A Look into the Small Bowel in Crohn's Disease
Article information
Abstract
Crohn's disease (CD) is an inflammatory bowel disease that can affect the entire gastrointestinal tract, with the small bowel (SB) being the most commonly affected site. In some patients, refractory inflammation or chronic strictures of the SB are responsible for a debilitating course of the disease that might lead to severely reduced quality of life. Therefore, SB imaging is a crucial element in diagnosing and/or managing SB CD, and continues to evolve because of technologic advances. SB endoscopy (capsule endoscopy and device-assisted enteroscopy) and cross-sectional radiologic imaging (computed tomography enterography and magnetic resonance enterography) have become key players to diagnose and/or manage CD. In everyday practice, the choice of the imaging modalities is based on the presence and availability of the techniques and of experienced operators in each institute, clinical usefulness, safety, and cost. Here, SB endoscopy and radiologic imaging in suspected or known CD patients will be addressed and discussed.
INTRODUCTION
Crohn's disease (CD) is an inflammatory bowel disease that can affect the entire gastrointestinal tract, with the small bowel (SB) being the most commonly affected site. The terminal ileum is the most common area affected by CD and it is usually accessible at the time of colonoscopy. However, proximal SB may be the only area of the gastrointestinal tract affected in approximately one third of patients with ileocolonic CD.1 In some patients, refractory inflammation or chronic strictures of the SB are responsible for a debilitating course of the disease that might lead to malnutrition and a severely reduced quality of life. Therefore, the SB warrants special attention in diagnosis and treatment of CD,2 and SB evaluation can also be helpful in differentiation of CD from inflammatory bowel disease of unclassified type.3,4
The SB lesions have transitionally been difficult to evaluate because of their inaccessibility to endoscopic exploration. Indeed, until recently, the endoscopic visualization of the SB was limited to the terminal ileum during colonoscopy and to the very proximal jejunum during push enteroscopy. The majority of the SB was not seen endoscopically and was evaluated with radiologic tests such as traditional radiological techniques (SB follow-through) in the diagnosis and management of CD.
In the last few years, the SB has come within reach of easy-to-apply endoscopy, that is, wireless video capsule (SB capsule endoscopy; SBCE) and device-assisted enteroscopy (DAE). In addition, several radiological techniques including computed tomography enterography (CTE) or magnetic resonance enterography (MRE) are now available for the evaluation of the SB. Each technology has its own strengths and weaknesses. They should be viewed as complementary studies and not mutually exclusive. The recent development of innovative imaging techniques has opened a new and exciting area in the exploration of the SB in CD patient. Among them, what is the best endoscopic or radiologic approach for the diagnosis and/or management of CD? Here, SB endoscopy and radiology in suspected and known CD patients will be addressed and discussed in detail.
SB ENDOSCOPY
SBCE
In 2000, Iddan et al.5 at Given Imaging published on a camera pill aimed at imaging the mucosal lining of the small intestine. By 2001, this capsule endoscope was officially approved by the United States Food and Drug Administration and was used on humans outside the clinical trials.
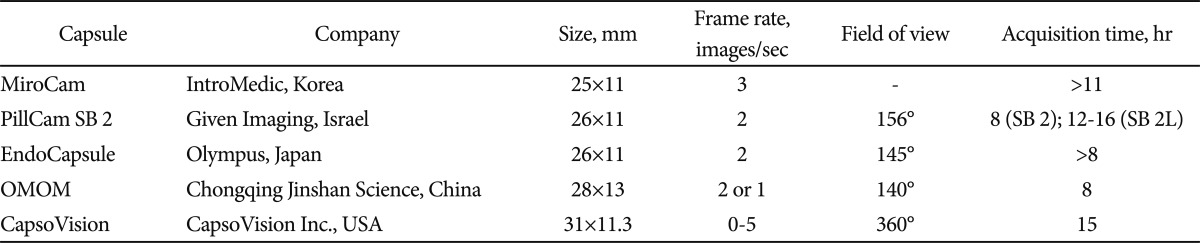
At present, there are several commercially available SBCE systems on the market that differ somehow in terms of technical details or software features: Miro-Cam, IntroMedic, Seoul, Korea (http://www.intromedic.com/); PillCam SB2, Given Imaging, Yoqneam, Israel (http://www.givenimaging.com/); EndoCapsule, Olympus Europe GmbH, Hamburg, Germany (http://www.olympuseuropa.com/endoscopy/); CapsoVision, Saratoga, CA, USA (http://www.capsovision.com/); OMOM, Chongqing Jinshan Science, Beijing, China (http://www.cqjs.net/) (Table 1).
