Fatal Cerebral Air Embolism Due to a Patent Foramen Ovale during Endoscopic Retrograde Cholangiopancreatography
Article information
Abstract
Fatal air embolism to the cerebrum during an endoscopic retrograde cholangiopancreatography (ERCP) has not been reported in a patient with a biliodigestive anastomosis and multiresistant extended-spectrum β-lactamase Escherichia coli (ESBL) bacteremia. A 59-year-old woman with a history of laparoscopic cholecystectomy and iatrogenic injury of the right choledochal duct, choledochojejunostomy (biliodigestive anastomosis), recurrent cholangitis, revision of the biliodigestive anastomosis, recurrent liver abscesses, and recurrent stenting of stenotic bile ducts, was admitted because of fever and tenderness of the right upper quadrant. On ERCP, a previously deployed covered Wallstent was replaced. Blood cultures grew ESBL. After stent removal 8 days later, the patient did not wake up and developed arterial hypotension and respiratory insufficiency, requiring mechanical ventilation. Computed tomography scans showed extensive air embolism to the liver, heart, and cerebrum. She died 1 day later. Although the exact pathogenesis of the fatal cerebral air embolism remains speculative, the nonphysiological anatomy and chronic infection with ESBL may have been contributory factors.
INTRODUCTION
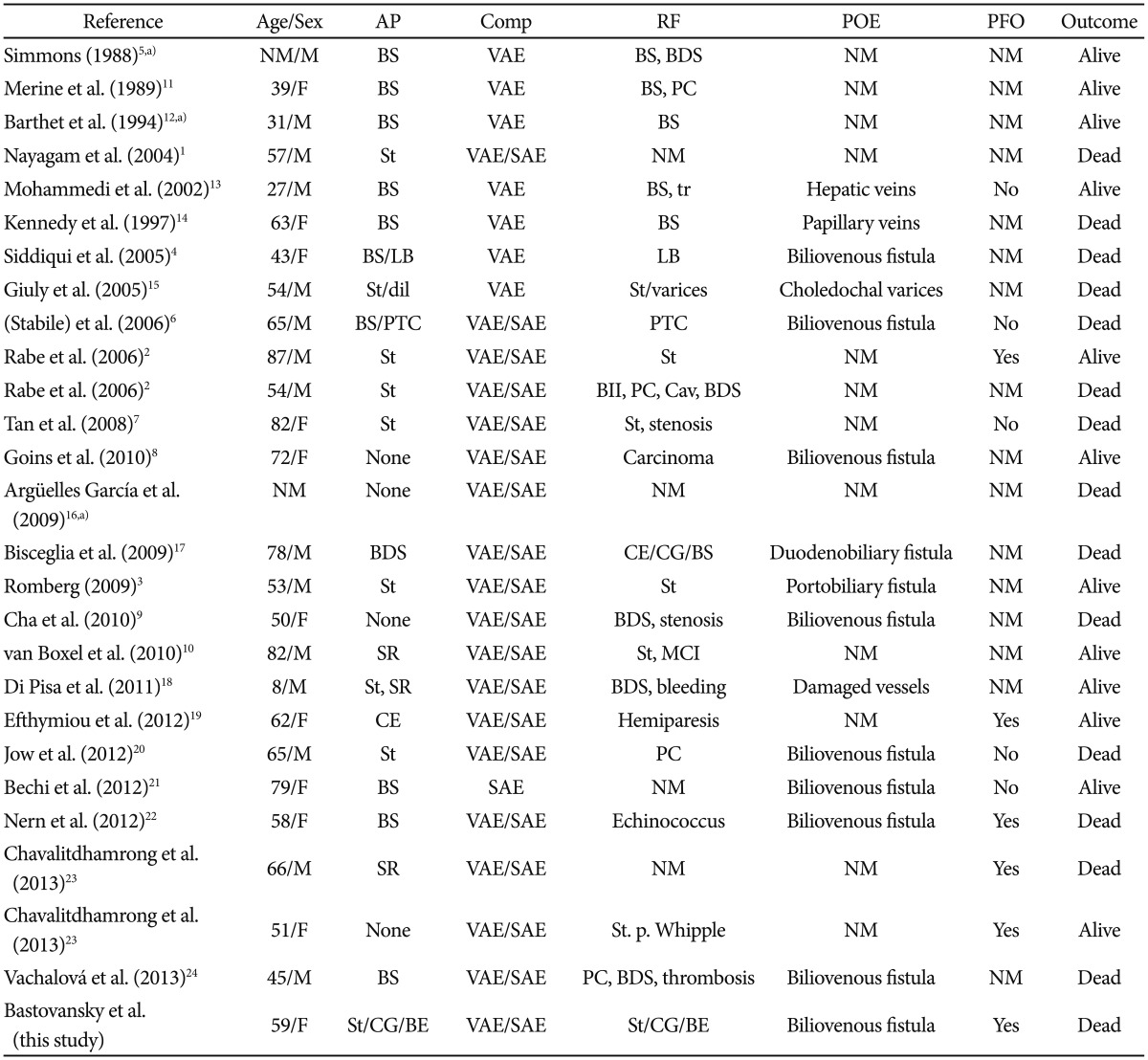
Air embolism is a rare but repeatedly reported complication of endoscopic retrograde cholangiopancreatography (ERCP)1,2,3,4,5,6,7,8,9,10 (Table 1);1,2,3,4,5,6,7,8,9,10,11,12,13,14,15,16,17,18,19,20,21,22,23,24 however, air embolism has not been reported together with chronic cholangitis, liver abscess, recurrent replacement of a bile duct stent, and bacteremia with multiresistant extended-spectrum β-lactamase Escherichia coli (ESBL).
CASE REPORT
A 59-year-old Caucasian woman underwent laparoscopic cholecystectomy at the age of 45 years. During the procedure, the choledochal duct was injured, requiring direct suturing. Postoperatively, a slight leakage persisted and a stenosis of the choledochal duct at the site of the injury and a choledochocutaneous fistula developed. Because conservative measures did not close the fistula, a high Roux-Y latero-lateral choledochojejunostomy (biliodigestive anastomosis) was set up 5 months after cholecystectomy. Despite this procedure, recurrent cholangitis developed, which responded favorably to antibiotics at each episode. As the frequency of cholangitic episodes increased 2 years after cholecystectomy (patient age, 47 years), the choledochojejunostomy was revised, the jejunum was partially resected, an adhesiolysis was performed, and intrahepatic gallstones were removed. Eight years after cholecystectomy, cholangitis