Should We Resect and Discard Low Risk Diminutive Colon Polyps
Article information
Abstract
Diminutive colorectal polyps <5 mm are very common and almost universally benign. The current strategy of resection with histological confirmation of all colorectal polyps is costly and may increase the risk of colonoscopy. Accurate, optical diagnosis without histology can be achieved with currently available endoscopic technologies. The American Society of Gastrointestinal Endoscopy Preservation and Incorporation of Valuable endoscopic Innovations supports strategies for optical diagnosis of small non neoplastic polyps as long as two criteria are met. For hyperplastic appearing polyps <5 mm in recto-sigmoid colon, the negative predictive value should be at least 90%. For diminutive low grade adenomatous appearing polyps, a resect and discard strategy should be sufficiently accurate such that post-polypectomy surveillance recommendations based on the optical diagnosis, agree with a histologically diagnosis at least 90% of the time. Although the resect and discard as well as diagnose and leave behind approach has major benefits with regard to both safety and cost, it has yet to be used widely in practice. To fully implement such as strategy, there is a need for better-quality training, quality assurance, and patient acceptance. In the article, we will review the current state of the science on optical diagnose of colorectal polyps and its implications for colonoscopy practice.
INTRODUCTION
Colorectal cancer is the second most cause of cancer related death in United States and majority of cancer arise from benign adenomas [1]. Colonoscopy offers real-time management of benign adenomas to break adenoma- carcinoma sequence, thus decreasing the possibility of colorectal cancer development. Polypectomy reduce the occurrence of colorectal cancer by approximately 50% in long term follow up [2]. More than 90% of the colorectal polyps are small (6–9 mm) or smaller (<5 mm) [3] and half of the them are non-neoplastic [4], only 1.7% of cases have advanced histology (villous or dysplasia) with lower possibility of developing colon cancer [3,5]. Therefore many polypectomies add unnecessary risk during colonoscopy. Current standard of care is to resect most polyps and sent for histology [6,7]. The ability to diagnose polyp histology in real-time during colonoscopy would allow leaving recto-sigmoid hyperplastic polyps (diagnose and leave) and resecting small adenomas without sending for formal histology (resect and discard). As cancer progression in small polyps is remarkably rare, we believe enhanced imaging technology may guide proper treatment decision at real-time during colonoscopy.
DIMINUTIVE COLORECTAL POLYPS : OPTICAL DIAGNOSIS
Optical diagnosis is a concept in which histopathology of colorectal polyps is determined at the time of colonoscopy by imaging technologies such as high definition white light (HDWL), narrow band imaging (NBI), or other narrow spectrum imaging technologies. This approach has potential benefits in terms of cost-effectiveness and efficiency of colorectal screening by decreasing the procedure time, costs associated with histology, and complications [8,9]. The American Society of Gastrointestinal Endoscopy (ASGE) established the Preservation and Incorporation of Valuable endoscopic Innovations (PIVI) process with regard to leaving recto-sigmoid hyperplastic polyps without resection as well as a resect and discard approach for small adenomas. A survey results from American College of Gastroenterology reported inconsistency in diminutive colorectal polyps management [10]. Most gastroenterologists (78%) reported that they leave small colorectal polyps in an average-high risk individual, but interestingly they leave them in a certain scenarios like advanced age, patients on anticoagulation, and appearance of polyps as non-adenomatous histology. In addition, gastroenterologists with greater experience and those confident to differentiate polyps’ morphology diagnose and leave diminutive colorectal polyps in place. In another multicenter cross-sectional study, a survey was conducted at three university centers in Europe and Australia in which patient’s inclination to accept resection of small colorectal polyps was studied. The main objective of the study was to measure the proportions of patients wishing to partake in a randomized trial that accede resection of small colorectal polyps. Results from this study showed that 50% participants were interested to participate in a clinical trial in which differing of resection of diminutive colorectal polyps would be applied (95% confidence interval [CI], 46%–55%) and 57% of partakers would be affable to accepting resection of diminutive colorectal polyps (95% CI, 51%–63%) outside of clinical trial. Factors associated for deferring diminutive colorectal polyp resection were higher education (p=0.001), more information on cancer risk (p=0.002), and the lower insight of cancer risk (all p<0.001) [11]. This was the first study to examine patient perspective whether they would be interested to defer resection of diminutive colorectal polyps while undergoing a colonoscopy. Results from this study also demonstrated that 50% would agree to not taking out of small colorectal polyps when the benefits and harm of this approach was provided. Note, that this included leaving diminutive adenomas in situ which is more expansive the current ASGE PIVI process of only leaving recto sigmoid hyperplastic polyps in situ. Published studies have shown that the risk of finding cancer in cross-sectional colonoscopy is about 1 per 2,000–3,000 polyps [3,5,12,13]. Regarding the progression of diminutive adenomas to cancer, it is likely that the latency phase is lengthier than the total life expectancy of the individuals in majority of cases. Studies have also demonstrated that most adenomas including non-diminutive adenomas appearing before the age of 65 rarely progress to cancer and merely 50% progress to size >10 mm in individual’s life span [14-16].