SBCE offers a noninvasive and easy-to-apply investigation of the SB. The video capsule is ingested and passes the intestinal tract by natural peristalsis, so SBCE does not have the ability to clean the mucosa or eliminate debris, bubbles, or bile during the procedure. Although bowel preparation should be used, there is no international consensus about patient preparation for SBCE.
Diagnosis of terminal ileal CD can be usually made at ileocolonoscopy, which should be performed before SBCE. A normal SBCE has a high negative predictive value (96% to 100%) for active SB CD. In other words, an unremarkable SBCE evaluation virtually excludes SB CD.6,7 Numerous articles showed the superiority of SBCE to barium studies or push enteroscopy in detecting lesions for SB evaluations. The latest meta-analysis demonstrated a significantly increased diagnostic yield with SBCE compared with SB follow-through, CTE, and ileocolonoscopy in patients with suspected CD or known CD, but only in patients with established nonstricturing CD being evaluated for SB involvement.8-10 Many prospective trials, including multicenter study conducted by Korean Capsule Endoscopy Study Group, have shown superiority of SBCE over SB follow-through for the evaluation of SB lesions.11 SBCE allows earlier diagnosis of CD of the SB and improves the diagnosis of colitis in patients in whom it is unclear whether the issue is CD or ulcerative colitis.
Although there are many advantages of SBCE, the main limitation of SBCE is the lack opportunity to take biopsies or to perform interventions, the difficulty to exactly localize identified lesions, and to control its movement. And SBCE shows high rate incidental findings, limited diagnostic accuracy in second portion of duodenum, and 25% of failure rate to reach cecum.
Persistent capsule retention is the feared major complication of SBCE. The incidence of capsule retention in the general population is between 1% and 2.5%, predominantly because of capsule retention at focal sites of intestinal stenosis. The incidence of capsule retention in cases of suspected CD was 1.6% in a previous study.12 On the other hand, capsule retention rate as high as 13% have been reported in patients with known CD.12 In a recent large-scale study, known CD was a risk factor for capsule retention (odds ratio, 9.39; 95% confidence interval, 3.32 to 26.54).13 Because of a propensity for intestinal strictures to develop, patients with CD are at increased risk of persistent capsule retention. For this reason, radiologic imaging of the SB is often recommended before SBCE. However, it is important to note that even in the presence of normal SB radiologic findings, there can still be significant undetected strictures. In an effort to avoid capsule retention, a dissolving test capsule called a patency capsule has been developed. This patency capsule is the same size as the SBCE. It is constructed of cellophane with a wax plug and a radiotag, which enables external scanning to verify its presence in the body. Data on a recently developed patency capsule suggest that it may significantly reduce (if not eliminate) the risk of complications and perhaps obviate the need for preliminary SB imaging. Patients with known CD should be informed before SBCE that despite of normal imaging they remain at risk of capsule retention.10 Because it is a metallic device, magnetic resonance (MR) imaging testing should not be performed until capsule passage is ensured clinically or radiologically. A warning remains against performing SBCE in patients with cardiac pacemakers or other implanted electromedical devices. However, recent data show no significant interaction between SBCE and pacemakers or implantable cardioversion devices.14-16 Capsule aspiration is a rare but potentially serious complication.17 Finally, intestinal perforation within a short time after capsule ingestion has been reported in a few patients with previously undiagnosed Crohn's strictures.18-20
DAE
Balloon-assisted enteroscopy (BAE)
Soon after capsule endoscopy was invented, Yamamoto et al.21 published his article on modifying a colonocope to perform a total enteroscopy using a two balloon system and an overtube. A commercial grade double balloon enteroscopy (DBE) scope and processor with safety controls was then developed by Fujinon, Saitama, Japan. to identify and treat SB conditions. BAE allows deeper intubation of the SB compared with push enteroscopy and ileocolonoscopy. BAE involves push-and-pull maneuvers for deep intubation of the SB, and single balloon enteroscope (SBE) and DBE are presently available (Table 2).2 SBE is virtually identical to the DBE except for its use of silicone material for the balloon and overtube and lack of the inflantable endoscope balloon. Rate of complete SB investigations seems to be more regularly achievable using double balloon instead of single balloon technique as reported in randomized studies, but therapeutic impact was similar between SBE and DBE. In patients with known CD, adhesion may limit examination by BAE, and in these circumstances, DBE may be preferred for the SB evaluation.
