Recent Advanced Endoscopic Management of Endoscopic Retrograde Cholangiopancreatography Related Duodenal Perforations
Article information
Abstract
The management strategy for endoscopic retrograde cholangiopancreatography-related duodenal perforation can be determined based on the site and extent of injury, the patient’s condition, and time to diagnosis. Most cases of perivaterian or bile duct perforation can be managed with a biliary stent or nasobiliary drainage. Duodenal wall perforations had been treated with immediate surgical repair. However, with the development of endoscopic devices and techniques, endoscopic closure has been reported to be a safe and effective treatment that uses through-the-scope clips, ligation band, fibrin glue, endoclips and endoloops, an over-the-scope clipping device, suturing devices, covering luminal stents, and open-pore film drainage. Endoscopic therapy could be instituted in selected patients in whom perforation was identified early or during the procedure. Early diagnosis, proper conservative management, and effective endoscopic closure are required for favorable outcomes of non-surgical management. If endoscopic treatment fails, or in the cases of clinical deterioration, prompt surgical management should be considered.
INTRODUCTION
Endoscopic retrograde cholangiopancreatography (ERCP)-related duodenal perforation develops in 0.4% to 0.6% of patients, with serious adverse effects on morbidity and mortality [1,2]. With the improvement of the endoscopic and surgical treatments of duodenal perforation, the outcomes have improved [3,4]. The clinical outcomes depend on the timing of the diagnosis and the efficacy of the treatment. Treatment strategy depends on the type of perforation such as perivaterian or ductal defects managed with nonsurgical management, or duodenal wall perforation treated with surgical repair [4,5]. However, with the development of endoscopic devices and techniques, the success rate of endoscopic closure in duodenal wall perforation was reported to be as high as 88% in a recent meta-analysis [6]. Endoscopic management is recommended for iatrogenic gastrointestinal perforation in selected patients with low level of evidence [2]. This review aimed to introduce advanced endoscopic management for ERCP-related duodenal perforation and to define its optimal management strategies.
CLASSIFICATION AND RISK FACTORS
Perforations related with ERCP were classified depending on their locations as follows: duodenal wall by the scope itself (type I), perivaterian by endoscopic sphincterotomy (EST) or endoscopic transpapillary balloon dilation (EPBD; type II), bile duct by a guidewire or basket (type III), and retroperitoneal air alone by compressed air leakage (type IV) (Fig. 1) [7]. This classification is widely used because it is appropriately related with clinical severity, perforation mechanism, anatomical location, and the need of surgical management [7]. The main causes of perforation are EST in 41%, the endoscope itself in 26%, the guidewire in 15%, dilation of a stenotic segment in 3%, ERCP devices in 4%, and stent insertion and migration in 2% [4].
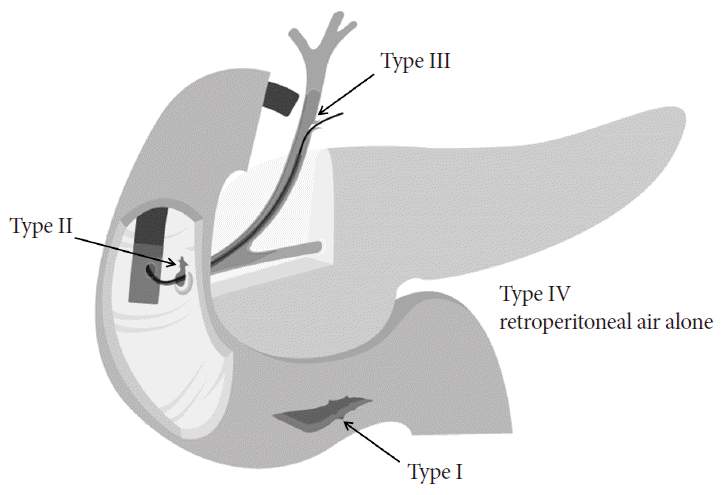
Classification of endoscopic retrograde cholangiopancreatography-related duodenal perforation. Type I, lateral or medial wall duodenal perforation; type II, periampullary perforation; type III, bile duct injuries; type IV, retroperitoneal air alone.
