Two Cases of Stress Cardiomyopathy during Esophagogastroduodenoscopy
Article information
Abstract
Esophagogastroduodenoscopy (EGD) is considered a relatively safe procedure. However, the procedure and the materials used in EGD with conscious sedation can cause stress to the patient. Adverse events during EGD have been reported, represented by cardiopulmonary complications. To date, five cases have reported worldwide to be associated with gastrointestinal endoscopy. Stress cardiomyopathy (SCMP) is a reversible cardiomyopathy that typically occurs in postmenopausal women due to stress and may resolve within a few weeks. SCMP resembles acute myocardial infarction but differs in terms of treatment and prognosis. Here, we describe two cases of SCMP with shock during EGD with conscious sedation.
INTRODUCTION
In general, esophagogastroduodenoscopy (EGD) is considered a relatively safe procedure [1]. However, during EGD, some adverse events such as heart failure and acute myocardial infarction (MI) may occur [1]. As far as we know, only five cases of stress cardiomyopathy (SCMP) have been reported in association with gastrointestinal endoscopy [2]. SCMP is a reversible cardiomyopathy that typically occurs in postmenopausal elderly women due to stress, and may resolve within a few weeks [3]. Here, we describe two cases of SCMP with shock during EGD with conscious sedation.
CASE REPORTS
Case 1
A 44-year-old woman with no relevant medical history underwent EGD for screening in late July 2014. She had previously undergone two EGDs without any complication. Her vital signs before the EGD were normal (blood pressure [BP] 110/70 mm Hg, pulse rate [PR] 84 beats per minute). Her electrocardiography (ECG) patterns and saturation of peripheral oxygen (SpO2) were normal. No signs of pain or discomfort were observed before the insertion of the EGD scope. For the EGD, lidocaine hydrochloride (20 mg spray) was administered to the throat, and 5 mg midazolam administered intravenously (IV) was used as a sedative. After the EGD scope was inserted through the pyriform sinus into the esophagus, however, she suddenly complained of chest discomfort and dyspnea. Her BP and PR increased to 180/160 mm Hg and 164 beats per minute, respectively. To awaken her from the sedation state, 0.25 mg flumazenil was immediately administered IV to reverse the effect of the sedative, and the EGD was stopped. She was moved to the emergency room (ER) for the evaluation of chest discomfort and dyspnea. Her 12-lead ECG showed ST elevation in the I and aVL leads, and II, III, aVF, and V4–6 ST depression in the ER (Fig. 1A). After 20 minutes, her BP was 62/24 mm Hg, PR was 67 beats per minute, SpO2 was 99% under nasal O2 (2 L), and respiration rate was 20 breaths per minute. As her BP was below 90/60 mm Hg, 2 L normal saline was administered IV and a vasoactive agent, norepinephrine, was started. The initial level of cardiac enzyme was normal. After several hours, her cardiac enzyme level increased: creatine phosphokinase MB (CK-MB) 19.1 ng/mL (reference range, 0.6 to 6.3) and troponin I (Tn-I) 5.39 ng/mL (reference range, 0 to 0.5). The transthoracic echocardiogram (TTE) showed only a hypokinetic mid-left ventricle with an ejection fraction (EF) of 58% (Fig. 2).
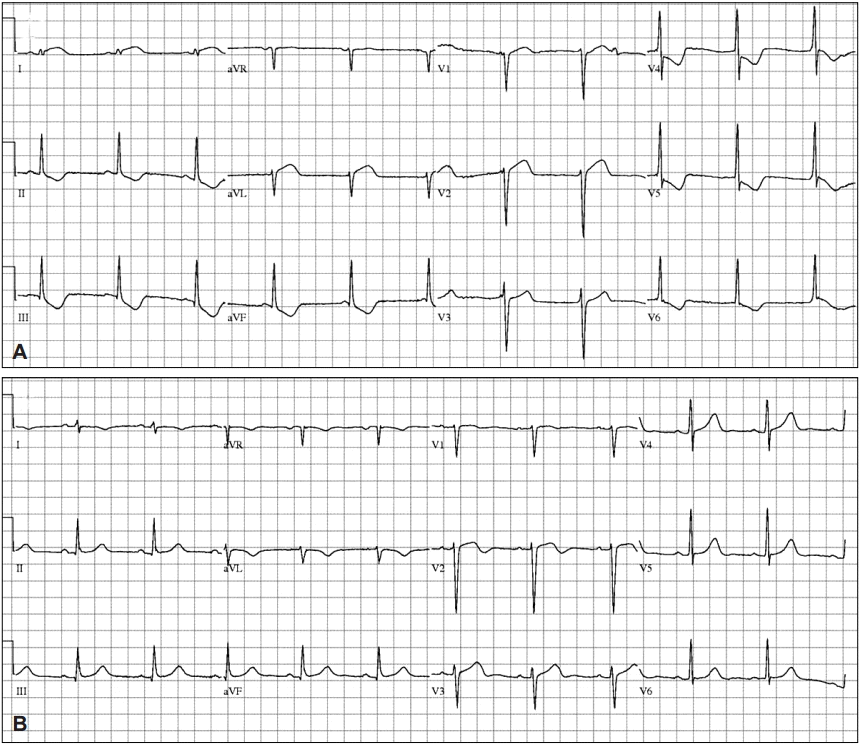
Electrocardiography (ECG). (A) ECG shows ST elevation in the I and aVL leads, and ST depression in the II, III, aVF, V4 to 6, (B) ST elevation and ST depression improvement.
