A Case of Early Gastric Cancer with Solitary Metastasis to the Pleura
Article information
Abstract
The incidence of early gastric cancer (EGC) has increased to >50% in Korea owing to a higher detection rate caused by rapid advances in diagnostic instrumentation. EGC with distant metastasis has been rarely reported. Here, we report the case of a 76-year-old woman in whom general EGC was initially diagnosed by endoscopy and endoscopic ultrasonography. She subsequently underwent endoscopic submucosal dissection (ESD). Histological examination of the ESD specimen revealed that neoplastic cells were located predominantly in the submucosal layer and submucosal lymphatic channels. Metastatic cancer cells were also found in the pleural effusion. After conducting all analyses, including immunohistochemical staining, we concluded that the patient had primary EGC with pleural metastasis.
INTRODUCTION
Gastric cancer is the most prevalent malignant neoplasm in Korea, comprising 20.8% of all cancers.1 As the incidence of gastric cancer has increased, many treatment options have been developed. Endoscopic submucosal dissection (ESD) is widely accepted as an important treatment option for cases of early gastric cancer (EGC) when the probability of lymph node metastasis is very low. The extended indications for endoscopic resection of EGC suggest that differentiated adenocarcinomas ≥30 mm in diameter are entirely free of nodal metastasis when lymphatic-vascular capillary involvement is absent or the submucosal penetration is ≤500 µm.2 More recently, Hirasawa et al.3 reported that intramucosal undifferentiated adenocarcinomas ≤20 mm in diameter without lymphatic-vascular capillary involvement or ulcerous findings present a negligible risk of lymph node metastasis. The incidence of lymph node metastasis is 1% to 3% for mucosal cancers and 11% to 20% for submucosal cancers.4 When invasion extends into the submucosal layer, the incidence of lymph node metastasis increases. The depth of invasion is also strongly associated with distant metastasis. EGC rarely metastasizes to distant organs. Pleural metastasis is not a common presentation of stomach cancer, and solitary pleural metastasis in EGC has not yet been reported. Herein, we report the case of a patient with primary gastric carcinoma with predominantly massive submucosal lymphatic channel invasion and metastasis to the pleura.
CASE REPORT
A 76-year-old woman with a 1-month history of epigastric discomfort was referred to the Department of Gastroenterology. She was being treated as an inpatient in the department of neurology after a diagnosis of Parkinson disease. Twenty days previously, she was found to have a gastric ulcer after having undergone upper gastrointestinal endoscopy in a local clinic. She continued to complain of abdominal discomfort, although she had been taking ulcer medications. On admission, her physical examination findings and laboratory data were unremarkable. A small amount of left pleural effusion was noted on a chest radiograph, and an abdominal radiograph showed no abnormality. Because she had upper respiratory symptoms, including cough, on admission, she had received antibiotics for suspected pneumonia. During her hospital course, a few days after starting the antibiotics, she showed improvement in respiratory symptoms. Symptoms associated with Parkinson disease and other upper respiratory symptoms had been well controlled with an oral antiparkinson drug and oral cough syrups followed by 3-day intravenous antibiotics. She did not report any upper respiratory symptoms, so the pleural effusion was expected to disappear soon.
Upper gastrointestinal endoscopy was performed to evaluate her symptoms. Endoscopic examination demonstrated a slightly elevated lesion (4 cm) with multiple shallow erosions, absence of a definite ulcer scar, and bridging or converging folds over the anterior wall of the antrum (type IIa) (Fig. 1). The endoscopic ultrasonographic (EUS) image revealed that the diffuse thickened hypoechoic lesion was limited to the mucosal layer, with no irregular narrowing or budding sign (Fig. 2). Histopathological examination of gastric biopsies showed a moderately well-differentiated adenocarcinoma. Abdominal computed tomography (CT) revealed no evidence of gastric wall thickening, enlargement of abdominal lymph nodes, or distant metastasis. The lesion was diagnosed as mucosal gastric cancer. She was transferred to our department for ESD, which was performed according to the expanded ESD criteria.
