A Case of Endoscopic Full-Thickness Resection in a Patient with Gastric High-Grade Dysplasia Unsuitable for Endoscopic Submucosal Dissection
Article information
Abstract
Gastric high-grade dysplasia is an important premalignant lesion in gastric epithelial cells and has a high possibility of transforming to adenocarcinoma. Therefore, biopsy-proven high-grade dysplasia should be treated with en bloc resection methods such as endoscopic mucosal resection or endoscopic submucosal dissection (ESD). We report the case of a 63-year-old male patient, diagnosed with gastric high-grade dysplasia at the angle and lesser curvature side of the lower body. The patient was initially treated with ESD, although histopathology subsequently showed horizontal margin involvement. Since the lesion was diffusely edematous and margins were uncertain because of the previous ESD treatment, we chose to treat the patient with laparoscopy-assisted endoscopic full-thickness resection (EFTR). EFTR is a recently developed procedure, which uses both endoscopic and laparoscopic techniques to resect the full-thickness of the tissue. The final pathologic report revealed high-grade dysplasia and a focal intramucosal carcinoma of 0.8×0.7 cm. We conclude that EFTR can be an effective alternative treatment in gastric high-grade dysplasia unsuitable for ESD.
INTRODUCTION
Gastric cancer is the fourth most common cancer, and the second most frequent cause of death from cancer worldwide.1 Early gastric cancer (EGC) is a malignant tumor confined to the mucosa or the submucosa, regardless of lymph node metastasis, and is known to comprise 40% to 60% of all gastric cancers thanks to advances in diagnostic techniques.2,3 Endoscopic resection, through endoscopic mucosal resection (EMR) or endoscopic submucosal dissection (ESD), have results similar to surgical treatment in cases with classic indications for endoscopic resection (well-differentiated mucosal cancer <2 cm, without ulceration).4 Therefore, early intestinal-type gastric cancer with no risk of lymph node metastasis is typically treated by endoscopic resection.
Precancerous lesions such as gastric epithelial dysplasia and particularly high-grade dysplasia (HGD) can progress to adenocarcinoma. HGD progression to adenocarcinoma was observed in 60% to 85% of cases during a 4.7-year follow-up.5,6 Many reports strongly suggest that HGD is highly predictive of invasive carcinoma, which either coexists, or appears shortly after biopsy;5,7 therefore, endoscopic resection or surgery should be considered.
Both surgical resection and repeated-ESD (re-ESD) may be effective in the treatment of recurrent EGC after failed ESD,8,9,10,11 though no definite guidelines exist for gastric HGD. As well, ablative therapy with argon plasma coagulation (APC) might be a viable alternative to surgical or endoscopic resection for dysplasia.12 Although HGD is highly predictive of hidden carcinoma, full surgical resection (e.g., gastrectomy), is too invasive. Therefore, we decided to use a new stomach-saving method: laparoscopy-assisted endoscopic full-thickness resection (EFTR). Here, we report the case of a patient with gastric HGD unsuitable for ESD.
CASE REPORT
A 63-year-old man visited our clinic for treatment of gastric HGD. The patient was referred to our clinic after a biopsy revealed a 1-cm active ulcerative lesion, identified as a microfocal tubular adenoma with HGD, after 2 months of proton pump inhibitor use and 1 week of Helicobacter pylori eradication treatment (Fig. 1). Upon physical examination, the patient exhibited no specific symptoms or abnormalities. The patient had no history of alcohol use or smoking, and no history of specifically diagnosed medical conditions. Blood tests showed that the white blood cell count was 5,340×103/µL; hemoglobin, 13.9 g/dL; and platelet count, 245×103/µL. All other laboratory findings were within normal limits. No specific gastric wall abnormalities or lymph node enlargements were noted on an abdominal computed tomography scan. The patient underwent a repeated upper endoscopy in our clinic for a more exact evaluation of the previously proven gastric dysplasia. A 7-mm, discolored, nodular lesion (previously found to be HGD) was noted at the lesser curvature side of the low body (Fig. 2A); therefore, we obtained two biopsy samples. A second elevated and nodular lesion was noted at the entire angle, and two biopsy samples were obtained from the more prominent nodular lesion located at the anterior wall of the angle (Fig. 2B). Both lesions revealed HGD; therefore, we planned to perform ESD for HGD treatment.