The PIVI guideline on evaluation of histopathology of diminutive polyps was developed by the ASGE to provide guidance on appropriate use of optical diagnosis. This strategy potentially has very large economic benefit. Based on simulated Markov modeling regarding “resect-and-discard” strategy of detected diminutive colorectal polyps, the economic benefit was $25 per person screened colonoscopy, but when projected to US population would result in annual savings of $33 million [9]. When the cost associated with “diagnose-and-leave” strategy is applied, in which each polypectomy cost approximately $179 per person, which would convert to the estimated stashes of $1 billion per year to the US health care system. This approach also has additional benefit by avoiding unnecessary adverse events related to polypectomy of small polyps.
To set guidelines for diminutive colorectal polyps management, the ASGE has developed a novel initiation in 2011 called PIVI. The main goals of PIVI initiative are to resolve important clinical questions and to establish diagnostic, and/or therapeutic inceptions for endoscopic skills and technologies. The main objective is to aid right integration of endoscopic skills and tools to optimize patient outcome. Thus, the ASGE has developed a PIVI threshold for optical diagnosis of diminutive colorectal polyps:
“1. For a technology to be used to guide the decision to leave suspected recto-sigmoid hyperplastic polyps 5 mm or smaller in place (without resection), the technology should provide a 90% or greater negative predictive value (NPV) (when used with high confidence) for adenomatous histology.
2. For colorectal polyps 5 mm or smaller to be resected and discarded without pathologic assessment, endoscopic technology (when used with high confidence) used to determine histology of these polyps, when combined with the histopathologic assessment of polyps larger than 5 mm, should provide 90% or greater agreement in assignment of post-polypectomy surveillance intervals when compared with decisions based on pathology assessment of all identified polyps [13].”
IMAGING TECHNOLOGIES FOR OPTICAL DIAGNOSIS HIGH DEFINITION WHITE LIGHT COLONOSCOPY
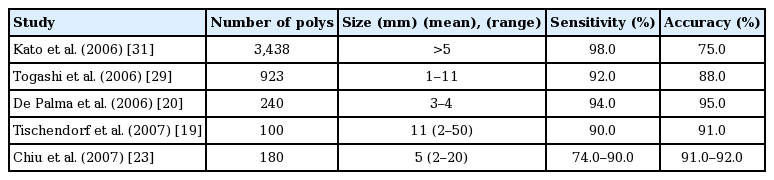
HDWL colonoscopy has been utilized to distinguish polyp histology at the time of colonoscopy and on still images. However, HDWL has low accuracy in distinguishing neoplastic vs non-neoplastic colorectal polyps [17-20] with an accuracy of only 59%–84%. Diagnostic values of HDWL for optical diagnosis of polyp histology (Table 1) [18-25].
Diagnostic Values for Optical Diagnosis of Colorectal Polyps with High Definition White Light Colonoscopy
Chromoendoscopy
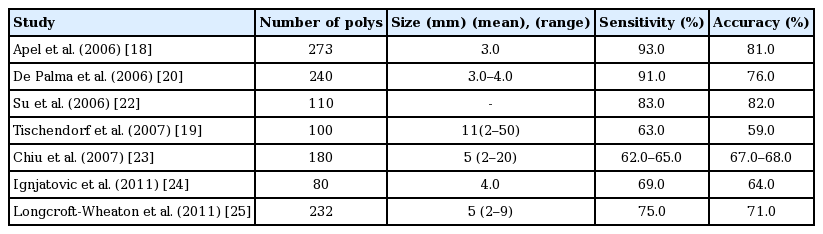
This technique was first utilized in Japan to describe histopathology of colorectal polyps at the time of colonoscopy. This technique provides the colonic pit patterns (Kudo pit pattern) and is widely used classification: [26] Kudo pit patterns 1 and 2 indicate= non-neoplastic lesion whereas Kudo 3 s, 3 L and 4 are considered as neoplastic lesion and Kudo 5 represents submucosal invasion. Kudo classification is precise in optical diagnosis (85%–96%) [19,20,27-29] when used with high definition colonoscope and chromoendoscopy. However, this technique needs additional training, equipment, time, and considerable learning curve [30]. Diagnostics value of Chromoendoscopy for optical diagnosis of polyp histology (Table 2) [19-21,23,29,31].