There are not enough data to recommend BAE, unless conventional studies including ileocolonoscopy and radiographic imaging of the SB have been inconclusive and histological diagnosis would alter disease management. The advantages of BAE compared with SBCE include the evaluation of atypical lesions, the ability to obtain biopsies for histopathology, and the potential for therapeutic intervention (e.g., dilation, retrieving foreign body, or treatment of bleeding lesions).22 The decision on whether SBCE or BAE should be performed first depends on the nature and location of the SB lesion, as well as local availability and expertise in suspected CD patients. Sometimes, SBCE provides information on the optimal route of approach (i.e., oral or rectal) by subsequent BAE.8
Despite of the advantages of BAE such as direct (real-time) inspection, biopsy, or therapeutic capabilities, BAE has a significant risk of complication (<5%). There is a close to 1% chance of complication in performing a diagnostic procedure, with pancreatitis being the most common issue. As expected, endoscopic treatment may lead to much higher change of perforation and bleeding.2 Even though SBE is less complex and has less anchoring capacity, it has been reported to have the same types of complications as those seen in DBE. Safety data on SBE are still scarce, but may be comparable to those of DBE.8
Active CD or previous intestinal surgery may increase the risk of perforation and hydrostatic balloon dilation of short fibrotic strictures in patients with SB CD has a small, but definable risk of perforation (0% to 3%). Additionally, BAE involves risks related to sedation, in contrast to SBCE where no sedation is required.
Spiral enteroscopy
Enteroscopy with the Endo-Ease System (Spirus Medical, Stoughton, MA, USA) uses a spiral-shaped overtube of 118 cm with a spiral of 0.55 cm high and 22 cm long and can be used with enteroscopes of less than 9.4 mm in diameter. The enteroscope is advanced or withdrawn with rotator clock-wise and counterclockwise movements of the spiral. Endoscopy of the SB by spiral enteroscopy is reported to be safe and seems to reduce the examination time, but the insertion extent is shorter in comparison to DBE. In CD patients, spiral enteroscopy has rarely been performed up to now.2 The feasibility of the technique has not been demonstrated in patients with CD, and no study has compared spiral enteroscope with other endoscopic techniques. Spiral enteroscopy is suspected by many to have a higher change of perforation and volvulus, but the very limited reports on this procedure does not provide us with a fair assessment of its safety. This form of procedure has recently become vastly limited because of the reported stoppage of manufacturing of the spiral overtube in favor of a motorized design with a short spiral.
Radiology in imaging SB
Cross-sectional enterography provides complementary information to ileocolonoscopy. Visualization of the SB with cross-sectional imaging methods requires distension of the intestines to identify the configuration of the bowel loops and to improve characterization of the bowel wall with luminal contrast. This is achieved by inserting a nasojejunal tube into the proximal SB (enteroclysis) or with oral intake of the luminal contrast medium (enterography). Conventional fluoroscopy (barium SB radiographs: SB follow through and SB enteroclysis) has thereby almost completely been replaced by cross-sectional imaging methods.
CTE and MRE
Among diverse diagnostic imaging modalities, during the recent decade, computed tomography (CT) and MR techniques have been optimized for SB imaging with increasing role in the evaluation of SB diseases, especially of CD. CT and MR are available as CTE or MRE with oral intake providing similar quality images but with an improved patient comfort over tube-assisted infusion of enteral contrast.
