Efficacy of Nasobiliary Tubes and Biliary Stents in Management of Patients with Bile Leak after Liver Transplantation: A Systematic Review
Article information
Abstract
Background/Aims
Bile leak is one of the most common complications of liver transplantation. The treatment options for bile leaks include conservative management, surgical re-intervention, percutaneous drainage and endoscopic drainage. We aimed to perform a systematic review to identify the efficacy of endoscopic treatment in the resolution of post-transplant bile leaks.
Methods
Two independent reviewers performed systematic literature search in PubMed, ISI Web of Science, grey literature and relevant references in May 2017. Human studies in English with documented post-liver transplant bile leaks were included.
Results
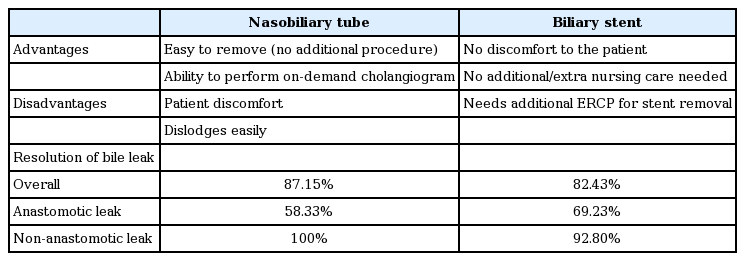
Thirty-four studies were included in the final analysis. The pooled efficacy of biliary stents for the resolution of post-transplant bile leaks was 82.43% compared with 87.15% efficacy of nasobiliary tubes. The efficacy of biliary stents was lower for anastomotic leaks (69.23%) compared to T-tube (90.9%) or cut-surface/ cystic duct stump related leaks (92.8%). Similarly, the efficacy of nasobiliary tube was also lower for anastomotic leaks (58.33%) compared to T-tube or cut-surface related leaks (100%).
Conclusions
In this systematic review, the overall efficacy was 82.43% in biliary stent group, and 87.15% in nasobiliary tube group. Both biliary stent and nasobiliary tube were more effective in managing non-anastomotic leaks compared to anastomotic leaks.
INTRODUCTION
Liver transplantation (LT) is the only treatment available for end-stage liver disease. Roughly 25,000 annual LTs are performed worldwide, and 6,000 of those are performed in the US [1]. Biliary complications, mainly bile leaks (BLs) and strictures are observed in 8%–19% of LT [2]. The incidence has been decreasing given improvements in surgical techniques for biliary reconstruction, patient selection and improved immunosuppression. A recent systematic review reported the overall rate of BLs was 8.2% [3]. In this review, the onset of BL ranged from post-operative day 1 to 6-months, and the incidence in deceased donor liver transplant (DDLT) and living donor liver transplant (LDLT) was 7.8% and 9.5%, respectively. In the earlier transplant experience, BLs were managed surgically, which resulted in significant morbidity and mortality [4]. As a result, minimally invasive approaches have been favored.
Endoscopic retrograde cholangiography (ERC) is the most common primary treatment approach for post-transplant BLs. There is significant evidence regarding the safety and efficacy of ERC in the management of post-transplant BLs in the last three decades, and it has become the standard care for management of such patients [5-7]. The primary endoscopic treatment strategy includes the placement of a trans-papillary biliary endoprosthesis that diverts the flow of bile preferentially into the duodenum, or externally. The endoprosthesis that have been used include plastic stents and nasobiliary tubes (NBTs).
Here, we reviewed the literature regarding the clinical efficacy of biliary stents (BS) and NBT placement for the treatment of post-transplant BL. The primary goal of the systematic review was to identify the overall efficacy (assessed by resolution of BL) of BS and NBT in post-transplant BLs. Secondary outcomes included healing time for the BL, adverse events associated BS and NBT and duration BS and NBT insertion. We further sought to assess the need for any additional ERCs, surgical re-interventions or interventional radiology procedures needed to treat BL.
