Primary Gastric Small Cell Carcinoma: A Case Identified as a Large Subepithelial Tumor from Invisible State in 6 Months
Article information
Abstract
Primary gastric small cell carcinoma (GSCC) is one of the gastroenteropancreatic neuroendocrine tumors. It is a rare cancer with a very aggressive behavior and a poor prognosis because of the high rate of metastases. It is usually found in far advanced stage. We experienced a case of GSCC which had developed into a large subepithelial tumor (SET) from invisible state in a short period. A 65-year-old man consulted our hospital because of early gastric cancer. He underwent endoscopic submucosal dissection for the early gastric cancer at high body posterior wall. After 6 months, the follow-up endoscopy showed a large newly developed SET-like lesion with central ulceration at the gastric cardia. Endoscopic biopsy revealed GSCC. Total gastrectomy was performed. One out of the 26 perigastric lymph nodes had a metastasis. He received 6 cycles of adjuvant chemotherapy with etoposide and cisplatin. He is still in good health 12 months after operation.
Introduction
Small cell carcinoma (SCC) usually occurs in the lung. The occurrence of SCC in extrapulmonary organs is rare, especially in the stomach [1]. Primary gastric SCC (GSCC) was first reported in 1976 [2]. GSCC has an aggressive biological behavior and poor prognosis because of its frequent metastases to regional or distant lymph nodes, as well as to the liver, even in the early stage [3]. It is commonly diagnosed in the far advanced stage. Clinical and endoscopic features of GSCC in the early stage are not yet well known. Here, we report a case of GSCC that progressed from an invisible state to a large subepithelial tumor (SET) with ulceration within 6 months.
Case report
A 65-year-old man was referred to Ulsan University Hospital because of an abnormal esophagogastroduodenoscopy (EGD) finding during his routine medical check-up. EGD revealed an ill-defined, flat nodular and erosive mucosal lesion measuring 2×2 cm at the high body posterior wall side of the stomach (Fig. 1A). The lesion appeared to be an early gastric cancer type IIc. Endoscopic biopsy revealed tubular adenocarcinoma. The patient had a history of appendectomy in his twenties; pneumonia, 4 years ago; and inguinal herniorrhaphy, 3 years ago. He had a family history of esophageal cancer, with two of his brothers also having esophageal cancer. The patient had been drinking approximately 50 g of alcohol daily for 40 years.
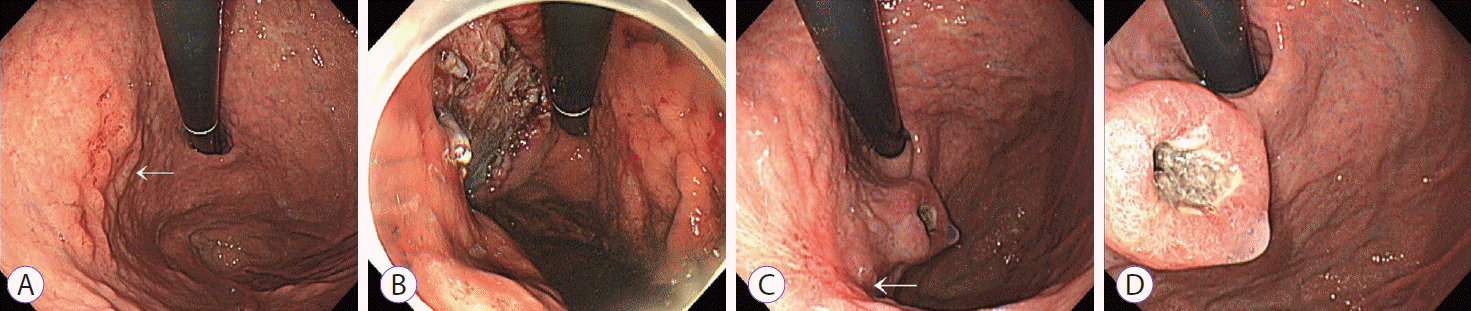
Endoscopic images. (A) Early gastric cancer lesion (arrow). (B) Ulcer after endoscopic submucosal dissection (ESD). (C, D) Follow-up endoscopy after 6 months. The image presents the post-ESD scar at the high body posterior wall side of the stomach (arrow) and the mass at the gastric cardia (C). (D) A closer image of the mass.