Narrow spectrum technologies
For last few years, several technologies are available for visualization of diminutive colorectal polyps that are better than HDWL. These imaging technologies include i-SCAN (Pentax, Mississauga, Canada), Fujinon Intelligent Color Enhancement (FICE; Fujinon Inc., Wayne, NJ, USA), and Narrow band imaging (NBI; Olympus, Tokyo, Japan), with a push button technologies integrated into the colonoscope. They are easy to use, quick, and user friendly.
Narrow band imaging
NBI (Olympus) is also called virtual chromoendoscopy with a narrow-band with “blue light” that has the capability to provide mucosal detail and vascular structures [32-35]. Adenomatous tissue is categorized by augmented angiogenesis, and appears as browner when visualized with NBI. The NBI International Colorectal Endoscopic (NICE) is the validated classification system for the categorization of diminutive colonic polyps (Table 3) [36].
Results of meta-analyses (by ASGE technology committee systematic review and meta-analyses) of NBI studies evaluating colorectal polyps histology in real-time, the pooled NPV for adenomatous histology was 91% (95% CI, 88–94) [13] and on high confidence the pooled NPV was 93% (95% CI, 90–96). On subgroup analysis, there was no significant difference was observed when compared to academic (91.8%, 95% CI, 89–94) versus community centers (88.3%, 95% CI, 82–94). In addition novice endoscopist reached PIVI threshold with high confidence (90%, 95% CI, 86–94) where has expert endoscopist exceeded PIVI threshold when assessment was done with high confidence (95%, 95% CI, 92–98) (Figs. 1, 2) [13].

Colonoscopy image of hyperplastic polyp with narrow band imaging. Features include lighter color than surrounding mucosa, black dot pattern, and absence of vessels.

Colonoscopy image of adenomatous polyp with narrow band imaging. Features include overall dense brown color, thick brown vessels surrounding tubular, oval and variable- shaped white mucosa.
In addition, regarding the post-polypectomy surveillance intervals based on NBI optical biopsy versus histopathology, the percentage of agreement was 89% (95% CI, 85–93). On subgroup analysis, higher agreement was observed at academic medical centers 91% (95% CI, 86–95) compared to community settings 82% (95% CI, 74–90), higher agreement with experts 92% (95% CI, 88–96) compared to novice endoscopist 82% (82%, 95% CI, 75–88), and higher agreement with high confidence diagnosis 91% (95% CI, 88–95) compared to no confidence level provided 79% (95% CI, 71–86). In addition, on high confidence diagnosis, expert exceeded PIVI thresholds 93% (95% CI, 90–96) compared to novice operators 87% (95% CI, 82–93) [13].
In one retrospective study, real-time optical diagnosis predicted accurate surveillance interval in 92.7% (95% CI, 91.4–96.2) and optical diagnosis was not determined only in 0.3% (4/1254). The NPV for diagnosis of adenoma from all polyps was 86.8% (95% CI, 82.9–90.0), but when restricted to recto-sigmoid region only, NPV was increased to 95.4% (95% CI, 91.8–97.7) achieving both the PIVI thresholds [37]. On subgroup analyses looking at the cost savings with resect and discard policy, the highest savings were achieved for all diminutive polyps saving $309 per patient by reducing histopathology assessment of all polyps.
In addition, published studies have reported NBI has similar sensitivity (92%–94%) and specificity (86%–88%) to chromoendoscopy when assessed by experienced endoscopist in academic medical centers centers [17,19,21-23,27,38-41].
i-SCAN
Meta-analyses (by ASGE technology committee systematic review and meta-analyses) of 8 studies evaluating the diminutive colorectal polyps using i-SCAN were compared to histology. The pooled NPV was 84% (95% CI, 76–91). On subgroup analysis, NPV for experienced endoscopist was 96% (95% CI, 94–98) compared to 72% (95% CI, 69–76) for novice endoscopist [13]. Regarding the level of agreement with histopathology for post-polypectomy surveillance (based on Multi Society Task Force post-polypectomy surveillance intervals), only one i-SCAN study assessed the post-polypectomy surveillance. Result from this study demonstrated an agreement of 69.5 % (95% CI, 63–75) and PIVI threshold was not met [42].