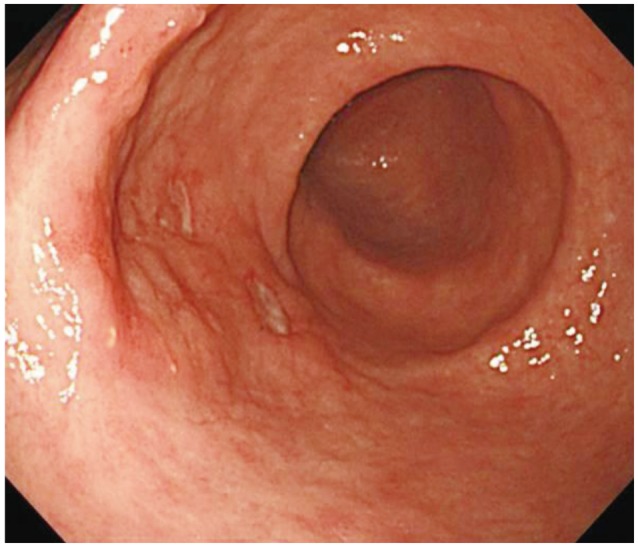
Endoscopic image showing a slightly elevated lesion (4 cm) with multiple shallow erosions, no definite ulcer scar, and bridging or converging folds over the anterior wall of the antrum.
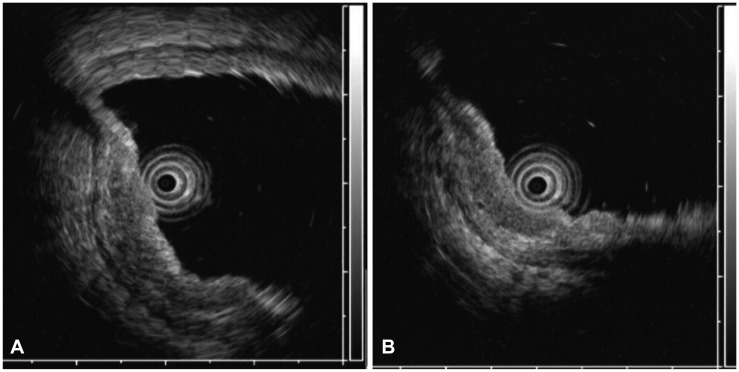
(A, B) Endoscopic ultrasonographic image showing that the diffuse thickened hypoechoic lesion was limited to the muscularis mucosa, with no irregular narrowing or budding sign.
On the day after ESD, the patient reported fever and frequent cough. A follow-up chest radiograph showed an increased amount of left pleural effusion (Fig. 3). Aspiration pneumonia was suspected, and a diagnostic thoracostomy was performed along with initiation of intravenous antibiotic therapy.
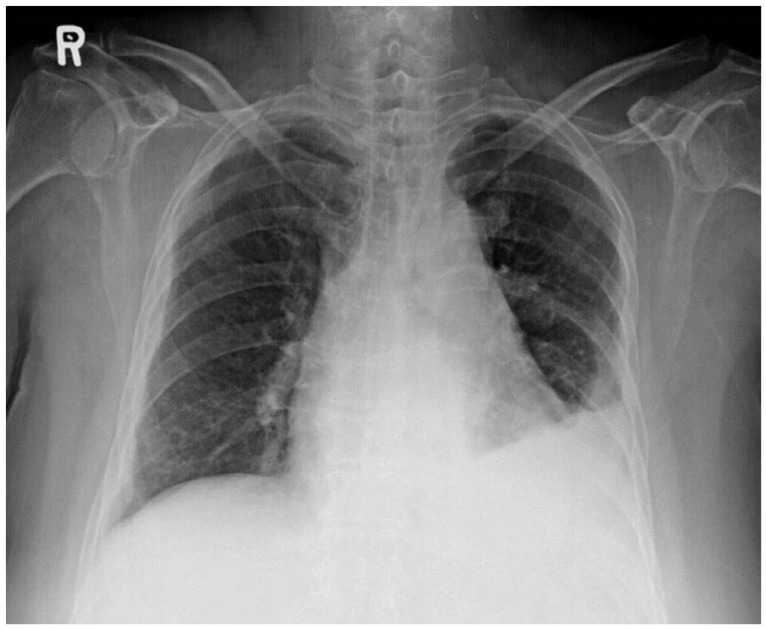
Chest radiograph obtained after endoscopic submucosal dissection showing left pleural fluid with subsegmental collapse of the left lower lobe.
The ESD specimen revealed a round, brownish, elevated lesion measuring 6×4×0.4 cm, with no surface change. Microscopic examination showed that the tumor was limited to the submucosal layer, with no involvement of the deep resection margin. The tumor showed clusters, or nests, of moderately well-differentiated adenocarcinoma, mostly confined to the large, dilated lymphatic channels with adjacent foci of stromal invasion (Fig. 4). The mucosal layer also showed multifocal lymphatic tumor emboli without stromal invasion.
