Plastic and Biodegradable Stents for Complex and Refractory Benign Esophageal Strictures
Article information
Abstract
Endoscopic stent placement is a well-accepted and effective alternative treatment modality for complex and refractory esophageal strictures. Among the currently available types of stents, the partially covered self-expanding metal stent (SEMS) has a firm anchoring effect, preventing stent migration and ensuring effective covering of a narrowed segment. However, hyperplastic tissue reaction driven by the uncovered mesh may prevent easy and safe stent removal. As an alternative, a fully covered SEMS decreases the recurrence of dysphagia caused by hyperplastic tissue ingrowth; however, it has a high migration rate. Likewise, although a self-expanding plastic stent (SEPS) reduces reactive hyperplasia, the long-term outcome is disappointing because of the high rate of stent migration. A biodegradable stent has the main benefit of not requiring stent removal in comparison with SEMS and SEPS. However, it still has a somewhat high rate of hyperplastic reaction, and the long-term outcome does not satisfy expectations. Up to now, the question of which type of stent should be recommended for the effective treatment of complex and refractory benign strictures has no clear answer. Therefore, the selection of stent type for endoscopic treatment should be individualized, taking into consideration the endoscopist's experience as well as patient and stricture characteristics.
INTRODUCTION
Dysphagia caused by benign esophageal strictures is a frequently encountered problem. It can negatively influence the patient's quality of life and may cause important complications such as malnutrition, weight loss, and aspiration.1 Benign esophageal strictures are caused by various disorders and procedures such as gastroesophageal reflux disease, surgery (anastomotic stricture), radiotherapy, ablative therapy, caustic ingestion, and pill-induced injury.2,3 Esophageal strictures can be divided into two types: simple and complex. Simple strictures are defined as short, focal, straight, and easy to pass through with a standard diagnostic endoscope, and are mostly caused by Schatzki rings, esophageal webs, or peptic injury. Complex strictures are longer (>2 cm), tortuous, angulated, or have a severely narrowed diameter (<12 mm) and often do not allow passage of an endoscope. Complex strictures can be mainly caused by surgery, radiotherapy, or corrosive injury.1,2 Of the two types of strictures, the complex type is difficult to treat and associated with a higher recurrence rate than the simple type. Refractory strictures have the following characteristics: an anatomic fibrotic esophageal restriction, absence of inflammation or motility disorder, and inability to achieve a diameter of ≥14 mm in five sessions of dilatations at 2-week intervals or inability to maintain a diameter of ≥14 mm for 4 weeks once this diameter has been achieved.4
