Mechanisms of Biliary Plastic Stent Occlusion and Efforts at Prevention
Article information
Abstract
Biliary stenting via endoscopic retrograde cholangiopancreatography has greatly improved the quality of patient care over the last 30 years. Plastic stent occlusion limits the life span of such stents. Attempts to improve plastic stent patency duration have mostly failed. Metal stents (self-expandable metal stents [SEMSs]) have therefore replaced plastic stents, especially for malignant biliary strictures. SEMS are at least 10 times more expensive than plastic stents. In this focused review, we will discuss basic mechanisms of plastic stent occlusion, along with a systematic summary of previous efforts and related studies to improve stent patency and potential new techniques to overcome existing limitations.
INTRODUCTION
One of the most remarkable achievements in the history of therapeutic pancreatobiliary endoscopy is the introduction of biliary stent placement to prevent obstruction during endoscopic retrograde cholangiopancreatography (ERCP). This has become established as a treatment to resolve obstructive jaundice by a non-surgical approach. As this technique allows the relief of preoperative acute biliary obstruction, it is useful for stabilizing patients before surgery and enhancing their quality of life by enabling internal drainage in those who are not eligible for surgery [1,2].
The main disadvantage of plastic stents is their limited diameter. This is set by the working channel in the duodenoscope, which prevents use of plastic stents with an outer diameter of more than 12 Fr [3]. This is not a major problem for patients who only require temporary stent placement, but can be a major issue for patients who require long-term placement of a stent. Patients with early stent occlusion before scheduled replacement can experience sudden obstruction of bile flow and complain of symptoms of recurrent biliary obstruction. This can also lead to acute cholangitis and sepsis, which can be life-threatening. Frequent stent replacement also increases medical costs and reduces the quality of life of patients with general illness.
In this focused review, we will discuss mechanisms of plastic stent occlusion, along with a summary of previous efforts and related studies to improve stent patency, and potential new techniques to overcome existing limitations.
MECHANISMS OF BILIARY STENT OCCLUSION
Biliary plastic stents were first introduced in 1979, and the mechanisms of biliary stent occlusion were thoroughly investigated 20 to 30 years ago [4]. Early stent occlusion within 30 days, although very rare, mostly occurred due to positioning error, blood clots, debris, or mucus from a mucin-producing tumor [5]. This review will focus on late stent occlusion (≥30 days post-placement).
The widely-accepted theory is that bacterial biofilm and biliary sludge both play major roles, along with the extent of bacterial infection and duodenobiliary reflux of dietary fiber [3,6-8]. Biliary sludge is different from cholesterol-rich sludge, which is generally related to gallstone formation. The biliary sludge that causes stent occlusion is mainly composed of crystals of calcium bilirubinate and calcium palmitate formed by bacterial enzymes [6-12]. It is also known that several types of proteins (such as fibronectin, vitronectin, laminin, fibrin, and collagen), which are derived from bacteria of unknown specific origin but are not naturally present in bile, form a conditioning film, making the bacteria more adhesive, and also work with the bacteria to produce biliary sludge [13-17]. The formation of a biofilm that occurs as a result of attachment of these proteins onto the inner surface of a stent is known to play a major role in the initiation of sludge accumulation, but its exact role in the process of stent occlusion is still unknown.
THE ROLE OF BACTERIAL COLONIZATION IN STENT OCCLUSION
The most important factor in the process of stent occlusion is bacterial colonization. Micro-organisms isolated from occluded biliary stents include anaerobic bacterial species, fungi, and aerobic bacterial species. The gram-positive Enterococcus species, and gram-negative Escherichia coli and Klebsiella species are most commonly encountered among aerobic bacteria, with Clostridium species among anaerobic bacteria. The species identified vary greatly among reported series, possibly affected by sites where stents were implanted, time to test after removal, interval antibiotic use, and inadequate techniques to identify anaerobic species. In any case, the synergistic effect between bacterial adherence and biofilm formation caused by these bacteria is known to trigger stent obstruction. Studies have suggested that stent patency may vary depending on the types of bacteria or the types of byproducts and proteins released by different bacteria. Other studies on scanning electron microscopy (SEM) have suggested that biofilm thickness may vary depending on their combined action [18].
