaticoduodenectomy can present with ascending cholangitis. This condition is related to a large volume of reflux through the biliary-enteric anastomosis and static materials with bacterial overgrowth in the afferent loop. Patients with afferent loop syndrome after pylorus preserving pancreaticoduodenectomy frequently cannot be confirmed as surgical candidates due to poor medical condition. In that situation, a non-surgical palliation should be considered. Herein, we report two patients with afferent loop syndrome presenting with obstructive jaundice and ascending cholangitis. The patients suffered from the recurrence of pancreatic cancer after pylorus preserving pancreaticoduodenectomy. The diagnosis of afferent loop syndrome was confirmed, and the patients were successfully treated by inserting an endoscopic metal stent using a colonoscopic endoscope.
INTRODUCTION
Afferent loop syndrome, defined by accumulation of bile acid and pancreatic juice and distention due to the obstruction of afferent loop of duodenum or proximal jejunum, can be developed as a complication after gastrectomy with gastrojejunostomy.1 And it can also occur after surgical resections including anastomosis between stomach and other foreguts such as pancreaticoduodenectomy. Althogh a few foreign case series of afferent loop syndrome after pancreaticoduodenectomy were reported,2,3 we could not find any report in Korea. Afferent loop syndrome after pancreaticoduodenectomy can be managed either surgically or non-surgically, but non-surgical treatment is preferred due to the patientsh medical conditions.2 Percutaneous approach, frequently considered for non-surgical management in past, has given the way to endoscopic treatment with the accumulating evidence of lower rate of procedure-related complications. However, it is not always easy to find an obstructed segment of the small bowel through endoscope due to the redundant properties of the small intestine and anatomical change in the abdomen after surgery. Herein, we report two cases of afferent loop syndrome after pylorus preserving pancreaticoduodenectomy, successfully treated by endoscopic confirmation of obstructive lesion in the afferent loop and metal stent implantation with peroral approach of colonoscope.
CASE REPORT
Case 1
A 52-year-old male visited emergency roomcomplaining of melena and general weakness. He was diagnosed chronic pancreatitis and pancreatic divisum 4 years ago. He received endoscopic biliary drainage with biliary stent for common bile duct stricture caused by chronic pancreatitis 3 years ago. Thereafter, he suffered repeatedly from acute exacerbation of chronic pancreatitis and in the meanwhile, a mass lesion in the head of pancreas was detected on abdominal computed tomography (CT) scan 2 years ago. He underwent pylorus preserving pancreaticoduodenectomy with the suspicion of pancreatic cancer and the pathologic review of the resected mass confirmed pancreatic adenocarcinoma with T2N0M0 stage. During the postoperative follow-up period, repeated endoscopic hemostases were required for recurrent gastric variceal bleeding due to portal hypertension combined with portal vein thrombosis. And from one year ago, palliative chemotherapy was installed for the metastatic recurrence of pancreatic cancer in liver and distant lymph node. However, the chemotherapy was abandoned after 3 cycles by the patient cycles y was a only conservative treatment has been continued.
On the physical examination at the admission, his blood pressure (BP) was 109/79 mm Hg, pulse rate (PR) 96 per minute, respiratory rate (RR) 20 per minute, and body temperature (BT) 36.7. His conjunctiva was pale and sclera was icteric. Direct tenderness on the whole abdomen was observed without rebound tenderness. Serum chemistry tests showed total protein of 7.2 g/dL, albumin of 4.1 g/dL, AST/ALT of 477/259 IU/L, total bilirubin of 4.5 mg/dL, alkaline phosphatase of 590 IU/L, amylase of 15 U/L, and lipase of 12 U/L. On plain X-ray of abdomen, there was no abnormality including abnormal gas pattern (Fig. 1A). On comparison of the images of abdominal CT scans with the images of the previous CT scan obtained 2 weeks before, the inflammation and remarkable distention of proximal jejunum and biliary tree dilatation, suggesting bowel obstruction by invasion of recurrent pancreatic cancer were noted (Fig. 1B, C). On the second day of admission, cholangiography with percutaneous transhepatic biliary approach revealed dilation of intrahepatic biliary trees and the contrast agent was found to drain smoothly, but did not pass through the obstructed lesion in the small bowel (Fig. 1D). With these findings, the patient was diagnosed afferent loop syndrome complicated with biliary trees dilation and suspecting ascending cholangitis. For the confirmation of the diagnosis and management, peroral endoscopic exploration of small bowel was performed with colonoscope. With this approach, the luminal stenosis and ulcerative lesions covered by white patches induced by extraluminal invasion of masses in proximal jejunum was found (Fig. 2A). For relieving the endoluminal occlusion, an uncovered metal stent, 24 mm of diameter and 6 cm of length (Hanaro stent; MI Tech Co., Ltd., Seoul, Korea) was successfully deployed after getting the contrast X-ray images (Fig. 2). Large amount of congested bile juice gushed through the stent. Afterward, the patient was recovered from abdominal pain and sepsis, and received 4th chemotherapy before the discharge.