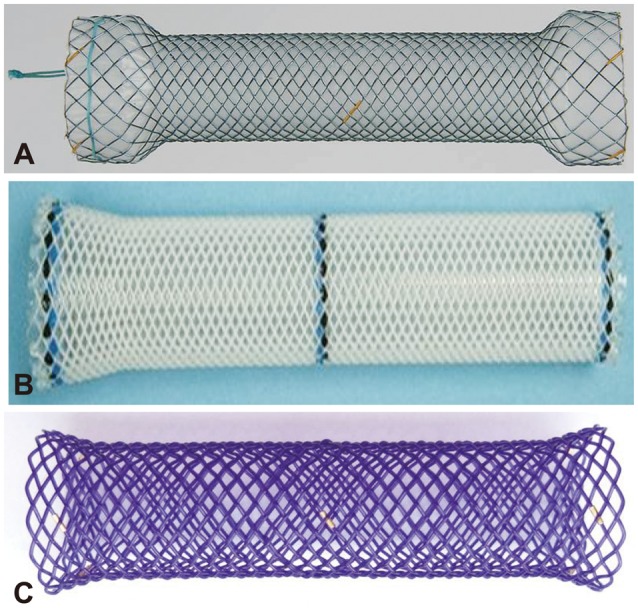
Until now, serial endoscopic dilatation with bougies or balloons has been the standard treatment for esophageal strictures. Although the immediate success rate for relieving dysphagia is 80% to 90%, the recurrence rate reaches 30% to 40% during long-term follow-up, especially in complex strictures, requiring repeated dilatations or even surgery.5,6 What is worse, up to 10% of patients will not experience any meaningful improvement and be unresponsive to repeated endoscopic dilatations. In these cases, repeated sessions of dilatation without long-term clinical success are closely linked to increased physical and emotional burden to patients; thus, an alternative treatment strategy should be introduced.7,8,9 As an alternative, surgical solutions such as gastric pull-up and enteral replacement are most effective; however, many patients are not good surgical candidates because of their comorbid conditions or unwillingness to undergo surgery because of a substantial risk of morbidity and mortality.7,10,11 Endoscopic temporary placement of a self-expanding esophageal stent has been proposed as a well-accepted and effective treatment for refractory esophageal strictures owing to its longer-lasting dilatation effects, ability to maintain luminal patency, and simultaneous stretching of the strictures in comparison with dilatation procedures; moreover, its favorable outcomes, as well as lower morbidity and mortality than surgery have been reported.12,13,14 Currently, three types of esophageal stents are available: self-expanding metal stent (SEMS), self-expanding plastic stent (SEPS), and biodegradable (BD) stent (Fig. 1).2,15 This review focuses on the current experience with esophageal stents, mainly SEPS and BD stents, for complex and refractory benign esophageal strictures.
SELF-EXPANDING PLASTIC STENT
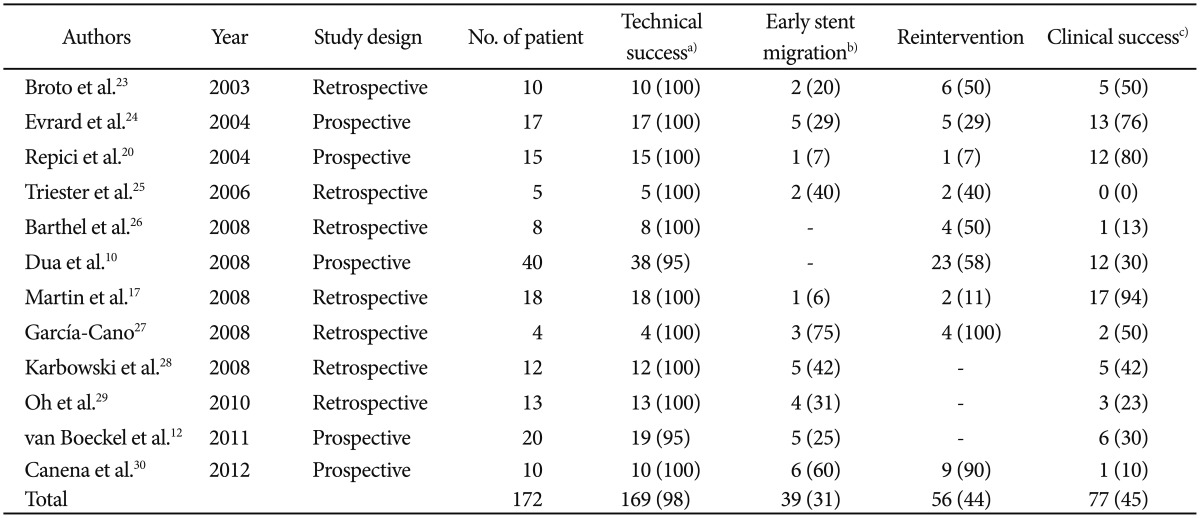
Recently, SEPS has been introduced to minimize the hyperplastic tissue reaction. SEPS can be easily removed and seems not to be inferior to metallic stent.16 Initially, SEPS was found to provide significant improvement of dysphagia and quality of life, and to decrease the number of dilatation sessions in patients with benign strictures.2,17 On the basis of these results, the use of SEPS in patients with benign esophageal strictures has been proposed as an alternative to SEMS.18 The currently