MATERIALS AND METHODS
Study selection
We selected studies that included patients undergoing LT for various indications and developed BL post-operatively. Studies including patients who underwent BS or NBT placement for the management of BL in adult and pediatric population were reviewed. We included controlled trials, prospective or retrospective cohort studies and case series. We excluded studies that (a) included patients with post-operative BL from surgeries other than liver transplant (b) case reports and (c) did not have a follow-up duration for the assessment of BL. In a case of duplicate or overlapping studies, most recent publication was used for inclusion and analysis.
Search strategy
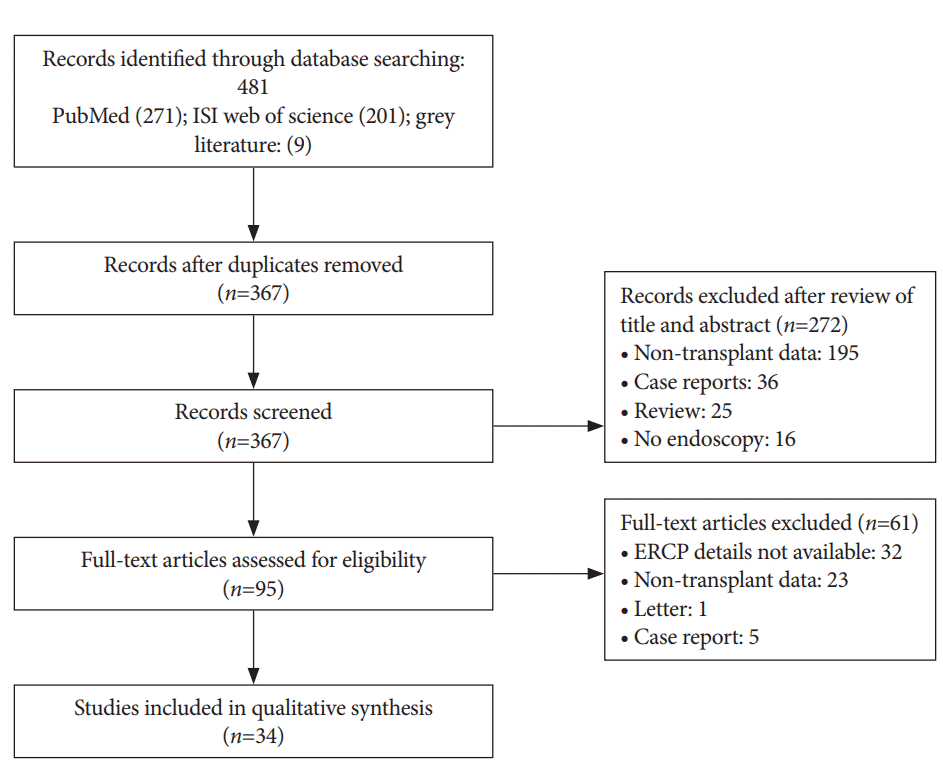
A systematic search was performed in multiple electronic databases (PubMed; ISI Web of Science), grey literature and review of pertinent references of included studies by three independent researchers (AR, AO and VG) in November 2016. Human studies in English published from 1991–2016 were screened. Boolean search was conducted using the terms “Liver transplant AND bile leak”. Mesh terminology used for the search was: “Liver Transplantation”[Mesh] AND “Bile”[Mesh] AND leak [All Fields]. Handsearching of the relevant evidence was also performed to ensure inclusiveness. Final exploration of the electronic databases was performed on May 26th, 2017 and the search results were updated (Fig. 1). Two additional researchers evaluated the titles and abstracts of initially screened studies to assess inclusion (SI and PK). Full texts of the eligible articles were then carefully examined to determine inclusion for qualitative analysis. Any inconsistencies among reviewers were resolved by consensus or discussion with a third reviewer (KK). One reviewer (AR) contacted the authors, if needed, for additional information.