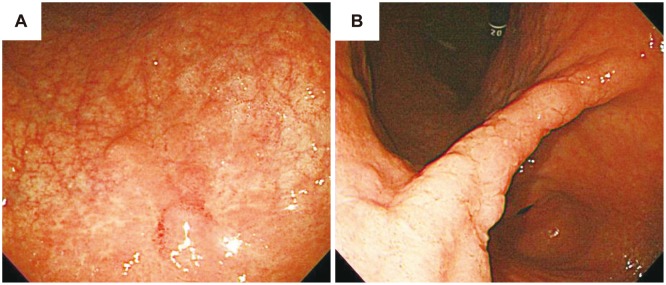
Endoscopic findings before endoscopic submucosal dissection. (A) Discolored and nodular lesion located at the lesser curvature of the low body. (B) Diffuse nodularity observed at the angle. Both lesions were confirmed as high-grade dysplasia after biopsy.
ESD was performed under conscious sedation, using midazolam and propofol, in the hospital on day 1. No complications such as bleeding or perforation were observed after the procedure. The first lesion (located at the lesser curvature side of the low body) measured 0.7×0.5×0.5 cm in size, and was found to be low-grade dysplasia. The second lesion, at the center of the angle, measured 1.2×0.7×0.5 cm in size, and was found to be HGD. However, lateral margin involvement was observed at both lesions. Postoperative diet was started at day 3, and the patient was discharged the same day. After 6 months, follow-up upper endoscopic biopsy was performed, and HGD was again detected. We attempted ESD a second time, but were not successful because of diffuse edema and uncertain margins.
If the results of the follow-up endoscopic biopsy had been EGC, we might have chosen a gastrectomy with regional lymph node dissection. However, since the biopsy result was HGD, we wanted to preserve as much of the stomach as possible. After detailed consultation, we undertook laparoscopy-assisted EFTR to treat the gastric dysplasia.
Surgery was performed with the patient under general anesthesia, and lasted 2 hours and 20 minutes. The patient received an antibiotic injection; then, a 12-mm initial access site was established at the periumbilicus. After a forward-viewing laparoscope was introduced through the periumbilical trocar, a pneumoperitoneum was created with carbon dioxide, and two additional trocars were placed. An upper endoscope with a transparent cap was also used. We marked the normal mucosa surrounding the lesion with APC (Fig. 3A), then injected fluid into the affected submucosa. An initial cut (precut) was made with a needle knife outside the sealed area; then, an insulation-tipped knife was inserted into the precut incision, and used to create a circumferential submucosal incision (Fig. 3B). A small puncture was made on one side of the lesion (Fig. 3C), and full-thickness resection was performed with an IT-2 knife around three-quarters of the circumference (Fig. 3D). A harmonic scalpel was used to liberate the remaining quarter of the circumference under laparoscopic supervision. The defective gastric wall was closed manually after a mini-incision, and a leakage test using endoscopic air inflation was carried out after the closure.
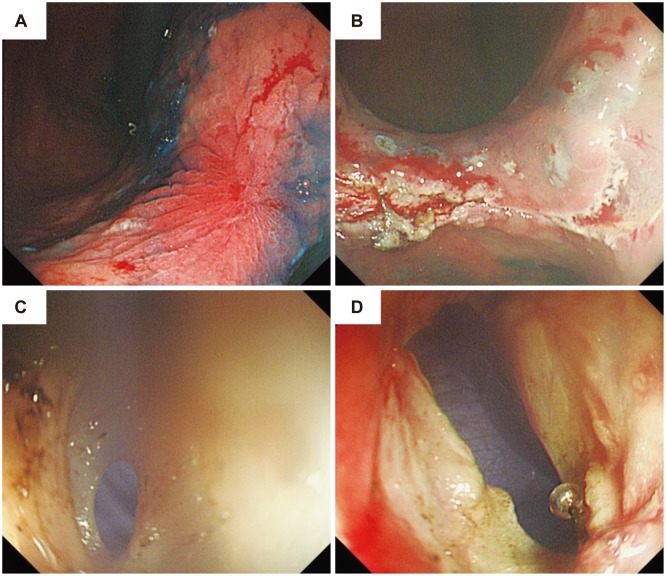
Endoscopic full-thickness resection after failed endoscopic submucosal dissection. (A) The successfully sealed lesion. (B) The circumferential submucosal incision. (C) The punctured lesion. (D) Completed full-thickness resection.
The postoperative diet was started at day 5, and patient was discharged at day 10 without complications. The excised portion of the lesion was measured at 4.2×3.0×1.2 cm. The final pathological report revealed HGD with a focal intramucosal carcinoma of 0.8×0.7 cm (Fig. 4). No lymphovascular invasion or resection margin involvement was detected. Therefore, we conclude that the lesion was completely resected.