available SEPS is the Polyflex stent (Boston Scientific, Natick, MA, USA), which is composed of a plastic wire and has a fully covered design with silicone and a proximal flare. This stent material has been suggested to reduce reactive tissue hyperplasia. As a result, the stent is easily removable and approved by Food and Drug Administration for the treatment of benign esophageal strictures. The use of Polyflex stents in malignant strictures has shown good relief of dysphagia, a major complication rate of 9%, and a recurrent dysphagia rate of 37%, mostly due to stent migration (13% to 29%), tissue overgrowth (10% to 30%), and food impaction (5%).2,16,19 For benign strictures, varying results have been reported. The initial study series in benign esophageal strictures showed promising results, with relief of dysphagia in 80% of patients.8,20,21,22 SEPS seems to result in a meaningful reduction of reactive tissue hyperplasia and safe stent removal owing to the fully covered design and the silicone material. However, results of a later series have shown a high migration rate (up to 62%) and low long-term relief of dysphagia (only 17% to 30% of patients).3,4,5,10 A recent systematic review of 130 patients with a benign esophageal stricture, obtained from 10 studies, showed that after a median follow-up period of 13 months, only 52% of the patients were dysphagia-free, the early migration rate was 24%, the endoscopic reintervention rate was 21%, and major clinical complications occurred in 9% of patients.3 Our systematic review of 172 patients with a benign esophageal stricture, including recent updated reports at the time of writing of this review article, shows the following results: technical success rate of 98%, clinical success rate of 45%, and early stent migration rate of 31% (Table 1).10,12,17,20,23,24,25,26,27,28,29,30
BIODEGRADABLE STENT
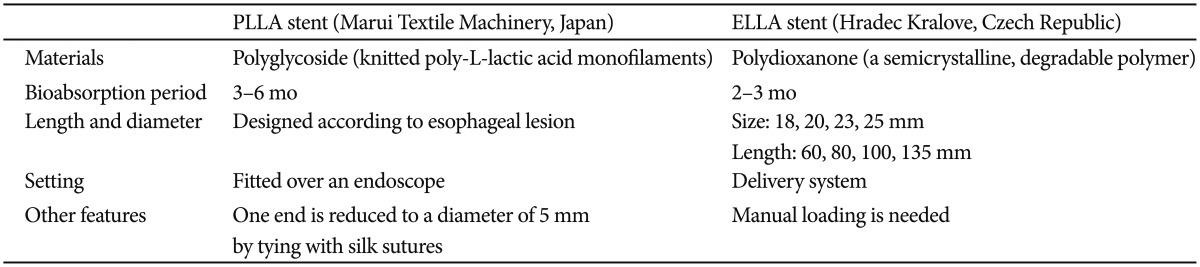
Considering that recent studies on SEPS have shown less favorable outcomes, including a disappointing long-term efficacy, high migration rate, and frequent needs for reintervention, the BD stent has been developed as an alternative to SEPS. The currently available BD stent designs are the ELLA-BD stent (ELLA-CS, Hradec Kralove, Czech Republic), which is composed of polydioxanone, a surgical suture material,31 and the poly-L-lactic acid (PLLA)-BD stent (Marui Textile Machinery, Osaka, Japan), which consists of knitted PLLA monofilaments (Table 2).32 These stents can be degraded by hydrolysis, which is accelerated at low ambient pH. Generally, stents begin to degrade after 4 to 5 weeks and dissolve during a period of 2 to 3 months. The major strength of BD stent over SEMS or SEPS is that it does not require removal, even when migrated, and it can be left until it is dissolved by gastric acid, which accelerates hydrolysis, thus avoiding further procedures and potential morbidity.31
