Diminutive and Small Colorectal Polyps: The Pathologist's Perspective
Article information
Abstract
Recent progress in advanced endoscopic imaging and electronic chromoendoscopy allows the real-time endoscopic estimation of the histologic type of polyps, mainly for the differentiation of adenomas from hyperplastic polyps. Accordingly, a "resect-and-discard" strategy applied to diminutive colorectal polyps is now one of the emerging issues among gastroenterologists. The strategy has a practical advantage in terms of the potential cost savings. However, it has a number of limitations in the medical, academic, and legal aspects. The major pitfalls include the endoscopic investigation of colorectal polyps with a wide variety of histogenetic origins, including serrated polyps, and the lack of a standardized method for polyp size measurement. Another issue is the importance of the pathologic diagnosis for legal purposes and medical research. Moreover, it is not certain whether the implementation of the strategy has economic benefit in countries with an undervalued reimbursement system for pathologic examination. There is no doubt that a highly confident optical diagnosis of polyp type is a novel valuable tool. It can provide a more steady symbiosis between gastroenterologists and pathologists to allow a more evident diagnosis and management of patients with colorectal polyps.
INTRODUCTION
Colonoscopy has become the primary method for colorectal cancer screening. It is effective in the detection and removal of adenomatous polyps,1,2 and is being increasingly and widely used.3 Accordingly, biopsy and final confirmative diagnosis of endoscopic procedures, including polypectomy, mucosal resection, and submucosal dissection, are growing practices in the field of surgical pathology.4 Histopathologic diagnosis of colorectal lesions plays a crucial role in patient management;5,6 therefore, accurate pathologic examination of colorectal lesions is of paramount importance. The current standard guideline for the colonoscopic management of polyps is to retrieve all resected tissue for pathologic assessment.7
Recently, the American Society for Gastrointestinal Endoscopy introduced the "resect-and-discard" strategy for diminutive colorectal polyps.8 The 2014 guideline of the European Society of Gastrointestinal Endoscopy suggests that virtual and conventional chromoendoscopy can be used, under strictly controlled conditions, for the real-time optical diagnosis of diminutive colorectal polyps as a replacement to histopathologic diagnosis.9 These documents were developed from evidence-based methods and are expected to offer substantial cost savings; however, they have a number of limitations.10 These guidelines should incorporate a multisociety-based consensus, most important the pathologist's perspective, concerning diminutive and small colorectal polyps.
CLASSIFICATION OF COLORECTAL POLYPS: HISTOLOGIC TYPE AND MEASUREMENT
Recent progress in advanced endoscopic imaging and electronic chromoendoscopy has allowed the real-time endoscopic estimation of the histology of polyps, and its main application is in the differentiation of adenomas from hyperplastic polyps (HPs).10 Pathologically, colorectal polyps may arise from mucosal glands, the lamina propria, or connective tissue, and can encompass a wide range of histogenetic origins. They can be neoplastic, hamartomatous, inflammatory, or of various reactive conditions (Table 1).11,12 Most of these are adenomas and HPs; however, other polypoid lesions of the mucosa and submucosa are readily detected at the time of colonoscopy. From the practical point of view, the endoscopic diagnosis of all of these polyps can be either adenoma or nonadenoma, and this can be enough to make a diagnostic decision; however, with this policy, we cannot reliably assess the infrequent but sometimes significant polyps, and therefore this may distort the occurrence rate of a variety of pathologic lesions described in the literature.