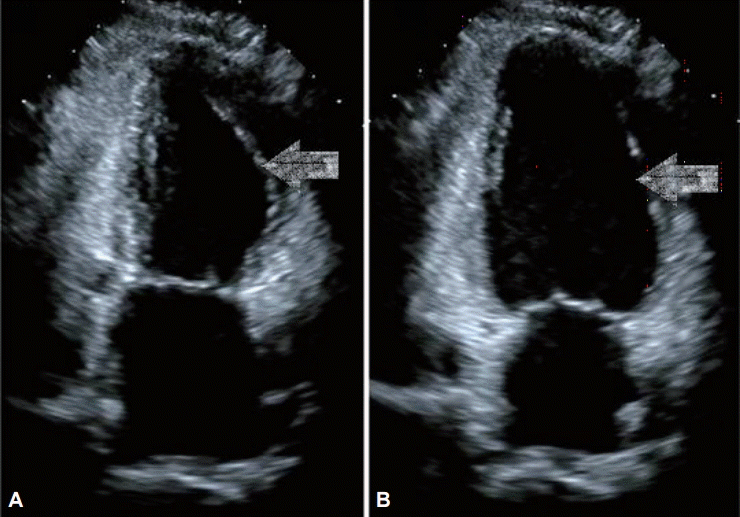
Transthoracic echocardiography (TTE). TTE shows hypokinetic mid left ventricle in (arrows) (A) systole and (B) diastole.
The first impression was ST elevation MI. Cardiac catheterization was carried out, but the result was normal. Also, the left ventriculogram showed a hypokinetic mid-left ventricle (Fig. 3)
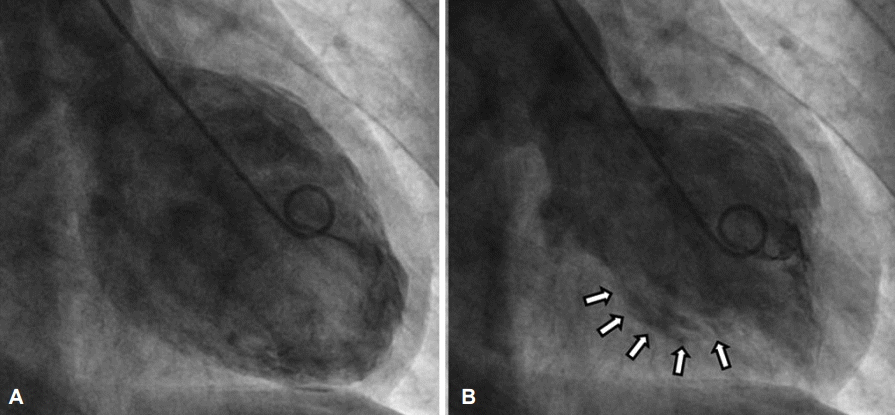
Left ventriculogram. Left ventriculogram shows hypokinetic mid left ventricle in (A) diastole and (B) systole (arrows).
Finally, SCMP was diagnosed and she underwent conservative treatment. Two days after EGD, her cardiac enzyme levels returned to normal. Three days after EGD, her SpO2 was >95% with room air and her ECG returned to normal (Fig. 1B). Her chest discomfort and dyspnea were resolved. The next day, the low BP recovered without a vasoactive agent. She fully recovered without other special events and then was discharged.
Case 2
A 45-year-old-woman underwent EGD for the follow-up of gastric gastrointestinal stromal tumor (GIST) in late April 2014. In the previous year, she underwent EGD with conscious sedation and endoscopic ultrasonography without any complication. She had no relevant medical history except for gastric GIST. Her vital signs, ECG pattern, and SpO2 before the EGD were all normal. For the EGD, lidocaine hydrochloride (20 mg spray) was administered to the throat, and 5 mg midazolam IV was used as a sedative. One minute after the insertion of the EGD scope, she suddenly complained of chest discomfort. Her BP and SpO2 decreased to 75/50 mm Hg and 80%, respectively, and heart rate (HR) increased to 120/min. To reverse the effect of the sedative, 0.25 mg flumazenil was administered IV and the EGD was stopped. After the withdrawal of the EGD scope, she spat out fresh blood with cough. After supplying 10 L O2 through a reservoir mask, her SpO2 was 90% to 91%. Normal saline (500 mL) was administered IV, and her systolic BP was 90 mm Hg. She was moved to the ER for the evaluation of chest discomfort, dyspnea, hemoptysis, and shock. Her ECG was normal; however, the cardiac enzyme levels were elevated: CK-MB 11.3 ng/mL and Tn-I 3.79 ng/mL. Chest computed tomography (CT) showed a multiple ground-glass opacity pattern and interlobular thickening, and the impression was pulmonary edema. TTE showed only a hypokinetic mid-left ventricle, with an ejection flow of 45%. To rule out MI, coronary artery CT was carried out and the result was normal. Moreover, the evaluation for pheochromocytoma—measurement of metanephrine and catecholamine in plasma and through a 24-hour urine collection—was performed, and the results were in the normal range.