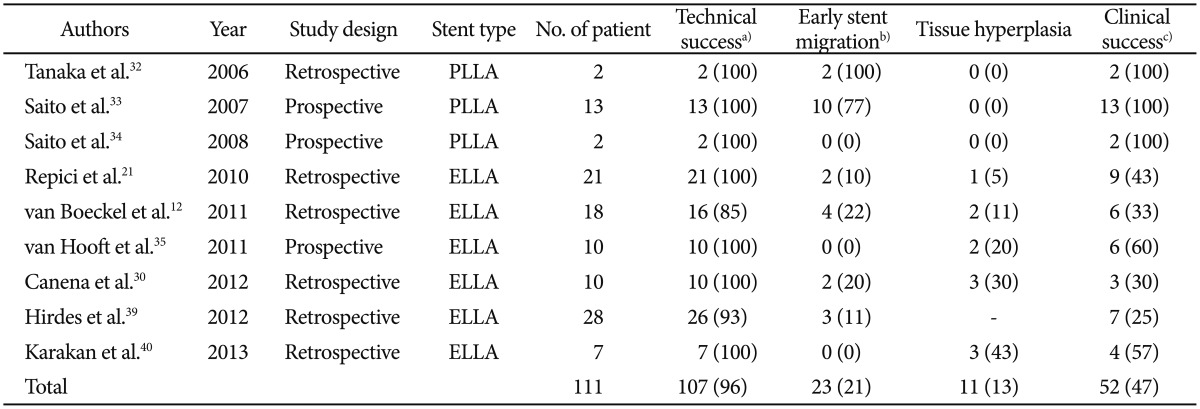
Subsequent studies have shown the availability of the PLLA-BD stent, with a low stent-related complication rate, compared with SEMS and SEPS.32,33,34 In these studies, there were no symptoms or need for reintervention within the follow-up period in all cases; however, most of the stents (10 of 13 cases [70%]) had migrated within 10 to 21 days after insertion. Because of this tendency of early stent migration found in those three studies, the natural history of degradation of the PLLA-BD stent within the esophagus and the tolerability of the degradation process over time were not adequately assessed. In contrast to the PLLA-BD stent, the ELLA-BD stent has shown some promising results. An initial case series with the ELLA-BD stent for esophageal strictures, including caustic, peptic, malignant, anastomotic, radiation-induced strictures as well as achalasia, showed a low migration rate (0% to 22%) and an acceptable clinical success rate (33% to 60%).21,31,35,36,37 However, the ELLA-BD stent, not unlike commercially available SEMS and SEPS, can induce significant hyperplastic tissue reactions.31,35,36,37,38 The uncovered design of BD stent allows stent embedding to the esophageal wall and significantly reduces the migration rate; however, it induces considerable reactive tissue hyperplasia. Our systematic review of 111 patients with a benign esophageal stricture obtained from nine studies showed the following results: technical success rate of 96%, clinical success rate of 47%, early stent migration rate of 21%, and tissue hyperplasia rate of 13% (Table 3).12,21,30,32,33,34,35,39,40
Recently modified ELLA-BD stents with a non-BD covering made of polyurethane were used in five patients with esophageal leaks or perforations.41 The initial clinical success rate was 80%; however, the stent migration rate was 60%. Therefore, the effect of the covered design on minimizing the hyperplastic tissue reaction remains an unsolved problem. Recently, drug-eluting BD stents have been introduced as a solution to reactive hyperplasia. Theoretically, localized delivery of drugs such as paclitaxel or rapamycin from drug-eluting stents is a promising treatment method for preventing restenosis or inflammatory cell proliferation. Until now, many experimental studies have been done with animal models.42,43 More studies on the clinical application of drug-eluting BD stents for human patients are needed.