The common risk factors related to duodenal perforation are old age and long procedure time [5]. Specific risk factors are altered surgical anatomy, including Billroth II gastrectomy and Roux-en-Y bypass surgery, in duodenal wall perforations, and biliary stricture dilation, sphincter of Oddi dysfunction, and endoscopic papillectomy in perivaterian or ductal perforations [5,8,9]. Whether diverticulum in perivaterian or needle-type papillotome is a risk factor remains controversial [2,10]. EST and EPBD showed the same risk of perforation in a meta-analysis [11].
RECOGNITION OF DUODENAL PERFORATION
Prompt recognition of duodenal perforation during a procedure is mandatory for favorable prognosis. However, many studies reported that 30% to 44.6% of perforations were found after the procedure [4]. Duodenal wall perforations can be detected by direct visualization on endoscopy, and perivaterian or ductal perforations are usually recognized on fluoroscopy [12]. The use of carbon dioxide for inflation may minimize extraluminal air; therefore, free air or retroperitoneal air is difficult to detect during the procedure [4]. Perforations should be suspected in the presence of abdominal pain, fever, and leukocytosis, even if a perforation was not detected at routine chest and abdominal radiography during or after the procedure. Clinical signs sometimes develop late, with peritoneal irritation signs and leukocytosis appearing at 3 to 4 and 12 hours after the procedure, respetively [4]. In addition, perforation is misdiagnosed as post-ERCP pancreatitis or abdominal distension with inflated air. Forty-three percent of patients with perforation revealed a concurrent post-ERCP pancreatitis [13]. Therefore, when perforation is suspected, a contrast-enhanced abdominal computed tomography (CT) scan should be performed, which is the most useful tool to detect perforation and provides useful information about pancreatitis and fluid collection (Fig. 2) [14].
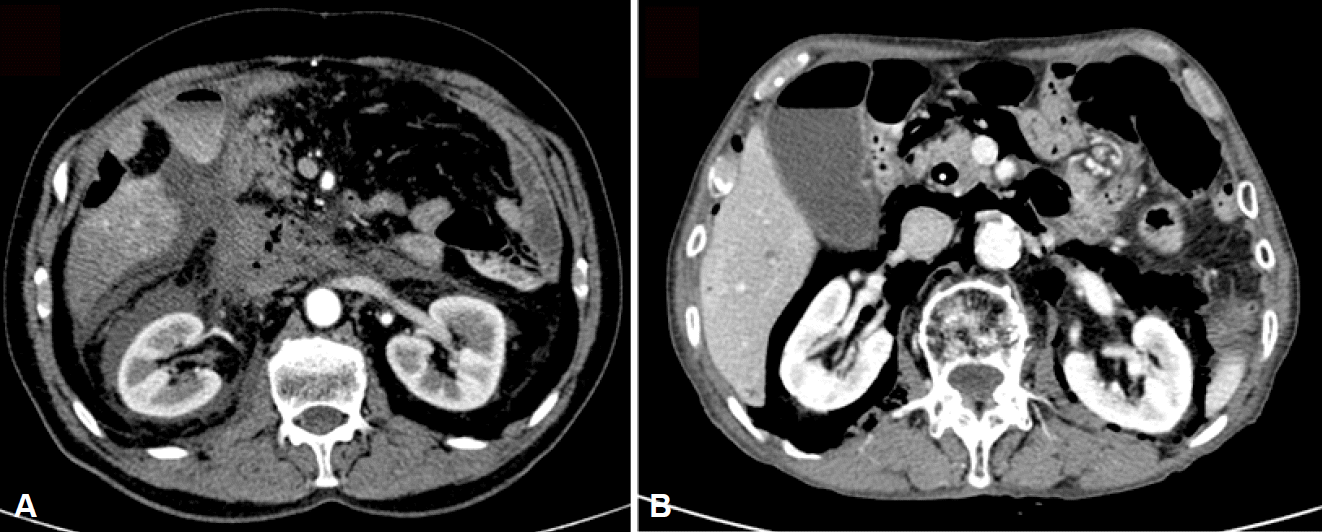
Computed tomography (CT) findings indicating a duodenal perforation. (A) An abdominal CT image showing massive fluid and air collection at the retroperitoneal space (type II perforation). (B) An abdominal CT image showing massive air leakage without fluid accumulation in the retroperitoneal space (type IV perforation).