DISCUSSION
Gastric epithelial dysplasia, particularly HGD, is considered a premalignant lesion that frequently progresses to adenocarcinoma. Therefore, biopsy-proven HGD should be treated with en bloc resection. Both EMR and ESD are widely used treatments for gastric dysplasia.13
After incomplete resection of gastric HGD by ESD, subsequent treatments might include re-ESD, careful observation, gastrectomy, wedge resection, or ablative therapy. Thirteen patients with gastric cancer who underwent gastrectomy after incomplete EMR/ESD were analyzed in one report.14 The positive residual tumor rate was only 15.4% (2 of 13) in positive lateral and/or vertical margin cases. A total of 84.6% of cases (11 of 13) had no residual tumor, despite positive margins. Lymph node metastasis was observed in 23.1% of cases (3 of 13) with submucosal cancers. The authors concluded that a gastrectomy is required for patients with incomplete resection after EMR/ESD, because of the risk of both residual tumor and lymph node metastasis. However, no reports of gastric dysplasia cases are available. In principle, gastric dysplasia is a benign epithelial lesion, confined to the mucosa, with no risk of lymph node metastasis. Therefore, when gastric dysplasia remains after ESD, a minimally invasive treatment that preserves the maximum amount of gastric tissue should be used.
Wedge resection is a minimally invasive treatment modality with acceptable results. However, wedge resection can create a larger resection than expected, and may lead to gastric stenosis or deformity. Local recurrence occasionally occurs, and positive surgical margin may also occasionally occur. Ablative therapy, including APC, is an alternative treatment, but its role is limited in the case of HGD.12,15
EFTR is a recently developed method, combining endoscopic and laparoscopic techniques. EFTR consists of four major procedures:16 1) a circumferential incision to the submucosal layer around the lesion, using the ESD technique; 2) an endoscopic full-thickness (from the muscle layer to the serosal layer) incision around three-quarters or two-thirds of the circumference under laparoscopic supervision; 3) laparoscopic full-thickness incision around the remaining one-quarter or one-third of the circumference from inside the peritoneal cavity; and 4) hand-sewn closure of the gastric-wall defect. Cho et al.17 demonstrated the benefits of EFTR and its use in all stomach locations.
In addition to the findings of Cho et al.,17 EFTR has many benefits. First, an appropriate surgical margin can be easily obtained using the initial circumferential submucosal incision with an ESD technique, making the subsequent full-thickness resection as small as possible. EFTR is also applicable to patients with gastric epithelial dysplasia or EGC, and those who have undergone an incomplete endoscopic resection, as our patient had. In these cases, repeated endoscopic resection is technically challenging, because of the severe ulcerative and fibrotic changes.18 In these cases, EFTR can remove benign and malignant lesions, even those originating from the deep layers.19 Moreover, selected patients with gastric cancer extending into a layer deeper than the submucosa, who are also at risk of lymph-node metastasis, may be treated or staged by EFTR with a lymphadenectomy,19 particularly if they are poor candidates for gastrectomy.
Despite the advantages of EFTR, the procedure is complex, costly, and may require conversion to gastrectomy. If unsuccessful, cancer cells may seed the peritoneal cavity. There are many reports regarding EFTR treatment for submucosal tumors, but there are few studies about EFTR in gastric cancer. Cho et al.17 described results from 14 stomach cancer patients who were treated with EFTR, and Nunobe et al.20 published a case report regarding a 70-year-old female patient with EGC who was successfully treated with EFTR. However, the number of patients was too small to prove the superiority of EFTR. In contrast, benign lesions, such as submucosal tumors or gastric dysplasia, do not seed cancer cells or metastasize to lymph nodes. Since HGD can coexist, or appear with, invasive carcinoma,7,9 the possibility of lymph node metastasis cannot be completely dismissed. However, it is unclear whether gastrectomy should be performed for a remnant lesion after incomplete EMR/ESD. Our patient did not require additional treatment, since the final specimen contained intramu-cosal adenocarcinoma within the indications for ESD. If the adenocarcinoma in the final specimen was beyond the indications for ESD, an additional gastrectomy with lymphadenectomy might have been required.
We have presented the first use of EFTR to treat incompletely resected HGD after ESD. In our case, EFTR enabled en bloc and whole-layer excision of lesions, with an adequate surgical margin. While the indications for EFTR are not well established, EFTR may provide a more reasonable and effective alternative to other laparoscopic procedures in cases of gastric dysplasia that have been incompletely resected by ESD.
Notes
The authors have no financial conflicts of interest.