PLASTIC AND BIODEGRADABLE STENTS: BETTER THAN METAL STENT
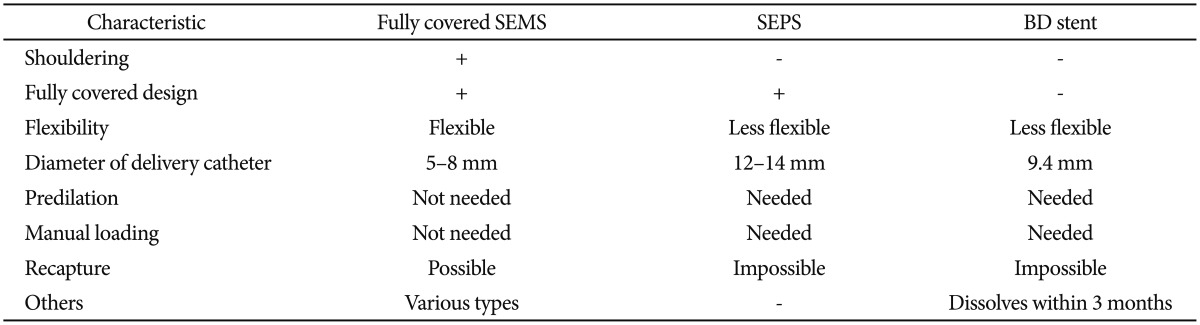
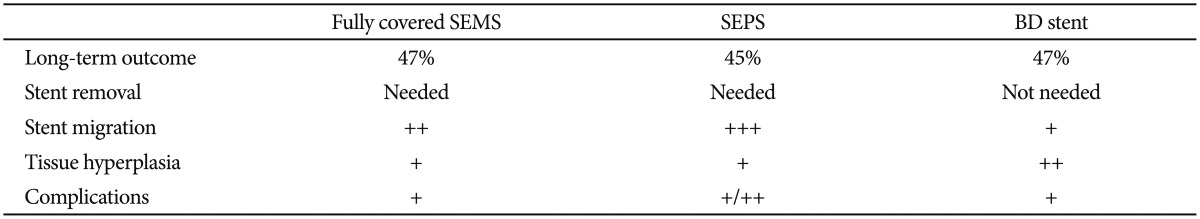
The main limitations of stent placement in benign strictures are hyperplastic tissue reaction, embedding into the esophageal wall, and stent migration. To avoid these limitations, an optimal stent must have a flexible, nontraumatic design and a diameter large enough for normal food passage. In addition, the procedure for insertion, repositioning, and removal should be simple and minimize stent migration and tissue hyperplasia.2 However, for the treatment of complex and refractory benign esophageal strictures, no ideal stent is yet available. Each type of stent has its own advantages and disadvantages according to the stent characteristics (Table 4). To date, only uncontrolled studies have evaluated the safety and efficacy of various stent designs in patients with benign esophageal strictures. On the basis of the studies published thus far, the long-term outcome according to the stent type is not different and is unsatisfactory (approximately 45%) (Table 5). As previously mentioned, although a partially covered SEMS has a high anchoring capacity owing to the uncovered design at both ends, it shows a high rate of hyperplastic ingrowth or overgrowth through the uncovered mesh, resulting in severe stent embedding and difficulty of safe removal endoscopically. On the other hand, the migration rate is higher when fully covered stents, either SEMS or SEPS, are used. This is because of their reduced anchoring capacity compared with partially covered SEMS. Meanwhile, the BD stent shows reduced migration rate owing to its uncovered design; however, hyperplastic tissue reactions have emerged as a main problem. Up to now, the question of which type of stent should be recommended for the effective treatment of complex and refractory benign strictures has no clear answer. Therefore, the selection of stent type for endoscopic treatment should be individualized, taking into consideration the endoscopist's experience as well as patient and stricture characteristics, especially including the location and cause of the stricture. In the case of sites with an increased risk of migration (i.e., distal esophagus or anastomosis site) or restenting after stent migration, the BD stent may be a reasonable option over SEMS or SEPS. To minimize tissue hyperplasia after BD stent placement, steroid injection or a drug-eluting BD stent may be an effective option. Larger, randomized, prospective, well-designed studies are needed to demonstrate the long-term efficacy and safety of stents at each applicable location within the gastrointestinal tract.
CONCLUSIONS
SEPS and BD stents were developed to decrease the limitations of SEMS for benign complex and refractory esophageal strictures. Early studies on both stents reported excellent results; however, our systematic review does not show a superior clinical outcome of these stents compared with SEMS. Although the ideal stent has not yet been developed, SEPS and BD stents have their own special merits such as decreasing tissue hyperplasia and eliminating the need for stent removal. Therefore, the selection of stent type should be individualized, taking into consideration the endoscopist's experience as well as patient and stricture characteristics. In the future, a new stent that has only the merits of SEMS, SEPS, and BD stents will need to be developed.
Acknowledgments
This work was supported by a 2-year research grant from Pusan National University.
Notes
The authors have no financial conflicts of interest.