Fujinon intelligent color enhancement
Meta-analyses (by ASGE technology committee systematic review and meta-analyses) of 8 studies evaluating the optical diagnosis using FICE scan and were compared to histopathology. Results showed that the pooled NPV was 80% (95% CI, 76–85). On subgroup analyses, NPV of FICE was not improved with endoscopist experience but FICE performance was improved with the use of magnification with NPV of 85% (95% CI, 79–91) [13]. Thus PIVI threshold was not met with this technology for optical biopsy.
Regarding the post-polypectomy surveillance intervals, only 2 FICE studies have been published [25,43]. Based on the two studies, agreement in assigning post-polypectomy surveillance intervals was 100% (95% CI, 91–100) and 97% (95% CI, 89–100).
ARTIFICIAL INTELLIGENCE AND BLUE LIGHT IMAGING IN COLORECTAL POLYP CHARACTERIZATION
Optical diagnosis using NBI in non-academic setting is below the ASGE PIVI threshold [44]. To overcome this drawback, artificial intelligence (AI) based diagnosis of colorectal polyp has been brought into the field of gastroenterology for polyp detection [45-50]. In one study AI based diagnosis using deep neural network-computer aided detection (DNN-CAD) was used to evaluate the NBI images of small colorectal polyps (Neoplastic or hyperplastic). Results from this study showed that DNN-CAD system accurately classified polyp histology with a positive predictive value of 89.6% and a NPV of 91.5% at a shorter time interval than the expert endoscopist [51], and less than half of novice endoscopist categorized polyps with a NPV of 90%. Thus, this automated diagnostic system could be the potent aide for gastroenterologists provided good NBI images. In another study effectiveness of CAD software for colorectal lesions was evaluated using magnifying NBI images. The NPV of neoplastic lesions was 95.6% [52]. The concordance between CAD and experienced endoscopist for optical diagnosis was 90.9%. Results from this study showed a fairly high categorization rate with CAD with magnifying NBI images and were not significantly different from those of experienced gastroenterologists. Although CAD has the potential to bring significant change in the field of endoscopy in future, endoscopists will not be substituted by CAD as they are the key person to correctly recognize suitable images for machine to learn. However, computers may simplify colonoscopy performance and help in prevention of colorectal cancer incidence and mortality.
Advanced endoscopic imaging technologies have made a significant improvement in colorectal polyp characterization. Currently only the NICE classification is used solely for the NBI technology [52] and is not reproducible when different technology is used [53]. In one study blue-light imaging (with or without magnification) was used to create a new classification for distinguishing colorectal polyps (hyperplastic, adenomatous, and invasive malignant lesions) [54]. Blue light imaging is selectively absorbed by hemoglobin and is based on the direct emanation of blue (without filter) light with shorter wavelength (410 nm). Magnification with zoom is up to ×135. On the basis of modified Delphi process, endoscopists created 12 factors into 3 main domains (that included morphological and pit/vessel findings) such as: (1) Polyp surface (mucus, yes/no; regular/irregular; [pseudo] depressed, yes/no), (2) Pit appearance (featureless, yes/no; round/non-round with/without dark spots homogeneous/heterogeneous distribution with/without focal loss), (3) Vessels (present/absent, lacy, peri-cryptal, and irregular) [54].
1. Interobserver agreement for polyp surfaces each domain as follows:
For the polyp surface domain for mucus: 0.92 with and 0.88 without magnification): Perfect
B) Polyp surface domain for the regular/irregular surface: 0.67 with and 0.66 without magnification): Substantial
2. Interobserver agreement for polyp pit pattern:
A) Pit pattern: 1 0.9 with and 0.8 without magnification: Good
B) Pit pattern round/non-round features: 0.77 with and 0.69 without magnification, but less reliable for the homogeneity of distribution: with/without optical magnification 0.58 [54].
3. Interobserver agreement for the vessel (0.81 – 0.85) [54]: Perfect
A) Although blue light imaging technology looks promising in detection of colorectal lesions, however future studies are needed to validate this classification and potential application in clinical practice.