His laboratory test results were unremarkable. Abdominal computed tomography (CT) revealed no abnormal findings in the stomach and no lymph node enlargement. Endoscopic submucosal dissection (ESD) was performed (Fig. 1B). A second-look endoscopy was conducted the following day, which revealed a post-ESD ulcer. Histopathological evaluation of the resected tissue revealed a well-differentiated tubular type IIb+IIc adenocarcinoma, 1.8×1.6 cm in size. The cancer was confined to the muscularis mucosa without lymphatic or vascular invasion. Both vertical and lateral resection margins were free.
Six months later, follow-up EGD was performed. The patient experienced no gastrointestinal symptoms since ESD. EGD revealed a previously unseen mass on the gastric cardia beside the ESD scar. The mass was approximately 2.5 cm in width with central ulceration and resembled a SET (Fig. 1C, D).
Biopsies were taken from the ESD scar and the mass. On microscopic examination, the specimen of the ESD scar did not show any remnant malignant cells. However, the mass was diagnosed as neuroendocrine carcinoma (NEC), consistent with SCC. Histopathological evaluation revealed small cells with scant cytoplasm, ill-defined cell borders, finely granular nuclear chromatin, and absent nucleoli. The tumor cells were positive for CD56, pancytokeratin, and thyroid transcription factor-1 and negative for P40 (Fig. 2). Chest CT revealed no abnormalities. Therefore, we could diagnose the gastric lesion as a primary GSCC. The tumor was not evident on abdominal CT, possibly due to the collapsed stomach (Fig. 3A). In contrast, positron emission tomography-CT revealed a hypermetabolic lesion on the gastric cardia (Fig. 3B).

Specimen obtained from endoscopic biopsy. (A) Histopathological evaluation reveals small cells with scant cytoplasm, ill-defined cell borders, finely granular nuclear chromatin, and absent nucleoli (Hematoxylin and eosin stainining, ×400). (B) Positive staining for CD56, ×40. (C) Positive staining for pancytokeratin, ×40. (D) Positive staining for thyroid transcription factor-1, ×40.

(A) Abdomino-pelvic computed tomography (CT) showing no abnormalities. (B) Positron emission tomography-CT showing a hypermetabolic lesion (SUV max: 3.8) in the stomach (arrow).
As a curative operation, laparoscopic total gastrectomy with Roux-en-Y esophagojejunostomy was performed (Fig. 4). The pathological tumor size was 2.8×2.4×1.0 cm. The cancer extended to the subserosa with lymphovascular and perineural invasions. One of the 26 perigastric lymph nodes had metastasis and was 8 mm in size without extranodal extension (pT3N1). Histopathological evaluation of the resected tissue revealed poorly formed trabeculae or sheets of small cells that were densely packed with frequent mitoses (Fig. 5A). Immunohistochemical staining results were as follows: Ki-67 labeling index: 90%, CD56: positive, synaptophysin: positive, chromogranin: negative, and p53: negative (Fig. 5B-D).
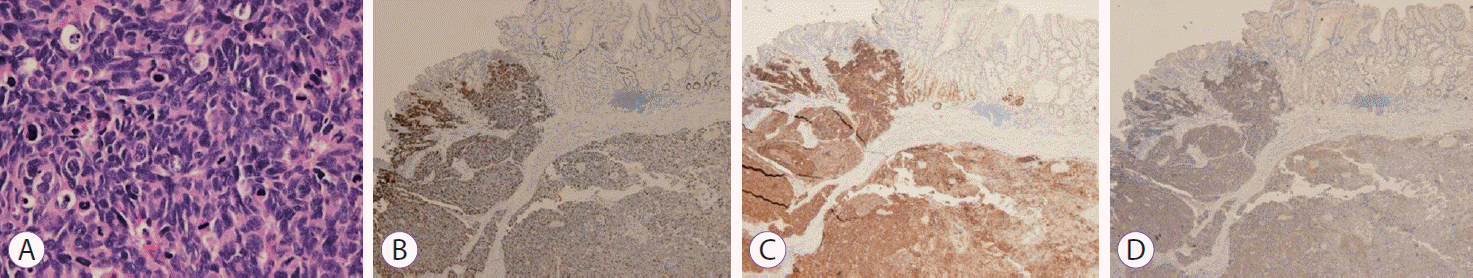
Histopathological evaluation and immunohistochemistry. (A) Microscopic finding showing mitosis of the tumor (Hematoxylin and eosin staining, ×400). (B) Ki-67: 90%, ×40. (C) Positive staining for CD56, ×40. (D) Positive staining for synaptophysin, ×40.