Data abstraction and definitions
A standardized form was used by three independent reviewers (AR, AO and VG) who extracted the following data from relevant studies: author, study name, type of study (retrospective or prospective), peer reviewed full publication or abstract, number of patients undergoing biliary stenting, or receiving NBT, demographic information of the patients, living donor or cadaveric transplant, type of biliary anastomosis (hepaticojejunostomy or duct to duct anastomosis), onset/recognition of BL, site of BL, adverse reaction from placement of BS or NBT, duration of BS or NBT insertion, need for further intervention for persistent BL (endoscopic, surgical or radiological). Any discrepancy was resolved with consensus or input from a third reviewer (KK). BL was suspected when a transplant recipient developed clinical symptoms (Abdominal pain, jaundice, fever etc.) or abnormal liver function tests. BL was confirmed based on the imaging (direct cholangiography via T-tube or NBT, hepatobiliary scintigraphy), ERC or bilious intra-abdominal drain out-put.
Quality assessment
Using the Newcastle-Ottawa quality assessment scale (NOQAS) for the cohort studies, we assessed the quality of included studies. We also included following ad hoc quality criteria: (i) total number of patients more than 10 and (ii) peer reviewed full publication.
Outcomes assessment
Primary outcome: Primary outcome was to assess the overall efficacy of BS or NBT in resolution of post-transplant BL in the recipients.
Secondary outcomes: Secondary outcomes included: (a) time to resolution of BL after NBT or BS insertion, (b) duration of prosthesis (BS, NBT) in patients with BL, (c) need for further interventions (endoscopic, radiologic or surgical) in patients who failed to heal the BL after BS or NBT placement and (d) complications arising from endoscopic BS or NBT insertion
Results
A systematic search of data was performed in November 2016, and the search was updated on May 26th, 2017. After removal of duplicates, 367 studies were identified by literature search. Review of title and abstract excluded 272 studies. Another 61 studies were excluded after full text review (reasons mentioned in the Fig. 1).