With the improved resolution of multidetector CT, CTE has become an important method of choice for evaluating SB diseases, determination of disease activity, the extent, and severity of inflamed bowel.23,24
On CTE, CT findings indicative of active inflammation include bowel wall thickening (thickness of >3 mm), mural stratification, mural hyperenhancement, increased attenuation in the perienteric fat and engorged vasa recta (comb sign).23,25
Although the diagnostic yield of CTE has been shown to be inferior to SBCE in suspected and established SB CD,26 CTE have high sensitivities (95.2%) for detecting active SB inflammation. In addition, both CTE and MRE equally provide excellent information on extraenteric manifestations and complications including abscess, fistula, and bowel obstruction, thus adding useful information on endoscopic investigations.27 However, because patients with CD are often young and CD is a chronic and relapsing disease, they may undergo repeated imaging examinations to assess the status of their disease. Thus, large lifetime dose of radiation is a concern particularly in young patients with CD. Radiation dose of more than 100 mSv may be observed in some patients. Efforts should be made to reduce radiation dose in these patients by minimizing CT examination, dose reduction (using the dose modulation option or advanced reconstruction techniques), or considering another diagnostic imaging modality.
For this reason, MRE has been introduced as a radiation-free alternative method to evaluate patients with CD.28,29 MRE, these days, is well suited to play an important role in the evaluation of SB disorders. In recent prospective studies, MRE was found to have a similar accuracy, area under the receiver operating characteristics curve and sensitivity for detecting active inflammation in CD compared with CTE.27,30,31 In addition, MRE has the potential advantage of providing functional and quantitative information about bowel wall (e.g., diffusion, perfusion, or motility) that cannot be obtained by CT. In some CD patients with strictures, it is important to distinguish between inflammatory stricture and fixed fibrotic stricture, as obstruction and spasm in active CD may be relieved by medical treatment whereas chronic strictures may require surgical intervention. MRE can provide useful information in this setting by differentiating between fixed fibrostenotic lesions by depicting fat-halo sign and inflammatory stricture.9
Early mucosal lesions such as aphthoid lesions, however, are not accurately visualized on CT or MR, making them less suitable as a first-line examination for suspected early diseases. Therefore, until now, CTE appears to be more cost-effective in the long term assessment and follow-up of patients, especially those with established CD. Although MRE is being used more frequently because of the advantage of lack of radiation, some limitation of MRE such as high cost, longer examination time, and slightly inferior spatial resolution than CTE, hardly makes it the initial imaging modality of choice in many adult patients.
OTHER MODALITIES FOR DETECTION OF SB CD
Percutaneous ultrasonography (US) is useful to detect SB CD and to reveal extraenteric complications, for example, abscess or fistula. Overall accuracy might be lower compared with endoscopy, but an experienced investigator can beneficially use US as an initial diagnostic tool for managing CD patients.32
The main US findings in CD are represented by thickening and stiffness of the gut wall, modifications or lack of its echostratification, reduction of peristalsis, mesenteric fibrofatty proliferation, lymph node enlargement; in case of complications, narrowing of the intestinal lumen, abscesses and fistula are usually easily detectable.33
Labeled-leukocyte scintigraphy in detecting active inflammation of CD has been evaluated. 99mTC HMPAO labeled white blood cell scan is a commonly used agent because of its greater availability, better image quality and lower radiation dose.34 The sensitivity and specificity of leukocyte-labeled scintigraphy has been reported to range 76% to 94.7% and 77.8% to 93.3%, respectively.30 Recent studies on 18F-fluorodeoxyglucose positron emission tomography (FDG-PET) in assessing active CD reveals lower specificity values compared to MR.35 The usefulness of PET in differentiating between active and indolent CD is still unclear.