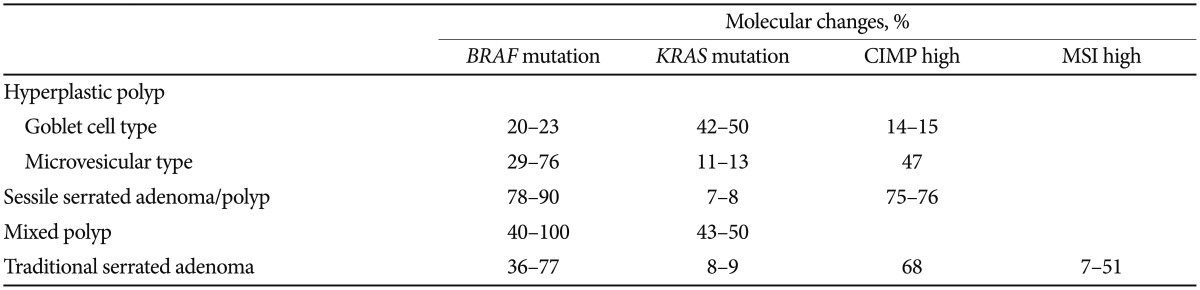
The classification and diagnosis of colorectal epithelial polyps became more challenging with the introduction of a third category, serrated polyps (SPs). SPs have emerged as precursor lesions in CpG island methylation phenotype (CIMP) colorectal carcinogenesis, known as a serrated neoplasia pathway, and account for 20% of all colorectal carcinomas.13,14 SPs include lesions with heterogeneous morphological and molecular features. This heterogeneous group comprises HPs, sessile serrated adenomas (SSAs)/polyps, mixed polyps, and traditional serrated adenomas. HPs were initially considered nonneoplastic lesions; however, subsequent identification of clonal genetic aberrations including BRAF mutation, KRAS mutation, and CIMP indicated that they are, in fact, neoplastic lesions (Table 2).11 SPs have a "saw-tooth" appearance on histology, as a result of crypt epithelial cell accumulation and luminal budding, secondary to inhibition of apoptosis. The classification of SPs is complicated by their morphologic subtypes and overlapping features, such as the absence or presence of varying degrees of dysplasia.15 The histologic differentiation of SSAs from HPs is predominantly architecture dependent, and a morphologic continuum exists between the two categories. The clinical significance and histologic criteria of these polyps, as with the search for reliable diagnostic biomarkers, are currently under investigation.16,17,18 Therefore, it is more important to create standardized diagnostic criteria and to understand the behavior of SPs than discard most of the small-sized SPs without verification. SPs are one example highlighting the importance of clinicopathologic correlation and communication between clinicians and pathologists.
The size of the colorectal polyp (especially the adenoma) is important in terms of its relation to the likelihood of malignant transformation, and to the risk of synchronous and metachronous adenomas and carcinomas.11 It is one of the major factors determining the risk groups of adenomas according to the 10-mm criteria.5,6 Small (<10 mm) polyps were further divided into diminutive and small polyps according to 5-mm dimensions.11 The resect-and-discard strategy is based on data showing a very low prevalence (<2%) of advanced histology in diminutive polyps.19 To adopt this size-based management strategy, accurate polyp size measurement is of paramount importance. Inaccuracy is almost never allowed in this act alone way of no return. However, endoscopic measurement of polyp size has been found to be inconsistent, and a substantial number of endoscopists overestimate or underestimate polyp size when the estimation is done visually or depending on the modality used for the measurement.20 Use of the postfixation measurement provided by pathologists would be a preferable alternative; however, tissue fixation may cause shrinkage or enlargement of the polyps.21 Another problem is that the malignant risk of a lesion in relation to its size is part of a continuum.21 Does something specifically occur at the 5-mm threshold? Is it reasonable to manage a 5-mm polyp and a 5.1-mm polyp differently without considering the measurement error? There has been, and will continue to be, a great deal of controversy in determining the standardized method of polyp size measurement, and this could be one of the problems that complicate the development of an official and legal standard.
PATHOLOGIC DIAGNOSIS AND INFORMATION: MEDICAL, PUBLIC, AND ACADEMIC ASPECTS
Surgical pathology is used in many branches of medicine because it provides a critical and definitive diagnosis. In the past, the pathologic diagnosis of cancer is primarily done on the basis of surgical resection in the case of advanced cancers. Recent medical advances have broadened the diagnostic technologies and management options for cancer, the most effective of which are prevention, early detection, and complete cure. Nowadays, endoscopic resection of precancerous lesions and early cancer has increased explosively; the same is true for the increase in the pathologic diagnosis for these lesions.4 This brought about the need for a more elaborate tissue diagnosis and an additional consensus for the diagnostic terminology and histopathologic grading of precancerous lesions in almost every human organs, including colorectal adenoma.22 Pathologists should be clearly aware of these facts and constantly strive to develop more applicable consensus criteria, as well as increase the interobserver agreement through consensus meetings, multicenter studies, and communicating with clinicians constantly, both informally and through interdepartmental conferences.4,23
In terms of the public health system, pathology reports are essential as they provide supporting confirmatory information about the performance of the medical procedure done on the patient, assign disease codes for national and private health insurance registration, and provide data for disease statistics, especially cancer.24,25 Pathologic examination, diagnosis, and storage of all resected human tissue can provide legal authentication of patient management.
Even a small human tissue such as a diminutive colorectal polyp harbors a vast amount of molecular information that may be crucial to medicine in the future although it seems trivial at the present time. Another important aspect of pathologic examination is the storage of human tissue for ongoing translational research. Pathologists can integrate the information from both the traditional morphologic examination and the newer techniques that are increasingly applicable to routinely processed tissue specimens.26 The discard policy of diminutive colorectal polyps may interfere with next-generation research that might be more beneficial in the field of medicine.