When a duodenal perforation is detected endoscopically, a comprehensive examination and clear report should be written about its size and location with a picture, the endoscopic treatment, whether carbon dioxide or air was used for insufflation, and the standard report information. Because air inflation itself during endoscopy may not worsen the clinical outcome, comprehensive endoscopic examination is essential for proper treatment [2].
ENDOSCOPIC MANAGEMENT
Duodenal wall perforation
Duodenal wall perforations require early closure because large amounts of intestinal contents leak into the peritoneum. Endoscopic techniques are determined based on the type and size of perforation and the endoscopist expertise available at the center [15,16]. The most important step before the closing procedure is thorough aspiration of the duodenal contents through the perforated hole.
A duodenal perforation of <10 mm in diameter can be closed easily with endoclips [17] by using a single-channel endoscope or lateral scope without changing the scope. Throughthe-scope (TTS) clips are widely used for closing luminal perforations [18], achieving technical success in 90% of gut perforations [6]. However, a large perforation (>20 mm) located at angulation is difficult to close by using TTS clips alone. When luminal closure with TTS clips fail, alternative methods using fibrin glue [19], endoclips and endoloops [20], purse-string suture [21], or band ligation [22] are applied [15,16]. Fibrin glue spray through a standard ERCP catheter between the clips could obliterate the perforation site and adhere multiple clips to each other [19]. Multiple endoclips and single or double endoloops are also effective for endoscopic closure of large duodenal perforations [20,21]. Another alternative method for duodenal closure is endoscopic band ligation (EBL) [22]. EBL could be easily performed for perforations 1 to 2 cm in diameter regardless of the site or angle when a perforation is detected during the procedure [23]. Gastroscopy attached with single- or multiple-band ligators in a transparent cap can approach the edge of the perforation. The perforated wall, along with peritoneal fat, is sucked into the ligator cup. One or two bands are subsequently released, resulting in closure of the perforation. Sucking together the duodenal wall and nearby omentum is helpful for complete closure of the perforation by making an omental patch endoscopically [24]. When a residual defect is suspected, additional endoclips can be applied at the edges of the perforation (Fig. 3) [25]. Duodenal wall perforation can be easily ligated because of a thin wall structure [26]. Endoscopic devices such as endoclip, endoloop, ligation band, and fibrin glue are usually available in an endoscopy unit and can be applied as soon as perforation occurs.

Endoscopic closure of a duodenal wall perforation by using ligation band and endoclips. (A) A large duodenal perforation on the lateral wall. (B) Endoscopic closure with band ligation and endoclips. (C) Endoscopic band ligation.
Specialized devices such as over-the-scope clipping device (OTSC) [27], endoscopic suturing (OverStitch; Apollo Endosurgery, Austin, TX, USA) [28], and open-pore film drainage [29] are reported as useful and effective for large luminal perforation.
OTSC revealed an 88% technical success rate [6]. OTSCs can close defects by up to 20 mm. Detailed OTSC techniques are as follows: OTSC in an endoscopic cap is placed over the mucosal defect. A twin grasper captures opposite sides of an open defect and apposes the tissue by using left- and right-sided graspers. The tissue in the graspers is pulled into the cap device, and then a clip is applied (Fig. 4). Compared with the TTS clip, the OTSC has higher compression force and enables capture of a larger tissue volume [30]. However, OTSC is not widely available and requires training before application in human patients [31].

