Prevention of Postendoscopic Retrograde Cholangiopancreatography Pancreatitis: The Endoscopic Technique
Article information
Abstract
Pancreatitis is the most frequent and distressing complication of endoscopic retrograde cholangiopancreatography (ERCP). Many recent studies have reported the use of pharmacological agents to reduce post-ERCP pancreatitis (PEP); however, the most effective agents have not been established. Reduction in the incidence of PEP in high-risk patients has been reported through specific cannulation techniques such as guide wire-assisted cannulation and the use of pancreatic stents. The present review focuses on ERCP techniques for the prevention of PEP.
INTRODUCTION
Pancreatitis is the most common complication of post-endoscopic retrograde cholangiopancreatography (post-ER-CP).1 The incidence of post-ERCP pancreatitis (PEP) varies widely from 1% to 7%. It is usually mild, but some severe cases (0.3% to 0.6%) result in pancreatic necrosis, multiorgan failure, and death. Many studies have used pharmacologic agents such as nonsteroidal anti-inflammatory drugs and protease inhibitors. These agents have shown preventive effects in some trials, but the optimal agents to prevent PEP have not been established. Improvement of equipment and experience has led to the development of advanced endoscopic techniques, and many recent studies have demonstrated that endoscopic techniques can effectively reduce the risk of PEP.
GENERAL CONSIDERATIONS ABOUT THE ENDOSCOPIC TECHNIQUE
Difficult cannulation, defined as 10 to 15 attempts at the procedure for >10 minutes, or five unintentional cannulations, indicates failure to perform selective deep biliary or pancreatic cannulation.2 Papillary trauma caused by difficult cannulation is an important, independent factor for PEP. Therefore, the number of cannulation attempts should be minimized to prevent PEP. In a large meta-analysis, pancreatic duct injection was found to be an independent predictor of PEP, although pancreatic duct injection was not found to be a significant risk factor for PEP in two more recent studies.3,4,5 Therefore, every effort should be made to reduce the number of injections and volume of contrast media as much as possible. Moreover, a movable catheter for biliary cannulation has been prospectively compared with the standard catheter in several randomized trials. All of these studies showed higher biliary cannulation success rates in the movable catheter group than in the conventional cannula. However, there was no difference in PEP rates.6
Sphincterotomy
The risk of PEP is generally similar for diagnostic and therapeutic ERCP. Biliary sphincterotomy; however, is not associated with an elevated risk of PEP. Although pancreatic sphincterotomy is generally known to be a significant risk factor for PEP, the incidence of severe pancreatitis is very low.7 The notion that the precut technique increases the risk of PEP is controversial because other factors such as the experience of the endoscopist and number of cannulation attempts may influence this risk. Precut techniques, including the standard needle-knife technique, fistulotomy, the use of a pull type sphincterotome, and the transpancreatic precut approach that involve the risk of pancreatic sphincter injury have been independently associated with an increased risk of PEP.8 However, a recent meta-analysis showed that pancreatitis developed in 2.5% of patients with an early precut compared with 5.3% of patients who underwent persistent cannulation attempts prior to the precut.9 Another meta-analysis similarly showed that an early precut with the needle-knife technique reduced the PEP rate.10 Moreover, a retrospective study showed that the PEP rate was lower when the precut technique was employed with <10 cannulation attempts than when precut technique is not used with ≥10 cannulation attempts.11 The decisions related to precut sphincterotomy concerning the timing and technique should be based on a patients' anatomy, indications, and the preference of the endoscopist. Early precutting should be considered the first alternative in patients with difficult cannulation, especially by experienced endoscopists.
Electrosurgical current
Thermal injury is thought to play a role in causing pancreatitis after sphincterotomy. A pure-cut current results in lesser edema than a blended current; therefore, a pure-cut current might reduce the incidence of PEP.12 A meta-analysis reported no significant difference in the incidence of PEP between pure-cut and blended currents, and the incidence of PEP does not seem to be influenced by the type of electrosurgical current used.13
Endoscopic papillary balloon dilation
Endoscopic papillary balloon dilation (EPBD) is a technique used to dilate the biliary sphincter while avoiding sphincterotomy to facilitate the removal of biliary stones. A randomized controlled trial (RCT) demonstrated that the technique was associated with a significantly increased risk of PEP, with two deaths occurring during the trial due to pancreatitis.14 In addition, a meta-analysis showed that PEP occurred more commonly in the EPBD group than in the sphincterotomy group (7.4% vs. 4.3%, p=0.05).15 Therefore, EPBD with <10-mm diameter balloons is generally in patients with a bleeding tendency or altered anatomy (such as Billroth II anastomosis) when sphincterotomy is difficult. Recently, several studies have demonstrated that large balloon dilation (12 to 20 mm) of the distal common bile duct and ampulla after sphincterotomy is a well-tolerated and effective technique for the removal of biliary stones without increasing the PEP rate.16,17 However, the size of the balloon should be selected according to the sizes of the ampulla, bile duct, and stone.