RESECT-AND-DISCARD STRATEGY: COST-EFFECTIVENESS
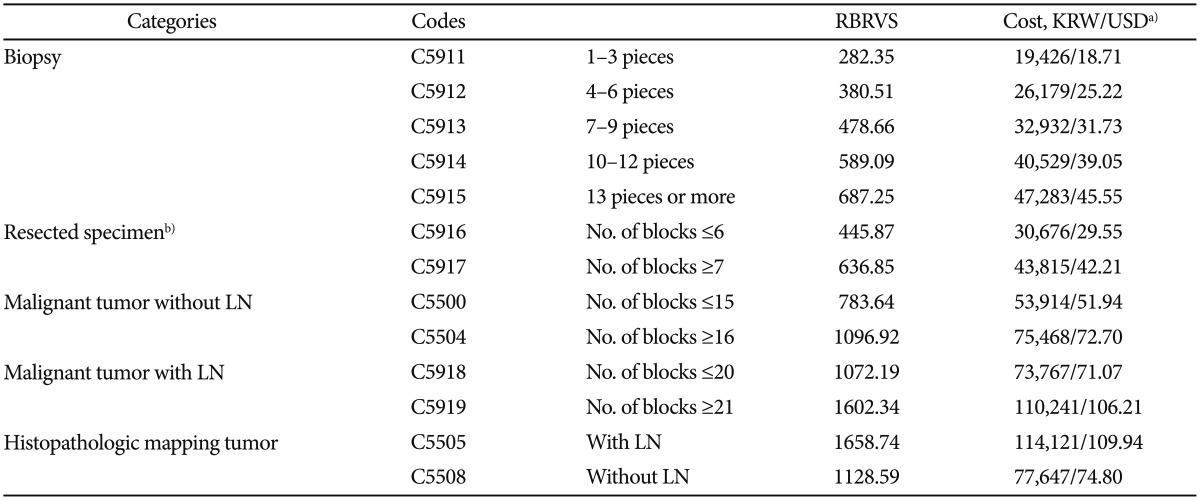
The major potential advantage of the resect-and-discard strategy for diminutive polyps is the reduction in the costs of histopathologic examination.8,27 This may be the reality in the United States; however, such benefit cannot be estimated in other countries with a different medical reimbursement system. For example, Korea has a generally undervalued reimbursement system for routine histopathology services. The classification of pathology services by the national health insurance system in Korea consists of 13 categories, and polypectomy specimens are coded as C5916 or C5917 on the basis of the number of resection or paraffin blocks (Table 3).28 Following the definition, resected polyps (from a single organ) that are six or fewer in number are considered a single unit of code C5916 and seven or more polyps (unlimitedly) are considered a single unit of code C5917, without any overlap. It is considerably different from the anatomic pathology coding by the American Medical Association, which defines individual specimen as a unit of code and then the actual fee is determined by multiplying the number of polyps and the corresponding price of the code.29 In a simulation model conducted in the United States, the resect-and-discard policy resulted in a substantial economic benefit according to the feasibility rate of in vivo differentiation of diminutive polyps and the cost of the pathologic examination.27 In another model, the overall net cost saving per patient was estimated to be US $174.30 Although there has been no cost-effectiveness analysis of the resect-and-discard strategy in Korea, the net cost saving per patient can be roughly estimated to be US $30 to 35. It is less likely that forgoing the pathologic examination of diminutive polyps has much economic benefit in Korea. Instead, the resect-and-discard strategy may bring about increasing medical cost due to the incorrect determination of the surveillance intervals.
CONCLUSIONS
Like many other paradigms of human activity, medical technologies and strategies are continuously and rapidly evolving. Recently, advanced colonoscopic imaging has been used to assess colorectal polyp histology, with a high prediction rate, particularly when done by an expert endoscopist. However, this new technique cannot provide information and function beyond histopathologic examination. In fact, the highly confident endoscopic estimation of polyp type is a novel valuable tool that can provide a symbiosis between gastroenterologists and pathologists to allow them to make a more evident diagnosis and management of patients with colorectal polyp. In the pathologist's point of view, the power of microscopic analysis and the amount of information that can be obtained even from a diminutive colorectal polyp represent a real acquisition, and there is no available technique that provides so much information in terms of data quality, quantity, and cost.
Notes
The author has no financial conflicts of interest.