Finally, SCMP was diagnosed and she underwent conservative treatment. One day after EGD, her SpO2 was >95% with room air. Two days after EGD, her chest discomfort, dyspnea, and hemoptysis were resolved. Three days after EGD, the elevated cardiac enzymes returned to normal levels. Six days after EGD, the follow-up TTE showed no abnormal contraction with an EF of 66%. Seven days after EGD, she had fully recovered without adverse events and then discharged.
DISCUSSION
EGD is relatively safe procedure that has been widely performed [1]. However, patients may usually experience anxiety and a feeling of discomfort when undergoing the procedure. Conscious sedation is associated with patient tolerance of and satisfaction with EGD; thus, EGD is mainly performed under conscious sedation. During the practice of EGD, undesirable complications may occur [1]. In a prospective study of 14,149 EGD cases, prompt cardiopulmonary incidents occurred in 2 per 1,000 cases. The 30-day mortality rate was 1 per 2,000 cases [1]. In another retrospective study of 21,011 EGD cases, cardiopulmonary complication occurred in 5.4 per 1,000 cases [1].
Acute MI is similar in clinical features to SCMP, as observed in our cases. SCMP is a cardiomyopathy that is a reversible left ventricular contraction failure caused by acute stress, without coronary artery disease and mainly occurring in postmenopausal elderly women [3-6]. In most cases, SCMP could be improved in a few weeks but could rarely cause pulmonary edema, cardiogenic shock, arrhythmias, heart failure, or death [5,7]. Therefore, SCMP should be distinguished from MI owing to the differences in treatment and prognosis. The current diagnostic criteria for SCMP have recently been published (Table 1) [7]. There are a few hypotheses about the etiology of SCMP: emotional stress, certain pharmacologic agents, exogenous catecholamine, and unstable condition of autonomic nervous system [8]. Stress factors such as the EGD procedure and sedatives may induce an unstable autonomic nervous condition. Insertion of the EGD scope from the pharynx into the esophagus, and from the lower esophagus to the cardia of the stomach, may induce tachycardia as a result of sympathetic nervous system hyperactivity [9]. The unstable condition of the autonomic nervous system due to EGD might have played a critical role in the development of SCMP in our cases. In one of our cases, during EGD insertion from the pharynx to the esophagus, the patient’s HR increased to 164/mm and she experienced chest pain and dyspnea. Globally, only five cases of SCMP associated with gastrointestinal endoscopy have been reported. Previous reports indicated one case of EGD in a postmenopausal woman with sedation, three cases of colonoscopy, and one case of simultaneous EGD and colonoscopy in a postmenopausal woman [2,9,10].
Compared with previous cases, our patients were premenopausal women without drug allergy, medication history, and cardiopulmonary disease, who had undergone several EGD procedures under conscious sedation. The SCMP in our patients is a rare cardiomyopathy but could occur during medical procedures such as EGD. Therefore, clinicians should know that SCMP could occur during EGD.
For the prevention and management of SCMP, assessment of cardiopulmonary status during the EGD, as well as a review of current drug use and drug allergies and physical examination of the patient should be performed [1]. Supplemental oxygen supply was reported to reduce the magnitude of oxygen desaturation [11]. Continuous ECG monitoring is appropriate in high-risk patients, in those with significant cardiopulmonary disease, in elderly patients, and when the procedure is expected to be long [1]. Capnography can be used to monitor the patient’s respiratory activity [12].
Furthermore, proper selection of sedatives and dosage is needed. The commonly used sedatives are benzodiazepines. Most endoscopists favor midazolam for its fast onset, short duration of action, and high amnestic properties. The usual total dose is 2.5 to 5 mg. Lower doses may be used in the elderly or in patients taking other central nervous system depressants [1].
In conclusion, physicians should be aware that SCMP could occur during EGD. For the risk minimization and management of SCMP during EGD with conscious sedation, assessment of the patient’s cardiopulmonary status, review of current drugs, and physical examination before EGD should be carried out, as well as continuous ECG monitoring and pulse oximetry with or without capnography in high-risk patients. Moreover, an appropriate titration dose of the sedative should be used depending on the patient’s risk.
Notes
Conflicts of Interest:The authors have no financial conflicts of interest.