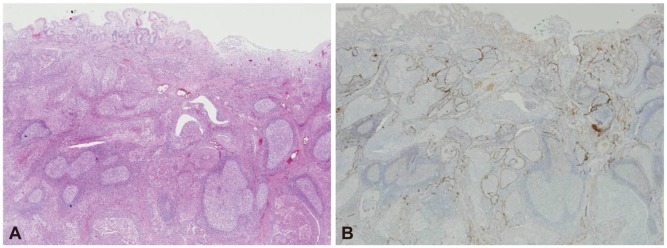
(A) Histological image of cancer cell infiltration into the lymphatics of the submucosal layer (H&E stain, ×200). (B) Immunohistochemical staining for D2-40 was positive in the lymphatic endothelium and negative in the capillary endothelium (×200).
Analysis of the left pleural fluid revealed the following findings: red blood cells, 32,000/mm3; white blood cells, 1,120/mm3; neutrophils, 4%; lymphocytes, 38%; adenosine deaminase activity, 15.4; and pH, 7.36. The ratio of pleural fluid protein to serum protein was 0.98. The ratio of pleural fluid lactate dehydrogenase (LDH) to serum LDH was 0.66. Metastatic adenocarcinoma cells were unexpectedly detected by cytological examination of the pleural effusion (Fig. 5).
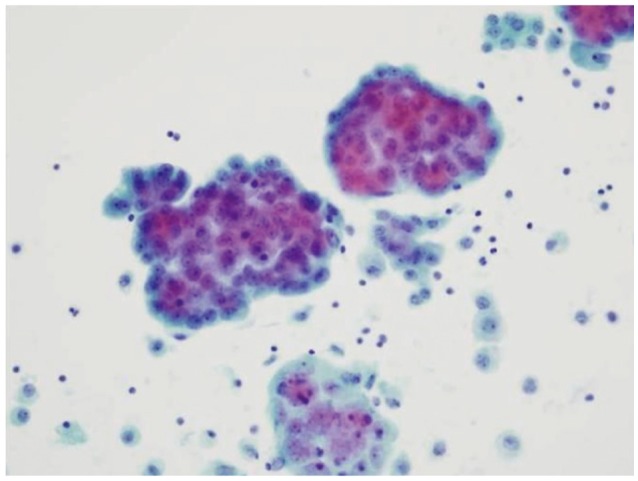
Cytological findings in the pleural fluid, obtained by thoracocentesis, showing metastatic adenocarcinoma (PAP stain, ×400).
To determine the primary site of metastatic adenocarcinoma, immunohistochemical (IHC) staining tests and positron emission tomography (PET)-CT were performed. EGC metastasis to the pleural space is rarely observed, but the ESD specimen from this patient showed this unusual histopathological feature.
The results of IHC staining tests were as follows: D2-40, positive; cytokeratin 7 (CK7), positive; CK20, negative; CD56, negative; synaptophysin, negative; human epidermal growth factor receptor-2, negative; and thyroid transcription factor-1, negative. The test results were negative for mucin (MUC) 1 and MUC2, and positive for MUC5A and MUC6.
PET-CT showed a focal abnormal hypermetabolic lesion in the aortopulmonary window, most likely representing malignant uptake; the standard uptake value (SUV) was 8.76. The SUV was elevated in the left lower lobe of the lung, with pleural effusion; the SUV of 4.43 indicated probable benign uptake.
After all analyses, instead of gastric metastasis, we concluded that the proper diagnosis was primary EGC metastasis to the pleura, with accompanying unusual histological findings. We planned to start chemotherapy, but she refused further treatment and wanted to undergo conservative management. She died 1 year after the diagnosis.
DISCUSSION
ESD is currently accepted as the standard treatment strategy for gastrointestinal neoplasms with no evidence of lymph node metastasis because it enables one-piece resection, even in the presence of large or ulcerous lesions. However, surgery is sometimes needed after ESD, because of incomplete dissection. Accurate determination of the invasion depth before intervention has become an indispensable part of treatment planning to avoid additional surgery after ESD. The degree of tumor invasion can be assessed by conventional endoscopy and EUS. The reported accuracy of endoscopy for the detection of EGC ranges from 90% to 96%.5 Sano et al.6 reported a 71.0% diagnostic accuracy of endoscopy in mucosal and submucosal cancers. EUS has been reported as the most reliable method in tumor and node staging of gastric cancer, with high accuracy rates.7,8 In the present case, the lesion was macroscopically noted as an elevated surface but showed no feature suggesting submucosal invasion, such as whitish patches, irregular nodular surface protrusion, or a deep depression in the elevated surface.9,10 For mucosal cancers without ulcerous changes on endoscopy, irregular narrowing and the budding sign in the third layer are useful for the diagnosis of submucosal invasion with EUS.11 In our patient, the endoscopic findings included an elevated lesion, without definite surface changes, and ulceration. No abnormal findings suggesting submucosal invasion were present. Therefore, the lesion of our patient was macroscopically similar to intramucosal gastric cancer, and ESD was performed. However, the ESD specimen showed massive submucosal lymphatic channel infiltration by tumor cells and a rare additional pleural metastasis. A retrospective review of this finding suggested that the diffuse thickened hypoechoic lesion limited to the muscularis mucosal layer and irregular thinned submucosal layer under the lesion could have represented massive submucosal lymphatic channel infiltration by tumor cells, which provided a route for the uncommon distant metastasis. In other words, the actual lesion was misperceived to be limited only to the mucosal layer on EUS.