Endoscopic closure of an anastomotic perforation by using an over-the-scope clipping device (OTSC). (A) OTSCs. (B) Jejunum perforation caused by Billroth II anastomotic leak. (C) Using a twin grasping forceps, the edges of the perforation wall are pulled back into the lumen of the cap attached to the endoscope. (D) The perforation site is successfully sealed with an OTSC.
Endoscopic suturing devices such as Eagle Claw (Olympus, Tokyo, Japan) and Purse String Suturing Device (LSI Solutions, Victor, NY, USA), which are attached to the end of a therapeutic double channel endoscope, allow full-thickness tissue apposition [32]. The device can be used multiple times without the need to remove the scope from the patient. However, endoscopic suturing devices are not widely available and are difficult to operate within the narrow duodenal lumen. They have been investigated in animals but have not been reported for clinical closure of duodenal perforations [33].
Another new technique is vacuum therapy with openpore film drainage, which is used for duodenal perforations that occur during ERCP [29]. The procedure consists of placing a drainage tube, which is wrapped with an open pore film, in the duodenal wall defect and application of vacuum for evacuation and collapse of the stomach and duodenum. Gastric, biliary, and pancreatic liquids are drained in an intraluminal direction continuously, and the transmural defect is closed simultaneously; thereby, preventing further extraluminal contamination (Fig. 5).
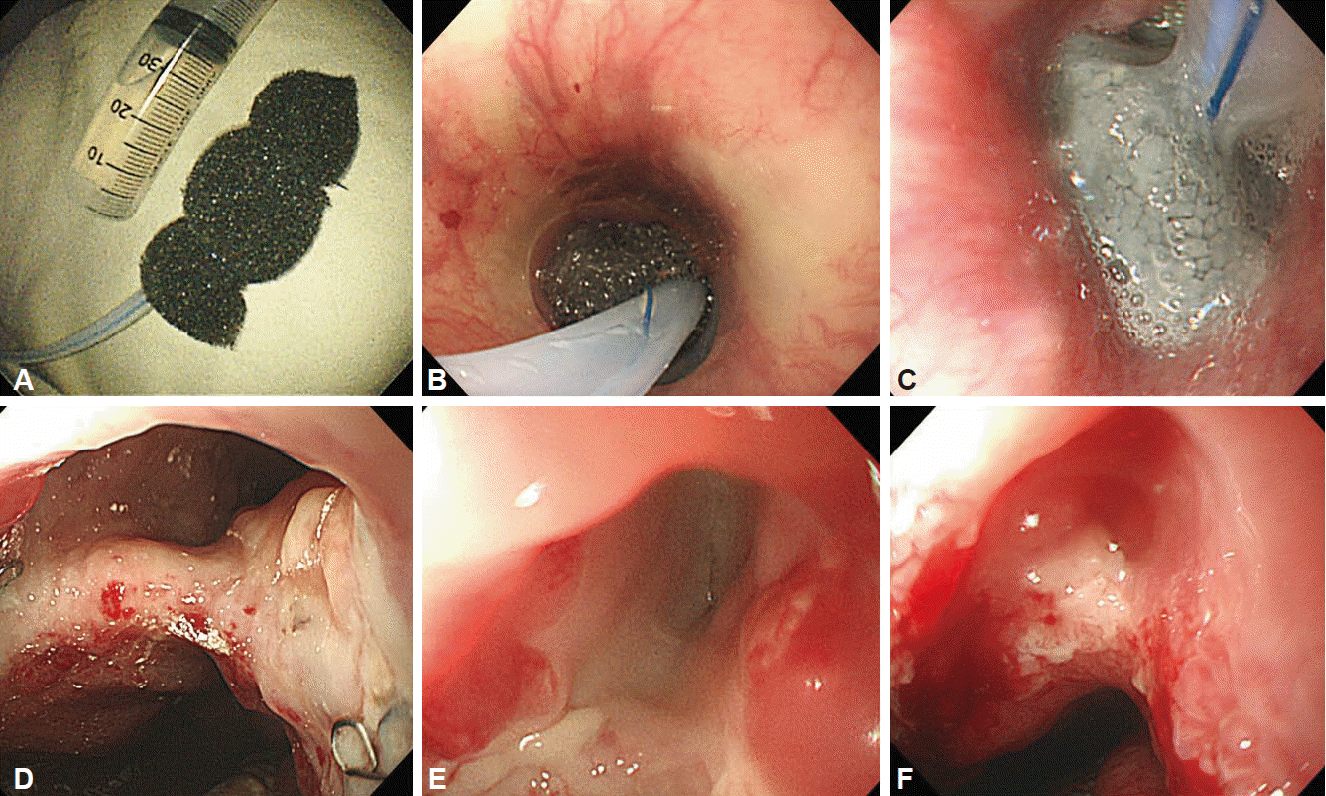