Sphincter of Oddi manometry
To reduce the risk of perfusion-related hydrostatic pancreatic injury, alternative catheters such as a modified triple-lumen perfusion catheter with simultaneous aspiration or a microtransducer catheter have been developed. Two RCTs showed a significantly lower incidence of PEP with the modified catheter than with the standard perfusion catheter (3.0% vs. 23.5%, p=0.01; 3.1% vs. 13.8%, p<0.05), and another RCT showed no episodes of PEP.18,19,20
SPECIFIC ENDOSCOPIC TECHNIQUES
Guide wire-assisted cannulation
The use of a guide wire as a primary cannulation device, either by pushing the wire directly into the papilla or by inserting the catheter into the papilla and then advancing the guide wire, has been increasing. Guide wire-assisted cannulation allows dye-free access. As the wires do not produce hydrostatic overpressure, careful guide wire entry into the pancreas during attempts at biliary cannulation does not increase the risk of pancreatitis. In an RCT, guide wire-assisted cannulation showed no PEP, with a cannulation rate of 98.5%.21 A retrospective study also reported that guide wire-assisted cannulation can be performed with a success rate of 97% and a PEP rate of 1%.22 In a meta-analysis, guide wire-assisted cannulation also showed a reduction in PEP compared with contrast-assisted cannulation (3.2% vs. 8.7%).23 In two meta-analyses, the odds ratios for the prevention of PEP were lower in the guide wire-assisted cannulation group in compared with the standard contrast cannulation group: 0.38 (95% confidence interval [CI], 0.19 to 0.76) and 0.23 (95% CI, 0.13 to 0.41), respectively.23,24 However, in another meta-analysis, no significant statistical difference in the incidence of PEP was observed between the two techniques (p=0.09).25 The most common methods involve the use of a single-guide wire technique (SGT) or a double-guide wire technique (DGT), in which a wire is inserted into the pancreatic duct and the cannulation device is passed alongside the guide wire (Fig. 1). In the first RCT, no cases of PEP occurred.26 However, another RCT showed a higher rate of PEP in patients undergoing DGT as opposed to SGT (17% vs. 8%).27 On the other hand, a retrospective study did not show a significant difference in the rate of PEP between the two techniques (5.3% vs. 6.1%).28 In an RCT, the incidence of PEP was lower in a 5-Fr pancreatic stent (PS) placement group than in a non-PS placement group after DGT (2.9% vs. 23%).29 Therefore, prophylactic PS placement should be considered when DGT is used in patients with difficult cannulation. Nowadays, many experienced endoscopists use a hybrid guide wire-assisted cannulation and standard contrast-assisted cannulation technique with minimal contrast medium to outline the distal ducts with wire probes. This type of hybrid technique may avoid pancreatic ductal injury, but has not been formally evaluated.
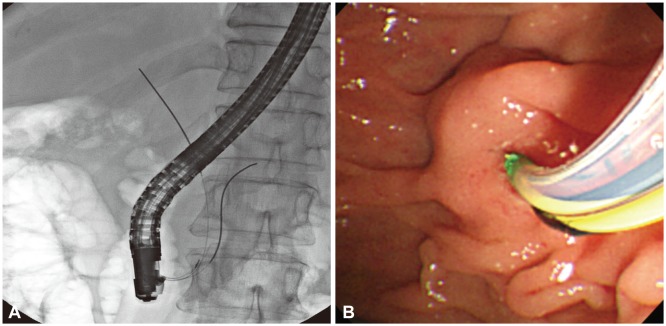
The double-guide wire technique was performed successfully in a case of difficult biliary cannulation. (A) Fluoroscopic image of each guidewire placed in the bile and pancreatic duct, respectively. (B) Endoscopic view of endoscopic sphincterotomy knife insertion into the bile duct alongside a guidewire placed into the pancreatic duct.
Pancreatic duct stent placement
PS placement decreases the incidence of PEP by promoting the drainage of the pancreatic duct and reducing pancreatic intraductal pressure resulting from papillary edema (Fig. 2). PS placement in biliary sphincterotomy for sphincter of Oddi dysfunction, pancreatic sphincterotomy, precut, balloon dilation, endoscopic papillectomy, DGT, and after difficult cannulation. Several RCTs have demonstrated the efficacy of PS placement (Table 1).29,30,31,32,33,34,35,36,37,38,39,40,41,42 Moreover, several meta-analyses have demonstrated its effect on PEP risk reduction compared with non-PS placement.43,44,45,46 In a recent meta-analysis that included six additional RCTs that were published since 2010, the incidence of PEP decreased from 19% in the control group to 7%.47 Despite the good efficacy of PS placement, pancreatic duct injury has been a major complication of PS placement. The reported overall complication rate is 4.4%.46 Ductal and parenchymal injury has been reported to occur when conventional 5-Fr or larger-caliber plastic stents have been used. Although such injuries have been assumed to resolve spontaneously, permanent stenosis and relapsing pancreatitis have been reported.48 Precautionary measures to avoid this complication include the use of smaller-caliber (<5 Fr) and softer plastic stents. However, one RCT concluded that wider 5 Fr stents were easier to place (9.2 minutes vs. 11.1 minutes) and required fewer guide wires than 3 Fr stents, with no difference in PEP rates.49 PS placement after ERCP is recommended for the prevention of PEP in high-risk patients. However, PS should be documented as acceptable by radiography or removed within a few weeks to reduce the possibility of complications. In addition, there are many issues concerning the efficacy of PS placement for low-risk patients, risk assessments, adverse events, optimal sizes and materials, and the timing of removal.

An endoscopic image of a patient who received a 5-Fr pancreatic duct stent after endoscopic papillectomy.
CONCLUSIONS
Minimal cannulation attempts and injections as well as a small volume of contrast medium are important for preventing PEP. Furthermore, specific ERCP cannulation and sphincterotomy techniques must be performed according to individualized risk assessments. Despite some debates, both guide wire-assisted cannulation and PS placement are useful endoscopic techniques that reduce the incidence of PEP in high-risk patients. The appropriate endoscopic technique can increase safety of ERCP. In the future, well-designed and well-executed studies with a large sample size that focus on each endoscopic technique and newly developed approaches should be performed.
Notes
The authors have no financial conflicts of interest.