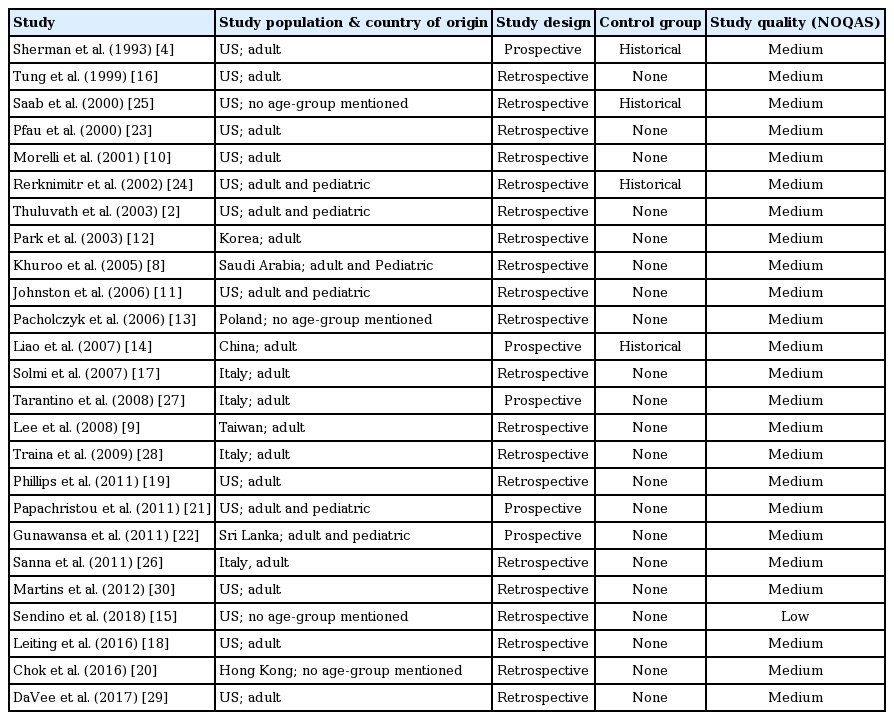
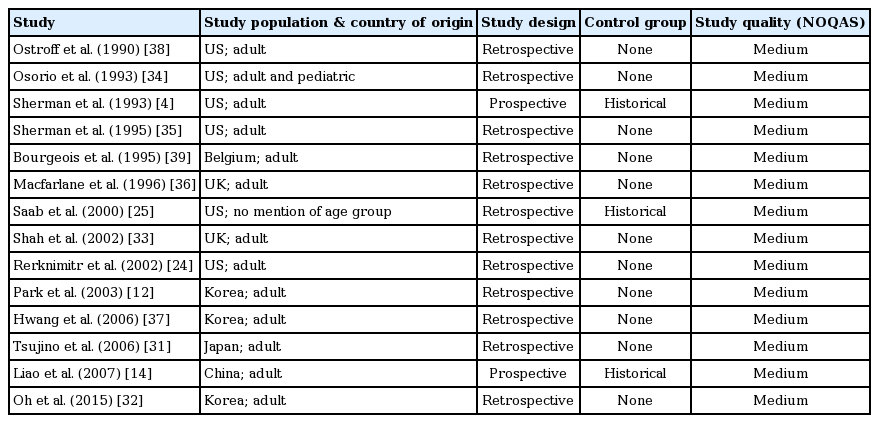
Thirty-four studies (29 retrospective, 5 prospective) were included in the final analysis [2,4,8-39]. Thirty- three studies were peer-reviewed full publications and medium quality as assessed by NOQAS, while one study [15] was a published research abstract and was low quality. Sixteen studies involved more than 10 patients. Two studies reported outcomes in both DDLT and LDLT [13,32], six in DDLT [4,11,19,21,30,39], four in LDLT [18,20,34,36] and twenty-two studies did not specify the type of transplant. A total of 370 post-transplant patients with confirmed BLs (anastomotic leak: 179, T-tube: 84, cut surface/duct of Luschka/cystic duct/others: 25, not reported: 82) treated with BS and a total of 179 post-transplant patients with confirmed BLs (anastomotic leak: 36, T-tube: 78, cut surface/duct of Luschka/cystic duct/others: 11, not reported: 54) treated with NBT, were reviewed.
Details of study design and characteristics are listed in Tables 1, 2 for BS; and Tables 3, 4 for BLs treated with NBTs.
Biliary stents
Efficacy of BS
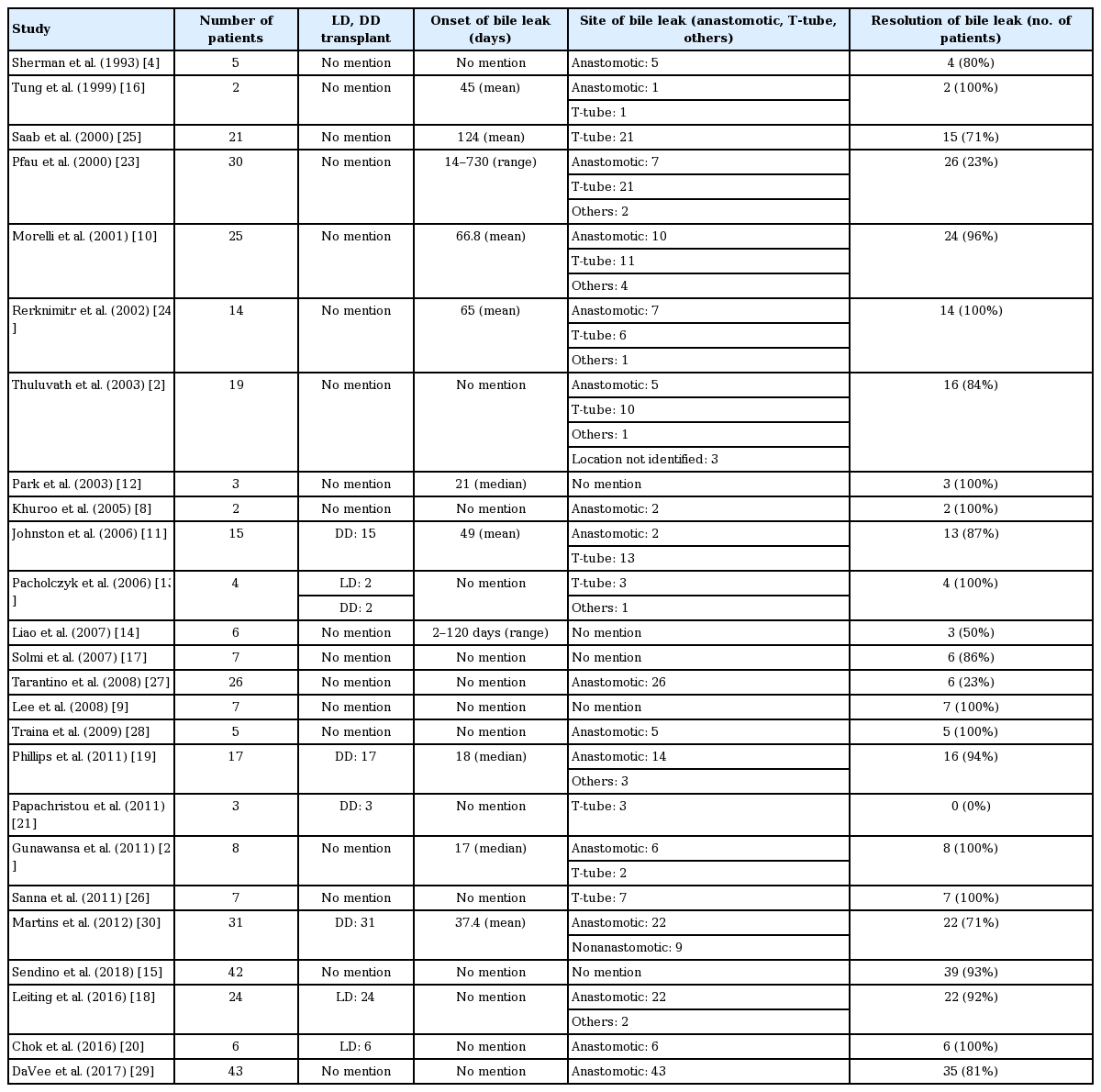
A total of 370 patients with confirmed post-transplant BLs who were treated with BS were identified. The pooled efficacy for the resolution of post-transplant BLs treated with BS was 82.43% (278/370). Among patients who had documented leak related to the choledochocholedochostomy, the efficacy of BS was 69.23% (90/130), the remaining 49 patients with anastomotic leaks did not have outcome mentioned in their subgroup analysis. Efficacy of BS among patients with documented T-tube related BL was 90.9% (68/74; remaining 10 patients with T-tube related leak did not have outcome mentioned in their sub-group analysis). Similarly, efficacy of BS for other causes of BL (cut surface, duct of Luschka and cystic duct stump) was also 92.8% (13/14, remaining 11 patients did not documented outcome of stenting in the sub-group analysis). The site of BL was not mentioned in the rest of the patients (82 patients). In cases where the type of transplant was specifically mentioned, BS was effective in controlling the BL in 93.7% (30/32) of LDLT and 76.47% (52/68) of DDLT patients.