DIAGNOSTIC STRATEGY OF SB IN DIFFERENT CLINICAL SITUATIONS
In suspected CD, ileocolonoscopy is still the reference standard in the diagnostic algorithm. It is used to diagnose terminal ileitis or colitis, followed by cross-sectional imaging including CTE or MRE to identify proximal CD or extraenteric lesions. SBCE is regarded a final identifier for detection of SB lesions that are reasons of unexplained symptoms. On the other hand, high negative predictive value of SBCE of 96% to 100% suggests using SBCE to exclude CD in suspected disease cases.8 SBCE could be considered an early step in suspected CD and nonconclusive ileocolonoscopy in the future.2
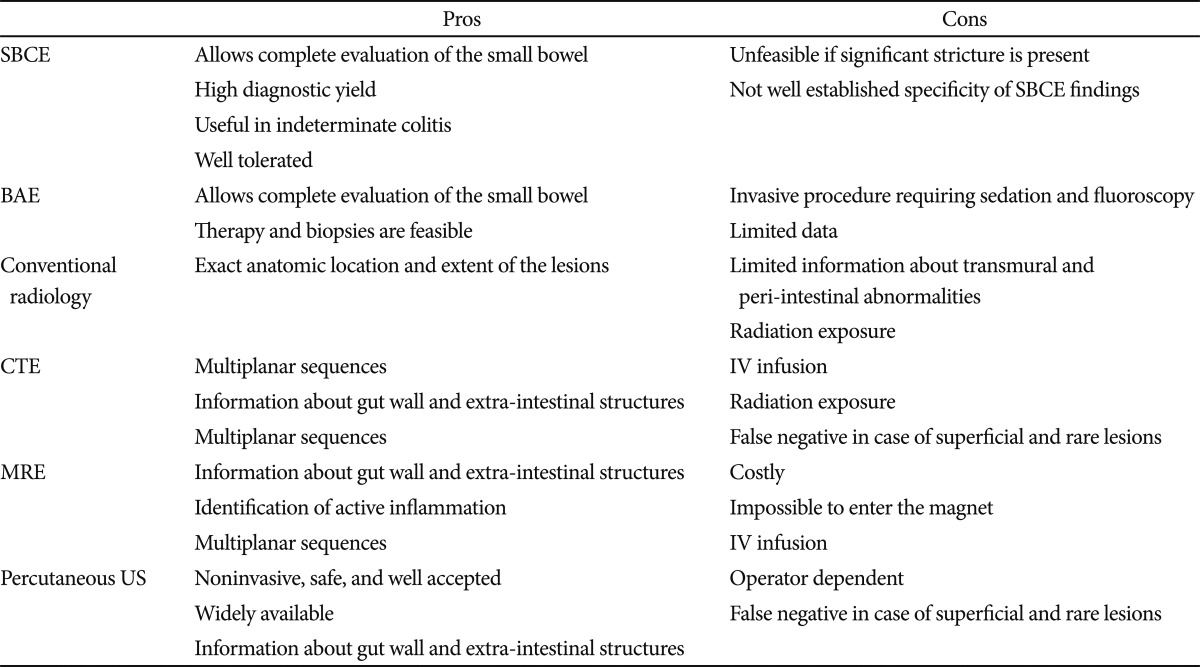
In established CD, value of cross-sectional imaging surpasses endoscopic information in many clinical scenarios such as septic patients and acute onset of severe complaints and pain. The role of SBCE in patients with established CD should focus on patients with unexplained symptoms when other investigations are inconclusive, if this will alter management. Radiographic imaging takes precedence over SBCE because it can potentially identify obstructive strictures, extraenteric disease, transmural nature, or anatomic distribution of disease.8 CTE or MRE may also give an indication of disease activity. The high potential for capsule retention in established CD should also be considered. Advantages and disadvantages of different SB imaging techniques are summarized in Table 3.
CONCLUSIONS
Although conventional ileocolonoscopy is the first diagnostic tool in patients with suspected CD, SB is the only site involved in as many as 30% of patients with CD. Therefore, SB imaging is a crucial element in diagnosing SB CD, and continues to evolve because of technologic and basic science advances. SB endoscopy (SBCE or DAE) and cross-sectional imaging (CTE or MRE) have become key players to diagnose and/or manage CD patients. SBCE shows the highest diagnostic yield in patients with suspected CD, but SBCE should be performed in patients with non-stricturing SB CD to avoid capsule retention. DAE, especially DBE, has advantages in therapeutic capabilities including balloon dilation or bleeding control. On the other hand, CTE and MRE are noninvasive modalities that allow both luminal and extraluminal evaluations. In everyday practice, the choice of the imaging modalities is based on the presence and the availability of the techniques and of experienced operators in each institute, clinical usefulness, safety, and cost.
Notes
The authors have no financial conflicts of interest.