SCANNING ELECTRON MICROSCOPY EXAMINATIONS
Biofilms generally do not become thick enough to cause complete stent occlusion. Removed biliary plastic stents evaluated by longitudinal sectioning show a less than 0.5-mm-thick inner layer of biofilm; the occlusion is mostly caused by debris, sludge, and food components (Fig. 1).
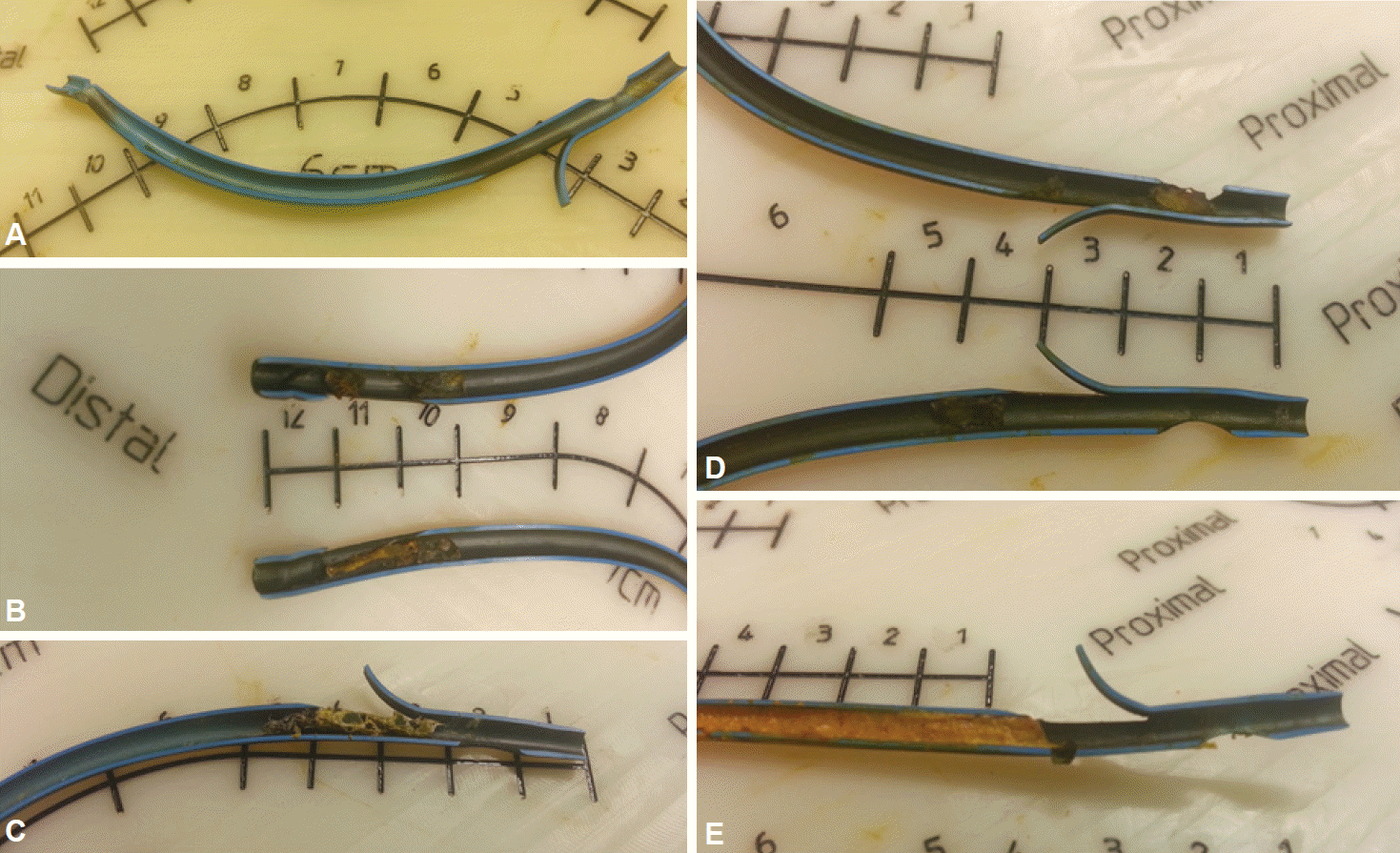
(A-E) Retrieved biliary plastic stents with the inner layer exposed by longitudinal cutting. The dark greenish biofilms do not contribute significantly to increased thickness of the occluded inner layer. Stent occlusion is mostly caused by debris, sludge, and food components.