Radiologic findings. (A) Abdominal X-ray shows normal bowel gas pattern. (B) Computed tomography (CT) scan shows intrahepatic duct dilatation with increased tumor size (arrow). (C) CT scan shows small bowel distention due to small bowel obstruction. (D) Cholangiogram shows intrahepatic duct dilatation and the contrast does not pass through the small bowel (arrow).
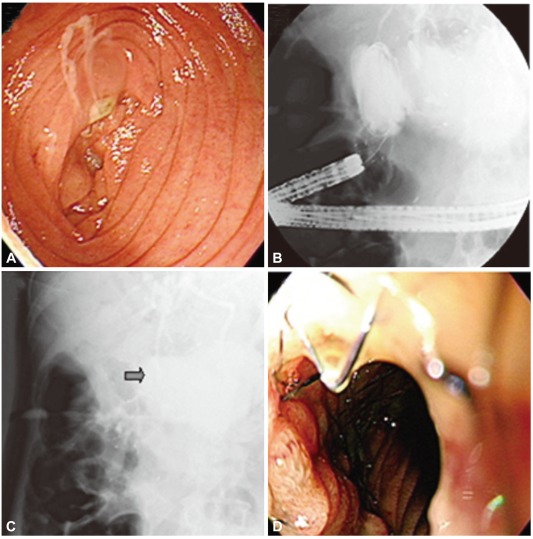
Endoscopic and fluoroscopic findings. (A) Endoscopy can not pass through the lumen. (B) A metal stent (Hanaro stent, uncovered, 6 cm) is inserted through the afferent loop. (C) The dye is drained well after stent insertion. (D) A dilated afferent loop of the small bowel is noted through the restored lumen of the occluded afferent loop.
Case 2
A 62-year-old female was presented with fever. She was diagnosed pancreatic head cancer (T3N0M0) 6 months before, and received pylorus prseserving pancreaticoduodenectomy after neoadjuvant concurrent chemoradiotherapy. However, she received ureteric stent insertion and chemotherapy just 2 weeks before the admission for ureter and liver metastases, detected on follow-up CT scan. On physical examination, BP was 156/73 mm Hg, PR 180 per minute, RR 15 per minute, and BT 38.2e. Pitting edema was observed on both legs. On complete blood cell count, white blood cell (WBC) was 230/mm3 (64/mm3 of neutrophil), hemoglobin (Hb) 10.2 g/dL and platelet 73,000/mm3. Blood chemistry tests were total protein 6.1 g/dL, albumin 3.2 g/dL, AST/ALT 28/41 IU/L, total bilirubin 1.3 mg/dL, direct bilirubin 0.9 mg/dL, alkaline phosphatase 181 IU/L, and glutamyl transpeptidase 384 IU/L. Routine radiologic exam of chest and abdomen revealed only small amount of pleural effusion on right thorax. She was diagnosed neutropenic fever due to chemotherapy and empirical antibiotics and granulocyte colony stimulating factor were started. On the third day of admission, fever was alleviated and blood culture was converted to negative for the microbial growth. From the seventh day of admission, intravenous antibiotics were changed into oral agents for the preparation of early discharge. But, the condition of the patient was abruptly deteriorated with spiking fever and decrement of blood pressure on 10th day of admission. The laboratory tests performed at that day showed WBC was 27,460/mm3, Hb 9.0 g/dL, platelet 142,000/mm3, AST/ALT 275/100 IU/L, alkaline phosphatase 235 IU/L, glutamyl transpeptidase 725 IU/L, and total bilirubin 5.2 mg/dL, suggesting obstructive jaundice and ascending cholangitis. Abdominal CT scan revealed an abscess pocket on right lobe of liver, diffuse dilatation of intrahepatic bile duct and distention of anastomosis site of small bowel, suggesting afferent loop syndrome due to small bowel invasion of pancreatic cancer (Fig. 3). The afferent loop obstruction site was confirmed with endoscopic exploration using colonoscope, and an uncovered metal stent (Niti-S D type stent; Taewoong Medical, Seoul, Korea) 20 mm in diameter and 10 cm in length was inserted (Fig. 4). The obstructive jaundice and ascending cholangitis was relieved after the procedure.