Vacuum-assisted closure of the esophageal defect due to an anastomotic leak. (A) Device made of porous sponge and attached to a nasogastric tube. (B) Positioning of sponge device at the esophageal defect. (C) After 3 days of therapy, the sponge device is filled with extraluminal fluid and gastrointestinal secretions. (D) Esophageal wall perforation before treatment. (E) Improved luminal defect with the sponge changed once after 3 days. (F) Closed wound with the sponge changed twice every 3 days.
Perivaterian or ductal perforation
Most cases of periampullary or ductal perforation are managed with biliary diversion and endoscopic closure to prevent retroperitoneal fluid leakage [13]. Biliary diversion is achieved with biliary stent or nasobiliary drainage. A fully covered self-expandable metal stent (FCSEMS) can be placed to drain the bile duct and occlude the leak site in perivaterian perforations (Fig. 6) [34]. A recent study reported that the benefits of FCSEMS in the perivaterian perforation area are painless course, lower leukocyte counts, and short hospital stay compared with conservative treatment alone [35]. The efficacy of TTS clips in closing periampullary perforations is unknown. However, it is technically difficult and has a risk of clipping the ampulla [36,37].
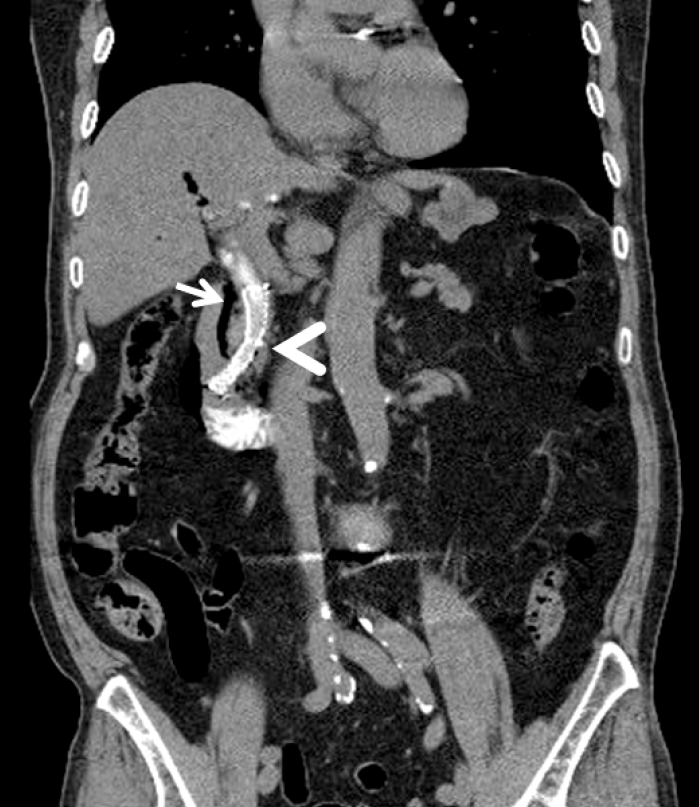
Abdominal computed tomography scan showing air collection in the periductal space (arrow). To block further bile and pancreatic juice leakage, a self-expandable stent (arrowhead) is inserted at the distal common bile duct.