A SEM examination of stents revealed interesting facts in terms of the change in biofilm depending on the time they were retrieved. Fig. 2 shows the inner surface of a stent retrieved approximately 4 weeks after implantation. The biofilm starts to appear on the inner surface of the stent (Fig. 2A); the biofilm itself becomes gradually thicker relatively evenly, and the surface becomes more solid (Fig. 2B-D). Fig. 3 shows the inside of a stent retrieved approximately 8 weeks after implantation. Sludge covers the biofilm, rapidly narrowing the inner diameter of the stent (Fig. 3A-C). It also appears that debris, presumably derived from causes other than sludge, is attached to the inner layer of the stent without the sludge (Fig. 3D).
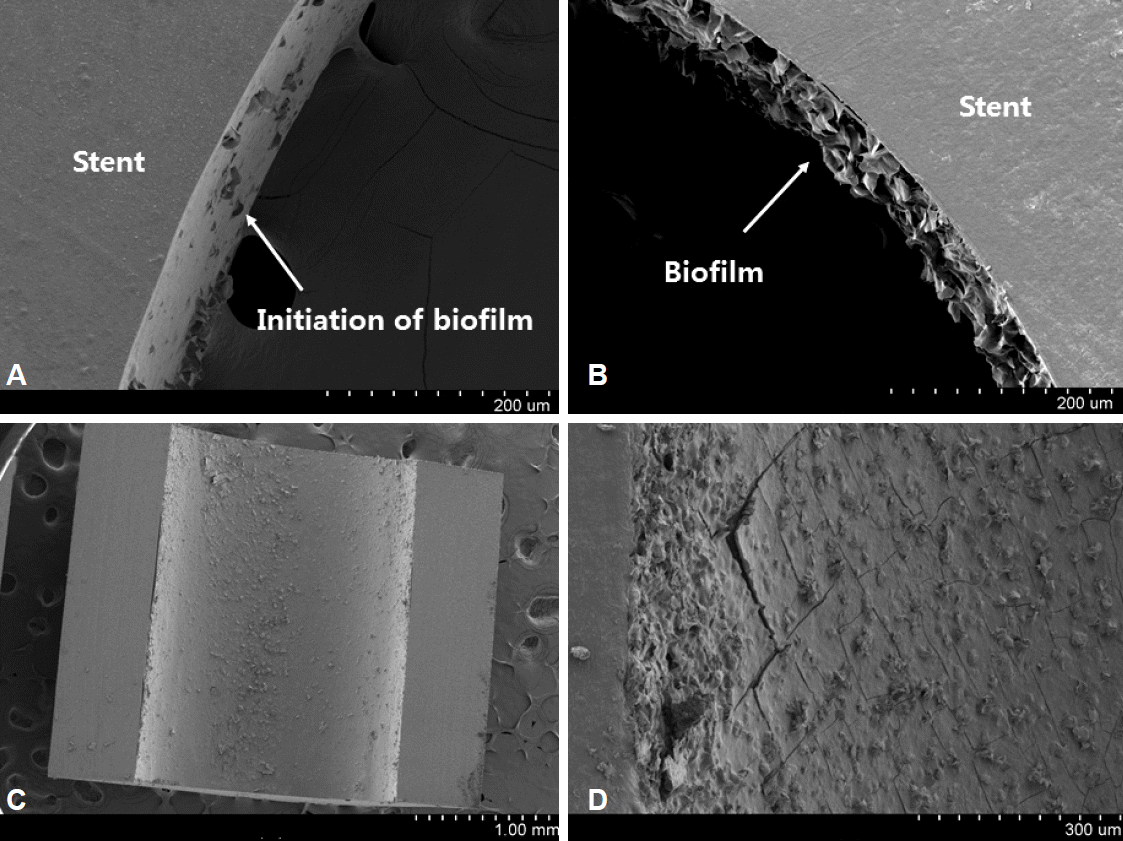
Scanning electron microscopy (SEM) examination of stent occlusion. SEM images of the inner surface of a stent retrieved at about 4 weeks. The biofilm starts to appear on the inner surface of the stent (A, ×250). The biofilm itself becomes gradually thicker relatively evenly (B, ×250; C, ×30). The surface becomes more solid and cracked in some areas (D, ×150).