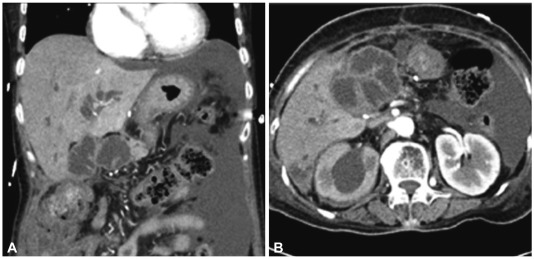
Radiologic findings. (A) Computed tomography scan shows anastomotic jejunal segment dilatation. (B) It shows anastomotic jejunal segment dilatation and hydronephrosis of the right kidney.
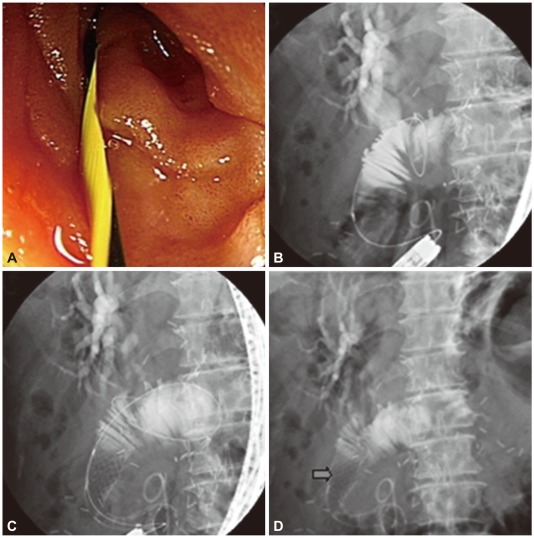
Endoscopic and fluoroscopic findings. (A) Through colonoscopic approach, cannulation is done. (B) After injection of contrast material, dye is not pass out and small bowel obstruction is noted. From biliary tract to small bowel, there is no obstruction. (C) A metal stent (Niti-S D type stent, uncovered, 10 cm) is inserted through the afferent loop. (D) The dye is drained well after stent insertion.
DISCUSSION
Afferent loop syndrome is defined as duodenal or jejunal obstruction at proximal anastomosis site of gastrojejunostomy.2 Afferent loop compression by abdominal adhesion after surgery, internal hernia, intussusceptions, volvulus, anastomotic site stricture, cancer recurrence and peritoneal metastasis are all contributing factors of afferent loop syndrome.1,2 Afferent loop syndrome can be classified into acute and chronic afferent loop syndrome. Acute afferent loop syndrome usually occurs within 1 week after surgery and accompanys abrupt epigastric pain, vomiting and acute clinical exacerbation in early phase of postoperative recovery. And, chronic afferent loop syndrome usually occurs several months or years after surgery, accompanying partial obstruction of afferent loop and abdominal distention which subsides with bilious vomiting 1-2 hours after meal. The elevation of intraluminal pressure of the partially occluded afferent loop with the secretion of pancreatic juice and bile acid after meal leads to dilate the affected bowel and causes the clinical symptom in chronic afferent loop syndrome. The cases in this report are rare types of chronic afferent loop syndrome, showing obstructive jaundice and ascending cholangitis caused by progressive obstruction due to cancer invasion, without distinct symptoms of gastrointestinal obstruction such as abdominal pain or vomiting.4,5 The initial presentation of the two cases were far from the typical afferent loop syndrome, and ascending cholangitis was suspected instead. Actually, CT scan with the suspicion of ascending cholangitis was helpful to confirm the afferent loop syndrome with the findings such as intraperitoneal metastasis and distension of proximal small bowel. In other words, afferent loop syndrome in these cases did not show any distinct symptom or clinical finding until afferent loop was completely obstructed and the stasis and infection within afferent loop lead to cholangitis. Obstructive jaundice caused by afferent loop syndrome leads to bile drainage impairment due to the increased pressure in afferent loop as high as 18 cmH2O, which causes dilatation of bile duct in turn.5 Especially, the afferent loop syndrome after pancreaticoduodenectomy can be presented with the symptoms of ascending cholangitis rather than the typical gastrointestinal obstructive symptom. This mechanism is attributable to the surgical characteristics of pancreaticoduodenectomy ancreaticanastomsis between bile duct stump and afferent loop of small bowel facilitates regurgitation more than normal bilio-enteric structure.6 We also need to point out that pancreatitis occurred less than cholangitis, although both bile duct and pancreas may be affected by the increased afferent loop pressure, suggesting that, although pancreatitis may occur ultimately, sphincter of Boyden (submucosal muscle layer enclosing pancreatic duct distinct from sphincter of Oddi) protects pancreas for a long time, causing cholangitis to appear first than pancreatitis.4