The patient received six cycles of adjuvant chemotherapy with etoposide and cisplatin. He remains in good health without evidence of recurrence 12 months after surgery.
Discussion
In 2010, the World Health Organization (WHO) classified gastroenteropancreatic neuroendocrine tumors (GEP-NETs) into NET grades 1 and 2, as well as NEC, based on the mitotic count and Ki-67 labeling index, regardless of the origin, size, or anatomical extent of the tumor [4]. NECs have a mitotic count of >20/10 high power fields and/or a Ki-67 labeling index of >20% and are categorized as small- and large-cell carcinomas depending on their pathological features [4,5].
NECs are rare in the gastrointestinal tract. GSCC is a GEP-NET and a rare disease, representing <0.1% of gastric cancers [3,6]. It is difficult to diagnose GSCC via endoscopic biopsy. Because the tumor has a tendency to grow beneath the mucosa like SET, there are less chances to obtain adequate tissues by endoscopic biopsies. In addition, the histological finding itself suggests malignant lymphomas or undifferentiated carcinoma [3,7,8]. Furthermore, sometimes mixed carcinomas are present, in which both neuroendocrine and adenocarcinoma components coexist [4]. Mixed adenoneuroendocrine carcinomas are composed of adenocarcinomas and NEC components, each comprising at least 30% of the entire tumor [4]. Mixed histopathology makes it difficult to diagnose GSCC. In this case, luckily, the tumor was diagnosed as SCC at first biopsy, and the surgical specimen revealed pure SCC.
SCC is diagnosed by hematoxylin & eosin histology. According to the WHO criteria, SCC consists of small cells with scant cytoplasm, ill-defined cell borders, finely granular nuclear chromatin, and absent or inconspicuous nucleoli [9]. Immunohistochemistry with neuroendocrine markers, such as chromogranin, synaptophysin, neuron-specific enolase (NSE), and CD56, is useful to prove its neuroendocrine origin [5].
Circulating tumor markers, such as plasma chromogranin A and NSE, are associated with certain types of NECs [1]. Plasma chromogranin A may be elevated in up to two-thirds of patients with advanced NEC [10]. NSE is higher in poorly differentiated tumors than in NETs. However, its utility in diagnosing diseases and predicting and monitoring outcomes has not been properly assessed in extrapulmonary NEC [1,11]. Screening for other hormonal markers is not justified unless clinically indicated [1]. Therefore, testing plasma-circulating tumor markers is not mandatory. In this patient, additional laboratory tests, including those for tumor markers and hormones, were not performed since the patient did not have any symptoms suggesting hormonal oversecretion, and the diagnosis was easily confirmed by biopsy.
Although a standard treatment for GSCC has not been established, once gastric NECs are confirmed, curative surgery is generally attempted in localized diseases [12]. GSCC is clinically extremely aggressive. Given the high relapse rate observed following radical surgery even in the early stage, adjuvant chemotherapy usually ensues. In addition, chemotherapy is the mainstay of care in advanced disease. Systemic chemotherapy has been proved to improve the median survival of GSCC patients in all stages [1,6,13]. On account of the similarity of the biological and clinical characteristics of gastric and pulmonary SCC, the disease has usually been treated with specific chemotherapeutic regimens for pulmonary SCC [12]. NECs with Ki-67 labeling index of >55% respond better to platinum-based chemotherapy than NECs with lower proliferative index [10]. Thus, the combination of etoposide and cisplatin is one of the most widely used regimens [10,14,15]. While more long-term follow-up needs to be performed, the etoposide and cisplatin regimen appeared to be effective in our patient.
To the best of our knowledge, this is the first case to demonstrate that the early developmental feature of GSCC is aggressive, rapidly growing, and exhibits SET-like behavior. The biological nature of GSCC suggests that even annual endoscopic check-ups cannot detect the tumor in the early stage.
Notes
Conflicts of Interest: The authors have no financial conflicts of interest.
Author Contributions
Funding acquisition: Hong Kil Jeon
Investigation: Kyung Bin Kim
Validation: Jae Wook Im, Sang Yu Oh, KBK
Writing-original draft: Yun Im Lee
Writing-review&editing: Byunggyu Kim