Scanning electron microscopy (SEM) examination of stent occlusion. SEM images of the inner surface of a stent retrieved at about 8 weeks. Sludge covers the biofilm and narrows the inner diameter of the stent (A, ×30; B, ×150). The biofilm is exposed in some areas that are not covered by sludge (C, ×150). Debris, presumably derived from other causes even before the formation of the biofilm, is attached to the inner layer of the stent (D, ×100).
What this suggests is that the biofilm is associated with the initiation of stent occlusion but that overall thickening of the biofilm is not the cause of total occlusion. It may be possible that a biofilm on the inner surface of a stent can make the surface irregular, facilitating the accumulation of sludge or debris. Various biological factors mentioned above and a number of physical factors (such as stent shapes, side holes, duodenobiliary reflux of different food components, inner coating status, bile composition status due to underlying disease, and preexisting cholelithiasis) are also thought to work together to promote sludge attachment to the inner layer. To date, antibiotics to inhibit the formation of biofilm have not improved stent patency.
METHODS FOR PREVENTION OF PLASTIC STENT OCCLUSION
Stent diameter
Stents with a larger diameter have longer patency because it takes longer for the inner cavity to fill with foreign substances [19]. However, it is impossible to endlessly extend the diameter of the working channel of an endoscope due to its design, and thus the maximum outer diameter of a plastic stent is 11.5 Fr at most. Then 10-Fr plastic stents require a duodenoscope with an accessory channel of 3.7 mm, and 11.5-Fr plastic stents require a duodenoscope with an accessory channel of 4.2 mm [20]. However, prospective studies found no major difference in the outcome between 10- and 11.5-Fr stents, and the mean duration of stent patency was 3 to 6 months [3,21-23].
Studies evaluating thinner-walled stents with the same outer diameter, and the use of single versus multiple plastic stents are needed.
Stent composition and shape
One can assume that the composition or shape of plastic stents might affect stent patency; however, previous studies have suggested that patency was not significantly affected by composition (Teflon, polyurethane, or polyethylene) or shape [24-27].
Straight plastic stents are thought to have a longer patency than pigtail plastic stents because they are much less resistant to flow. However, compared to the inner diameter, which limits the time to stent occlusion, the effect of stent shape does not seem as significant. Pigtail stents show lesser migration than straight stents because anchoring is well-maintained [20], there is less likelihood of food clinging because they have multiple drain holes on the side, unlike the flap of the straight type, and there is less likelihood of decreasing bile flow velocity when a distal part touches the duodenal wall after partial migration (Fig. 4).

Factors causing plastic biliary stent malfunction. (A, B) Distal ends of the straight stents are touching the duodenal wall as a result of partial distal migration. This may lead to flow disturbance and sludge accumulation, causing occlusion. (C, D) The straight stents have not migrated, but dietary fibers are clinging to the side flaps, causing stent occlusion. (E, F) Distal ends of the pigtail stents are touching the duodenal wall or partially migrated, but the side holes still allow bile flow.
Scheduled stent exchange
Unexpected stent occlusion may lead to recurrent jaundice and cholangitis, or even sepsis without immediate treatment, which may be life-threatening. The best known preventive measure is replacing the stent before it becomes occluded [28].
The median patency of 10-Fr plastic stents is 4 to 5 months in general, and the risk of stent occlusion increases significantly after 3 months. Many centers recommend stent replacement every 3 months on a scheduled (not as needed) basis [29,30]. However, this depends on underlying pathology, because stent occlusion is rare when death is imminent in patients with a malignant disease [6]. Therefore, it seems wise to aggressively use this method in patients with benign diseases who require repeated stent replacement [31].