Afferent loop syndrome does not show a specific finding on laboratory tests or simple X-ray in the absence of cholangitis or pancreatitis. Abdominal ultr asonography and CT scan is useful for rapid diagnosis of afferent loop syndrome based on findings such as obstruction or distention proximal to the obstructed lesion of afferent loop. However, ultrasonography or CT scan may not be informative when the bowel obstruction is partial or the distended bowel loop is decompressed with vomiting. In these conditions, cholangiography may have diagnostic value,5 with the contrast agent draining smoothly within the intrahepatic duct, but not passing through the obstructed site. Our cases were assumed as afferent loop syndrome by abdominal CT, and the first case required cholangiography for definite diagnosis.
Afferent loop syndrome complicated with cholestasis after pancreaticoduodenectomy can be managed either by surgery or by non-surgical treatment.2,7 However, the syndrome caused by the recurrence of malignant diseases like the patients of this report cannot be appropriate for surgical managements including Braun anastomosis (jejuno-jejunal anastomosis) or Roux-en-Y anastomosis.8 For non-surgical treatment, percutaneous transhepatic drainage catheter or endoscopic stent insertion within the obstructed afferent loop are available options.2,3 Percutaneous catheter insertion to the blind loop for the purpose of drainage and decompression might increase the risk of peritoneal leakage of enteric gases and contents.5 Recently, endoscopic management of afferent loop syndrome has been emerging as an excellent alternative for terminal cancer patientswho are unsuitable for surgical treatment.2,3 Especially, the both cases in this report were in need of urgent treatment of sepsis caused by cholestasis, and endoscopic stent insertion was the best choice of treatment rather than surgery.
Pancreaticoduodenectomy has been used for the treatment of pancreatic head and periampullary disease.7 According to the study performed by Yamaguchi et al.9 with 1,066 patients who received pylorus preserving pancreaticoduodenectomy, delayed gastric emptying was the most common early complication within 1 month after the surgery, late complications included ulcer at junction area, bile duct and liver abscess and diabetes. Even though cholangitis was not considered as a major concern in previous reports, it should be handled as an infrequent, but significant complications of pancreaticoduodenectomy. In addition, the 2 cases we reported here experienced increased bilirubin level and dilatation of intrahepatic duct without stenosis at bilio-enteric anastomosis site, which could be explained by mechanical obstruction of small bowel due to the recurrence of malignancy. In the first case, the cholangitis was not improved after percutaneous transhepatic biliary drainage, suggesting the increased pressure in afferent loop caused consistent bilious regurgitation through bilio-enteric anastomosis, which was resolved after treatment of afferent loop syndrome.
To make a short story, afferent loop syndrome should be considered as the fundamental cause of recurrent cholangitis after pancreaticoduodenectomy in particular when accompanying diffuse dilatation of intrahepatic bile duct and jaundice without stenosis at bilio-enteric anastomosis site. This study also suggests that endoscopic stent insertion can be an effective treatment.
Notes
The authors have no financial conflicts of interest.