BARRIERS TO IMPLEMENTATION
Cost efficiency is a hot topic in the field of gastroenterology. Current strategies for management of colorectal polyps recommend that all identified polyps be removed and the reimbursement to endoscopist is based on therapeutic procedures performed and pathological analysis of polyps. If resect and discard strategy is applied this may impact the reimbursement in an environment where physicians are compensated extra for polypectomy and also some group of gastroenterologists which own or share in the revenue of pathology services. It is important to note that resection of hyperplastic polyps adds unnecessary costs for resection and pathological evaluation where malignant transformation is exceedingly rare. In the other hand, the resect and discard strategy is only recommended by endoscopists when there is agreement of >90% between the optical diagnosis and pathological assessment of polyps which may be applicable in academic centers only [13]. Therefore, AI based CAD may help in accurately diagnosing colorectal polyps to avoid inappropriate resection, and, further, may help in saving cost associated with pathological evaluation and potential applicability in community settings too [51].
Another important concern is not resecting diminutive polyps may interfere the measurement of adenoma detection which is one of the quality metrics in colonoscopy. One landmark study showed the proportional decrease in risk of colorectal cancer with increase in adenoma detection (1% increase in adenoma detection rate (ADR) is associated with a 3% decrease in the risk of colon cancer) [55]. Endoscopists who have high ADRs are those who identify and remove all the smallest of polyps. This indicates that removing all visible polyps prevents from cancer. However it is still unclear whether resection of all polyps that is preventive, or it is simply a consideration of more comprehensive mucosal inspection for major lesions [56]. Thus, the best alternative could be calculation of ADR based on optical diagnosis although this is more subject to gaming.
Another aspect to address is the patient anxiety after proposing the concept of diminutive polyp deferral. In one cross sectional survey, an assessment of patient approval of optical diagnosis and their answers was conducted [57]. In this study, 49.6% patients backed diagnose and leave strategy and 60.8% supported resect and discard strategy. However, only 41.7% of patients supported both the strategies. Factors related to refusal of resection and discard were missed cancer diagnosis in family (odds ratio, 0.59; p=0.03), and bowel cancer history (either personal or in family) (odds ratio, 0.7; p=0.4). Results also showed that patients who didn’t support resect and discard strategy were more likely to ask compensation if a mistake occurred [57]. In another cross-sectional survey study on outpatient’s colonoscopy, factors regarding the acceptance of resect and discard were evaluated. Results showed that younger, white and ambulatory surgery center patients were more prone to agree the resect and discard strategy. Patients who declined “resect and discard” were more prone to be agreeable to pay more amount of out-of- for pathological evaluation of diminutive polyps (97.1% vs. 44.5%) [58]. Thus, although optical diagnosis is lucrative for healthcare economy, this approach is still not assuring and quite variable from patient perspectives.
Leaving polyps may also cause anxiety to patient and thus may need to provide adequate information to patient on both the risk and benefit of this approach. Further studies are needed to measure and improve patient acceptance of the optical diagnosis before widespread use in clinical practice.
CONCLUSIONS
The improvement in endoscopic imaging technologies has increased our ability to detect colorectal polyps, including very small, low risk lesions. While there are clearly benefits to higher adenoma detection and resection, the resection and pathological analysis of such a low risk lesions has increased the cost and risks of colonoscopy procedure. The correct assessment of diminutive polyps at real time would allow accurate decision making either to resect or discard for small adenomas without sending for pathological analysis or to leave recto-sigmoid hyperplastic polyps with negligible risk.
Based on PIVI guideline, for “diagnose-and-leave” approach for optical diagnosis of small recto-sigmoid polyps, the NPV be 90% or higher with high confidence. Results from the meta-analysis, the ASGE technology committee concluded that NBI meets the PIVI threshold and supports a “diagnose-and-leave” strategy for predicting neoplastic polyps in the recto-sigmoid colon.
For a “resect-and-discard” approach for 5 mm or smaller adenomas and when combined with histopathological analysis of polyps larger than 5mm, the meta-analyses demonstrated that NBI exceeded 90% or higher agreement for post-polypectomy surveillance intervals with high confidence (91%) in academic centers and experienced endoscopists. Thus, NBI imaging modality can be used for “resect-and discard” strategy for 5 mm or smaller colorectal adenomas.
Although PIVI has developed a threshold for resect-and-discard policy for optical diagnosis of diminutive colorectal polyps, the implementation still poses challenges such as widespread application of this approach in community practice, establishing the standard for the use of tools and skills among endoscopists, and the development of quality assurance program.
Notes
Conflicts of Interest: Michael B. Wallace reports consulting income from Olympus Corp and grant support from Boston Scientific and Cosmo pharmaceuticals. Other author has no financial conflicts of interest.