Antibiotics with or without choleretics
As bacterial colonization was found to be the most important cause of biliary plastic stent occlusion, in vitro and in vivo studies have been conducted with various antibiotics; however, none were found to prolong stent patency [18]. Attempts have been made to induce synergistic effects by combining antibiotics with choleretic agents such as ursodeoxycholic acid or terpene. However, the Cochrane review including a meta-analysis of 5 randomized trials reported no significant effect on stent patency or mortality rate [32].
Antibacterial coating or hydrophilic coating method
Similar to antibiotic administration, studies evaluating stents that incorporate antibiotics failed to show prolonged patency with in vitro or pilot studies [33-35]. These unfavorable results may be associated with technical issues such as inability to release antibiotics for a prolonged period of time, or because of bacterial resistance [6].
In vitro experimental studies have demonstrated that Teflon stents with a low coefficient of friction or stents with a hydrophilic coating can inhibit bacterial colonization and sludge formation [36,37]. Because bacterial adhesion to plastic stents is associated with surface hydrophobicity, studies have started to explore hydrophilic coatings [38-41]. Earlier studies reporting promising results had received much attention as a break-through; however, subsequent prospective large-scale studies failed to show prolonged patency [42,43]. The possible explanations are as follows: (1) the coated surface could have been damaged by guide wire manipulation during stent placement or by duodenobiliary reflux, and (2) the hydrophilic coating could have been degraded over time even before the initiation of stent occlusion.
In vitro studies were conducted to explore silver coating in the same context, but a clinical study has not been reported [44,45].
Stent design: stent without side holes
Many straight plastic stents have side holes created on purpose or added in the process of making the anti-migration flap. Coene and colleagues [46] found that sludge accumulation was more frequent around side holes, and devised and studied a new stent without side holes. A known mechanism of increased encrustation of sludge around side holes is the creation of microturbulence, affecting the friction coefficient of bile flow [46,47]. This observation stimulated other studies, but prolonged stent patency was not seen [24-26,48,49].
Stent design: antireflux valve
The hypothesis that duodenobiliary reflux may induce biofilm or sludge formation has led to the introduction of a plastic stent with an antireflux valve. This plastic antireflux stent showed 1.5-times longer stent patency than the existing standard type [50]. A follow-up study used a similar antireflux plastic stent [51]. Larger, prospective follow-up studies are needed.
An antireflux valve was also investigated with self-expandable metal stents (SEMS) with the same idea, but studies failed to show improved results [52-54]. This was possibly because (1) the valve was deformed by gastroduodenal secretion, or (2) the valve was compressed by the duodenal wall as a result of bowel motion or partial migration, causing valve malfunctioning and bile flow disturbance, as is also expected with antireflux plastic stents.
Stent position
An animal study reported improved patency by placing a stent above the papilla, preventing bacterial colonization by food reflux from the intestine [55]. This led to a prospective randomized study in humans, but the results showed increased stent migration rather than improved patency [56]. This was also investigated with SEMS with the same idea, but again stent migration increased without improving patency [57].
CONCLUSIONS
Endoscopic biliary stenting has been extensively investigated since the development of therapeutic endoscopy. For more than 30 years, however, no studies have produced any remarkable results to prolong stent patency, other than the implantation of larger-caliber stents or SEMS. As plastic biliary stents are easy to insert and remove, and financially less of a burden than SEMS, plastic stents with improved function to reduce occlusion are awaited. In the future, we also hope to see more studies on the following: (1) the development of self-expandable plastic stents or bioabsorbable plastic stents, ensuring larger calibers; (2) the development of new types of plastic stents with combined benefits of both straight and pigtail types, to eliminate the effect of food components on the flap without affecting flow velocity; (3) the development of new materials effective for preventing biofilm formation as a special coating agent on the inner surface; or (4) if it is not possible to inhibit biofilm effectively, methods of coating the inner surface with substances that can prevent surface irregularity caused by the biofilm itself, so that debris or sludge cannot be incorporated; (5) development of a larger-diameter channel ERCP scope; (6) testing of single versus multiple stents for routine malignant biliary obstruction (Table 1).
Notes
Conflicts of Interest: The authors have no financial conflicts of interest.
Acknowledgements
The following author received research support for this study from the Industrial Technology Innovation Program (Advanced Technology Center Program) funded by the Korean Ministry of Trade, Industry & Energy: Chang-Il Kwon.