When 14 studies with low quality and less number of patients (less than 10) were excluded, the pooled efficacy of BS for the resolution of BL was 77.6% (204/263). Sub-group analyses for BS efficacy in 11 medium quality studies with more than 10 patients showed: 64.7% (66/102) in duct-to-duct anastomosis, 90.5% (57/63) in T-tube related leak, 90.9% (10/11) in other causes (cystic duct stump, duct of Luschka etc.) of leak, 91.7% (22/24) in LDLT and 76.2% (48/63) in DDLT.
Efficacy of covered self-expandable metal stents
Four studies reported data on covered self-expandable metal stents (CSEMS) [19,28-30]. Three studies [19,28,29] described outcomes in fully covered self-expandable metal stents (FCSEMS) while one study [30] reported outcomes for both FCSEMS and partially covered self-expandable metal stents (PCSEMS). Two studies exclusively included patients who failed previously placed plastic stents [28,29], and the other two studies reported patients with endoscopic treatment failure (previous plastic stents) as well as treatment naïve patients [19,30]. Overall efficacy CSEMS for resolution of BL was 87.5% (49/56). Efficacy of CSEMS for treatment naïve and refractory BL was 93.75% (15/16) and 86.7% (13/15) respectively, while refractoriness of BL was not mentioned in the remaining 25 patients. Efficacy of FCSEMS and PCSEMS for resolution of BL was 86.8% (46/53) and 100% (3/3) respectively. Frequency of complications from CSEMS included: 18 (32%) biliary strictures after the stent removal (2 patients managed with surgeries; 12 with repeat plastic or metal stents; 2 conservatively and no treatment mentioned in 2 patients); 6 biliary pathologies like ulcerations, inflammation or hyperplasia at stent site; one cholangitis and one stent migration.