recurred, multiple liver abscesses developed, and blood cultures grew Streptococcus intermedius, Pseudomonas aeruginosa, and E. coli. Twelve years after cholecystectomy, cholangitis relapsed owing to the recurrence of bile duct stenosis at the site of the previous injury, which was dilated during an ERCP without complications. Despite this measure, cholangitis recurred 2 weeks later but responded to appropriate treatment. Three months later, cholangitis recurred and blood cultures grew ESBL. Stenosis at the site of the anastomosis was dilated and, for the first time, a plastic stent was deployed by means of ERCP. Three months later, cholangitis recurred. On ERCP, the previously placed plastic stent was no longer visible and a new one was deployed into the right hepatic duct without complications. Cholangitis recurred again 6 and 7 months later. During consecutive ERCP, a liver abscess was detected, and as the previous plastic stent was no longer visible, a 10-mm-long Wallstent (metal stent) was deployed.
Two weeks after ERCP, the patient was readmitted because of fever and tenderness of the right upper quadrant. Results of blood chemical investigations during hospitalization are presented in Table 2. During ERCP on hospital day (hd) 1, the old Wallstent was replaced with a new one. During a further ERCP under sedation with propofol 1% (10 mL) and midazolam (2.5 mg), 7 days later (hd8), the previously deployed Wallstent was removed. Because the patient did not wake up after the procedure, flumazenil (0.25 mg) was given, with no effect. Additionally, low blood pressure and respiratory insufficiency developed, requiring intubation and artificial ventilation. A computed tomography (CT) scan of the thorax showed air in the right atrium and ventricle, and pericardium (Fig. 1). Abdominal CT showed no bile duct stent; a widened choledochal duct; and air within the bile duct system, the liver, and the subcapsular veins (Fig. 1). Magnetic resonance imaging of the brain showed multiple hypointense lesions in the subcortical white matter, which were interpreted as being septic emboli or air emboli (Fig. 2). A CT scan of the cerebrum confirmed the presence of multiple air emboli within the parenchyma. Transesophageal echocardiography failed to detect a patent foramen ovale (PFO). Hyperbaric therapy was not available. A cerebral CT scan because of mydriasis, 1 day later (hd9), showed massive cerebral edema, with a shift to the left, and ongoing herniation of the brain stem (Fig. 2). Antiedematous therapy was ineffective. The patient died on the same day without regaining consciousness. At autopsy, the entrance of air into the venous system could not be detected neither inside nor outside of the liver; however, a PFO was found.