Stomach cancer typically spreads to nearby tissues and distant lymph nodes, or metastasizes to other organs such as the liver, esophagus, lung, and lymph nodes. In order of frequency, the most common metastatic sites of stomach cancer are the liver, peritoneum, and lymph nodes. This cancer may metastasize to the spleen, adrenal glands, ovary, brain, and skin. Stomach cancer seldom produces distant metastasis until it reaches stage T3. Distant metastasis of EGC has been rarely reported, and the prevalence is about 0.14% among all stomach cancers.12 However, distant metastasis to the liver, peritoneum, lymph nodes, or bone was diagnosed at the time of initial EGC diagnosis in some cases. Although gastric cancer can metastasize to the lung, it is a rare cause of malignant pleural effusion, representing about 2% of such cases, after cancers of the lung and breast and lymphoma.13 Therefore, we performed IHC staining tests to clearly determine the primary site. MUCs and CKs are helpful in predicting the primary sites of digestive cancers. MUC1 is a membrane-bound MUC expressed in colorectal adenocarcinomas.14 MUC2 is a large secretory MUC expressed in intestinal-type tumors and mucinous carcinomas. It is also expressed in goblet cells and early colorectal cancer. MUC5AC and MUC6 have been well investigated in gastric carcinomas. MUC5AC expression is observed in gastric foveolar epithelium, which constitutes major gastric MUCs. MUC6 is also a major gastric MUC. CKs are the principal intermediate filament proteins of normal epithelia and epithelial tumors. CK7 shows a positivity of 75% in the stomach. On the other hand, CK20 is highly expressed in the colon. In the present case, the results of IHC staining tests were as follows: CK7, positive; CK20, negative. The test results were negative for MUC1 and MUC2, and positive for MUC5A and MUC6. Taken together, these results showed that the most possible primary site might be stomach, and the cancer spread to the pleura. Pulmonary metastasis can be considered a systematic disease, in which tumor cells spread to the whole body. A solitary lesion is rare; multiple forms usually occur together with liver metastasis or peritoneal dissemination. Lung lymphatic drainage from the pleural space is the predominant mechanism. The neoplastic involvement of the efferent flow from the pleural space leads to an accumulation of fluid within the pleural space. A strong relation was found between carcinomatous infiltration of the mediastinal lymph nodes and the occurrence of pleural effusion.15 Furthermore, malignant effusion from a nonpulmonary primary lesion, as in our case, is a manifestation of a considerably advanced disease, not early stage disease, and is associated with limited survival. In our patient, gastric cancer presented as EGC, not as an advanced stage, because the cancer cells were predominantly in the submucosal layer with no deep marginal involvement. However, massive lymphatic system involvement by cancer cells was present in the form of clusters and nests. Ichikawa et al.16 reported that severe lymphatic invasion represents a high risk of relapse and metastasis because gastric cancer with lymphatic invasion has a higher proliferative activity and a greater ability to metastasize to distant organs. Massive tumor clusters in the lymphatic channels of the submucosa could be an important route for metastasis to the pleura through the mediastinal lymph nodes, although EGC was responsible in our case. With the increasingly widespread application of ESD for the treatment of EGC, precise pretreatment staging has become mandatory to assess the appropriateness of the procedure in relation to curative treatment. Accurate prediction of the depth of tumor penetration is important for selecting the treatment modality in EGC. When EUS reveals a diffuse thickened hypoechoic lesion in a patient with EGC, a strict staging workup is necessary at an early stage, as is done with advanced gastric cancer, because this finding could be suggestive of massive submucosal layer invasion. Moreover, when there are unusual features without ruling out rare metastasis, ESD should be postponed until a clear evaluation with all possible examinations is achieved.
Notes
The authors have no financial conflicts of interest.