Onset of BL and duration of BS placement
The pooled mean onset of BL in the studies reporting “mean onset” was 46.4 days (range, 1–184 days) after transplant. Two studies reported the median onset of BLs (17 and 21 days), and two studies provided range for the onset of BL (2-days–4-months; 2-weeks–2-years). The BS was kept in place for as long as it was deemed necessary to heal the BL. If BL was refractory to repeated stenting, patients further underwent surgery or radiological intervention. Two studies reported median duration for which stents stayed in biliary tree (37 days, 150 days), another two studies reported the range (6–8 weeks, 1–8 months) and six studies mentioned the mean duration (97.8 days). In the included studies, an ERC was generally performed after 4–12 weeks of the index procedure for assessment of BL and stent removal/exchange.
Additional interventions when BS failed
All patients with resolution of BL after first ERC underwent a repeat procedure for stent removal. In patients without BL resolution at the second ERC, they either underwent multiple subsequent ERCs with plastic or fully covered metal stents, surgeries (13 patients needed biliary reconstruction, 7 needed re-transplant and 10 patients underwent hepaticojejunostomy) or radiologically placed percutaneous trans-hepatic biliary drainage catheter. Only few studies mentioned the number or follow-up of surgical or radiological procedures when endoscopic therapy failed.
Complications of BS placement
Very few studies reported ERC-related complications that included: pancreatitis, bleeding, stent migration and stent occlusion resulting in cholangitis.
Nasobiliary tubes
Efficacy of NBT
A total of 179 patients with confirmed post-transplant BLs who were treated with NBT insertion were identified. The pooled efficacy for the resolution of post-LT related leaks treated with NBT was 87.15% (156/179). Among patients who had documented leak related to the choledochocholedochostomy, the efficacy of NBT was 58.33% (21/39). Efficacy of NBT among patients with documented T-tube related BL was 100% (78/78). Similarly, efficacy of NBT for other causes of BL (duct of Luschka and cystic duct stump) was also 100% (11/11). The site of BL was not mentioned in the rest of the patients (51 patients).
In cases where the type of transplant was specifically mentioned, NBT was effective in controlling the BL in 67.44% (29/43) of LDLT and 100% (22/22) of DDLT patients. All 22 patients in the DDLT group had leaks originating from the anastomosis. In the LDLT group, 34/43 patients had leaks associated with the anastomosis. In this subgroup, NBT were effective at managing post-operative leaks in 19/34 patients (55.8%).
Onset of BL and duration of NBT placement
The pooled mean onset of BL in the studies reporting “mean onset” was 109.5 days (range, 72–159 days) after transplant [4,25,36,39]. One study [12] reported the median onset of BLs (21 days), and one study [14] provided range for the onset of BL (2 days–4 months). The pooled mean duration the NBT stayed in place was 10.1 days in the seven studies that mentioned it, while one study mentioned the range for the duration of NBT insertion (3–14 days). The remaining studies did not mention how long an NBT stayed in place prior to its removal.
Additional interventions when NBT failed
In those patients with persistent leaks despite NBT placement (23 patients), specific treatments were mentioned in 12 patients. Five patients underwent surgical intervention for the repair of BL. Indication in these patients included persistent BL despite multiple endoscopic attempts. One patient underwent surgery due to perforation related with NBT placement. Five patients underwent endoscopic placement of plastic BSs for persistent leaks. One patient underwent percutaneous biliary drainage due to the presence of biliary stricture.
Complications of NBT placement
There were three ERC-related complications: one patient developed perforation and underwent surgery, while two patients had bleeding. There were no procedure-related deaths noted in the patients. No studies reported post-ERC pancreatitis.