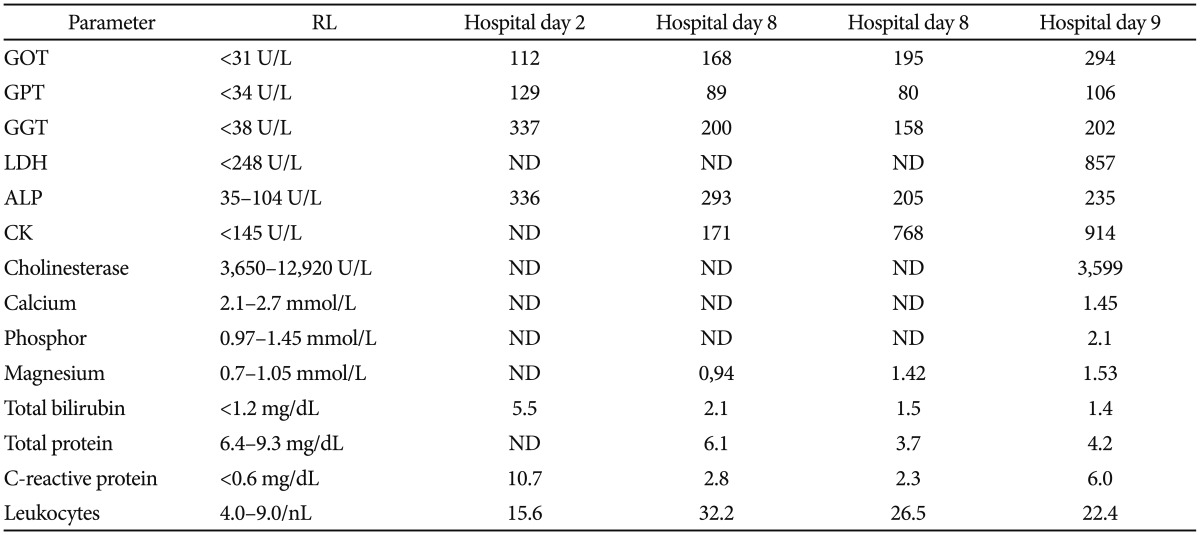
Results of Laboratory Investigations during Hospitalization (Hospital Day 8: Day at Which Fatal Air Embolism Occurred)
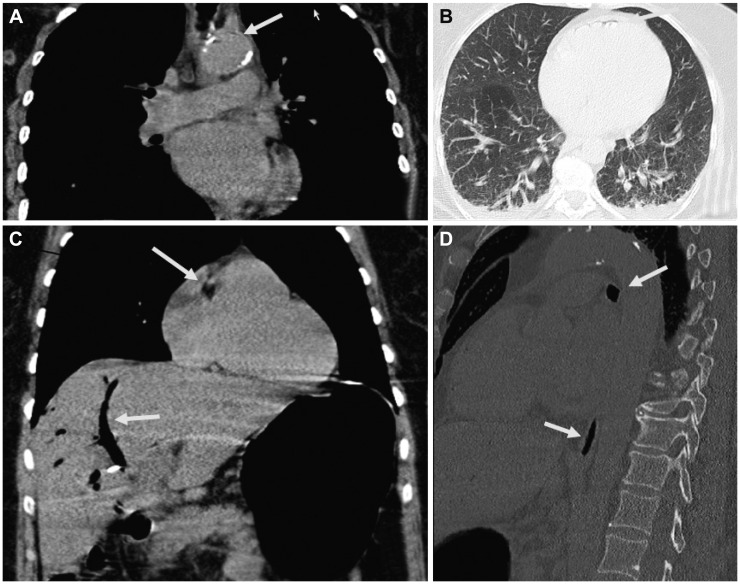
Computed tomography scan of the thorax and the abdomen showing air (arrows) within (A) the aorta, (B) pericardium, (C) bile ducts and right atrium, and (D) inferior caval vein.