Ductal perforations are usually small in size and improve with conservative management [3]. Perforation of the distal bile duct often can be managed by placement of plastic stents and, more recently, by FCSEMS [13]. If endoscopy fails to treat the perforation, percutaneous biliary drainage above the level of the perforation is an alternative treatment [2].
Retroperitoneal air alone (type IV) is developed by compressed air leakage [38,39]. It does not need treatment. Pneumoperitoneum can be present in up to 29% of asymptomatic patients who undergo uncomplicated procedures [40].
CONSERVATIVE MANAGEMENT
When a perforation occurs during an ERCP procedure, ERCP should be switched to carbon dioxide insufflations and decompression of tension pneumoperitoneum or tension pneumothorax before performing endoscopic procedures [15]. Switching from air to carbon dioxide for insufflation in iatrogenic perforations may prevent tension-pneumothorax, tension-pneumomediastinum, or tension-pneumopericardium, and abdominal compartment syndrome. Tension pneumoperitoneum should be decompressed immediately with an 18- or 20-gauge needle near the umbilicus, with the patient in the supine position [2].
Conservative managements are nil-by-mouth and intravenous administration of proton pump inhibitors, broad spectrum antibiotics, fluids, and on-demand pain medication. For the diversion of luminal content, nasogastric or nasoduodenal aspiration is used in all patients and total parenteral nutrition is needed. Cross-sectional imaging should be performed during follow-up, and if a liquid collection is disclosed, percutaneous drainage should be considered. Oral intake can be resumed after the fourth day if no further leakage is identified [2].
THERAPEUTIC ALGORITHMS FOR ERCPRELATED DUODENAL PERFORATIONS
When a duodenal wall perforation is detected during the procedure or earlier (<12 hours), endoscopic/conservative management could be performed [2]. When duodenal perforation is diagnosed later (>12 hours), endoscopic management could be performed in selected patients who are in good condition without extravasation of contrast medium or persistent large fluid collection on CT [2]. Perivaterian or ductal perforation could be started with nonsurgical management [16]. During conservative management, surgery should be considered in the conditions of failed endoscopic closure, serious leakage of contrast material, development of sepsis during conservative management, peritonitis, undrained fluid retention in the peritoneum, and worsening patient condition [41]. As salvage operation is related with worse outcomes than those in cases treated initially with operative repair, patients who require surgical repair at the time of diagnosis should be identified [1].
PROGNOSIS
The overall mortality of ERCP-related duodenal perforation is reported to be 7.8% to 9.9% [1,2]. The prognosis of ERCP-related duodenal perforation is determined based on the type, interval between perforation and recognition, and early luminal closure to reduce serous fluid leak. The amount of extraluminal air is not proportional to the size of the perforation and clinical severity [42]. Non-operative treatment was successful in 79% to 93% of patients with periampullary injuries and in all the patients with ductal perforation [3,32]. Endoscopic closure for duodenal wall perforation was applied and succeeded clinically in only 22% of cases. However, the clinical success rate after successful endoscopic closure was 94% [2]. Patients treated with surgery showed poor prognosis with delayed diagnosis, salvage operation, repeated operation, or old age [1].
CONCLUSIONS
The incidence of ERCP-related duodenal perforation has decreased, and the outcomes of nonsurgical management have improved [1,4]. This progression is achieved by early diagnosis, proper conservative management, and immediate endoscopic closure using various endoscopic devices. Endoscopic therapy could be instituted in all patients with intraprocedurally identified perforations [2,6]. However, the mortality in ERCP-related perforations still remain at 7.8% and salvage operation was associated with poor prognosis [1,4]. To reduce morbidities and mortalities in ERCP-related perforations, evaluation of risk factors, early detection of perforation during ERCP procedures, and training in endoscopic closure techniques are mandatory. During nonsurgical management, a multidisciplinary approach by endoscopists, radiologists, and surgeons is recommended to determine when and who should receive operation.
Notes
Conflicts of Interest: The author has no financial conflicts of interest.