Discussion
We report that biliary stenting and NBT placement are both effective treatment options for the management of posttransplant BLs. In our systematic review, the overall efficacy of BS was 82.43% compared to 87.15% efficacy of NBTs. Both BS and NBT were highly efficacious for T-tube and cystic duct stump leaks, but the efficacy appears lower for anastomotic leaks (Table 5). DaVee et al. [29] looked at the refractory BLs and noted that hepatic artery disease and portal vein thrombosis were significant contributing factors for the refractory leaks. Hepatic vascular compromise leads to stricturing of the bile duct, which in turns leads to the anastomotic leak. More studies looking at in-depth analysis of the other contributing factors underlying the low success rate for anastomotic BLs are needed. There are certain advantages and disadvantages of both treatment modalities. Disadvantages of NBTs include patient discomfort, easy dislodgement and extra nursing care for the management of the tube. Advantages include ability to do a cholangiogram on demand to look for evidence of healing prior to removal and no need for an additional endoscopic retrograde cholangiopancreatography (ERCP) for its removal. BS avoids the patient discomfort associated with NBT, but almost always needs repeat ERCP for the removal.
BL is one of the most common post-transplant biliary complications. ERC is considered the gold standard for the diagnosis of BL, and also provides opportunity for therapeutic intervention if there is any evidence of anastomotic stricture [40]. The primary aim of the endoscopic prosthesis insertion is to decompress the biliary system and divert the bile flow away from the leak site [34]. In this systematic review, the overall efficacy of BS for the resolution of post-transplant BLs was 82.43%, the efficacy was much lower for anastomotic leaks (69.23%) compared to T-tube related BLs (90.9%) and other causes (92.8%) of BL (cut surface, duct of Luschka and cystic duct stump). The overall efficacy of NBT was 87.15%. The resolution of leak appears to be higher for T-Tube related leaks (100%) and cystic duct stump/duct of Luschka leaks (100%) compared to leaks specifically related to the anastomosis (58.33%). We further noted a higher efficacy for NBT for resolving post-transplant BLs in patients who underwent DDLT (100%) compared to LDLT (67.44%). As there are no randomized controlled prospective trials comparing NBT to plastic stenting, there cannot be a direct comparative conclusion from this study regarding NBT vs. plastic stents.
Publication bias is an inherent issue of the systematic review. We only selected studies reporting outcomes in English, so language bias could also affect the results of our study. Most important limiting factor for our systematic review was that we included studies with significant heterogeneity in patient population, study design and outcomes. The included studies in this systematic review had multiple variabilities which could potentially interfere with the results and introduce bias, for example, some studies in our systematic review did not mention variables such as: site of BL, type of stent used, catheter size for the NBT, duration of follow-up, number of additional ERCs to achieve resolution of BL, complications from ERC and management of refractory BLs.
Despite all these factors, this is the best available evidence for the efficacy and safety of the BS and NBT in BL. The results from this review may be of great value for the management of BLs, and designing the future studies.
For future studies, we encourage implementation of protocols that include the patient characteristics, procedure details and outcomes in post-transplant BLs. These protocols should include detailed information about surgery performed (DDLT vs. LDLT, type of biliary reconstruction, use of splinting stents or T-tubes, catheter size for NBT, need for ductoplasty, intra-operative complications and hepatic artery injury); degree of BL (low-grade or high-grade); site of BL (anastomotic, T-tube or cut-surface); details of the endoscopic procedure (size endoprosthesis, duration of follow-up, complications) and salvage therapy if initial endoscopic treatment fails. It will be difficult to conduct a prospective randomized controlled trial comparing the efficacy of NBT to BS, as BS has largely replaced the NBT to become a standard treatment for post-transplant BLs in the last decade.
In conclusion, we report that both BS and NBTs are effective for treating post-transplant BL. Further, we note that the efficacy is greater for leaks not associated with the anastomosis. Both BS and NBT can be considered as an initial strategy for post OLT leaks, with serial cholangiography determining subsequent treatment approaches for persistent leaks. While CSEMS appear to be an emerging treatment option, the lack of comparative trials demonstrating clear efficacy is needed prior to widespread use.
Notes
Conflicts of Interest:The authors have no financial conflicts of interest.