DISCUSSION
Air embolism to the cerebrum during ERCP has been reported as a periprocedural complication during visualization of the bile ducts,1,6 after endoscopic sphincterotomy,5 during replacement of biliary stents,2,7 4 months after percutaneous biliary drainage and ERCP,6 and after percutaneous liver biopsy and ERCP (Table 2).4 The venous or systemic air embolism, including to the cerebrum, in these cases was explained by various pathomechanisms. Some authors attributed the occurrence of air embolism to the mechanical irritation of the bile duct walls by the endoscope and the development of biliovenous shunts.3 Others attributed the occurrence of air embolism to inflammation of the mucosa and the muscular wall resulting in transgression of air to the surrounding veins.1,3 Again, other authors speculated that air embolism resulted from irritation of the bile duct walls by the contrast medium and consecutive leakage of air into the venous system. According to another reported mechanism, air embolism could have resulted from damage of the bile duct mucosa and bile duct wall from the high pressure with which air was insufflated into the investigated cavities.1 A further cause of air embolism could be mechanical irritation of the bile duct wall by bile duct stones or from the removal of a metallic bile duct stent.3 It is also conceivable that there are preexistent biliovenous fistula, which are opened mechanically, from the increased pressure, or from systemic bacteremia. Another speculation relies on the assumption that air migrates through a patent sphincterotomy to the portal vein.3 It is also possible that sutures from a previous surgery are mechanically opened or that they expand spontaneously. All these pathomechanisms assume that air embolism results from the transgression of air through the bile duct wall or the sphincter into neighboring veins or even arteries.
Concerning the present patient, it remains speculative which of the above-mentioned pathomechanisms apply. Transgression of air from the bile duct system to the vasculature either occurred spontaneously or was due to mechanical irritation and rupture. Whether air embolism in the present case resulted from mechanical irritation by the endoscope or the manipulation of the stent remains speculative.2,6 Manipulations could have caused rupture of the bile duct wall and the surrounding veins, and the development of a biliovenous shunt. Enhancing pathogenetic factors could be the chronic inflammation of the bile duct walls, inflammation of the surrounding veins, and insufflation of air under pressure into the bile duct system during the procedure. The bile duct walls might have been particularly vulnerable because of chronic cholangitis, the recurrent abscesses, previous surgery, repeated stenting, repeated ERCPs, and the systemic systemic bacteremia. The walls of the periductal veins may have been affected by the neighboring chronic cholangitis and the bacteremia. Infection with ESBL may have predisposed the patient to the vulnerability of the bile ducts and the surrounding veins and the consecutive development of a biliovenous shunt. Another predisposing factor could be the nonphysiological anatomy after the previous surgery.
Irrespective of the underlying pathomechanism, cerebral air embolism as a complication of ERCP is only comprehensible in the presence of a PFO, arteriovenous shunts in the lung, or insertion of the caval veins directly into the left atrium. In the absence of such abnormalities, air embolism during ERCP should be impossible, with the consequence that patients scheduled for ERCP should first undergo contrast-enhanced transthoracic or transesophageal echocardiography to exclude or confirm a right-to-left shunt at the cardiac or pulmonary level. Patients with such a shunt should undergo ERCP only with precautionary measures, such as the immediate availability of a pulmonary artery catheter to acutely drain air from the right atrium or the availability of a hyperbaric facility. The air embolism to the cerebrum in the present patient was most likely only possible because of the presence of a PFO, as documented at autopsy. Transgression of air through pulmonary arteriovenous shunts or abnormally inserting caval veins is rather unlikely as such abnormalities were not found on autopsy.
In conclusion, this case shows that ERCP may be rarely complicated with a fatal cerebral air embolism, and that previous surgery or chronic systemic ESBL infection may have a contributory role in addition to chronic cholangitis, liver abscess, or mechanical irritation of the bile duct wall by the stent or the endoscope. If patients do not wake up after ERCP, air embolism should be considered; a cerebral and thoracic CT scan should be ordered; and appropriate measures, including aspiration of air from the right ventricle through an acutely floated pulmonary artery catheter or hyperbaric oxygenation, should be initiated. In patients with a PFO or pulmonary arteriovenous shunts, ERCP should be carried out with caution and only if emergency facilities are available. In patients with chronic infections or non-physiological anatomy of the bile ducts, a PFO shoud be excluded before ERCP or the procedure reconsidered.
Notes
The authors have no financial conflicts of interest.