Endoscopic Ultrasound-Guided Vascular Procedures: A Review
Article information
Abstract
Since the 1980s, endoscopic ultrasound has advanced from being purely diagnostic to an interventional modality. The gastrointestinal tract offers an exceptional window for assessing the vascular structures in the mediastinum and in the abdomen. This has led to a rapidly growing interest in endoscopic ultrasound-controlled vascular interventions as a minimally invasive alternative to surgical and radiological procedures.
INTRODUCTION
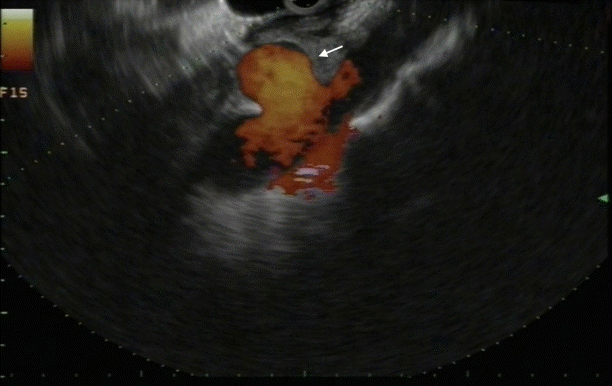
For the past 40 years, the role of endoscopic ultrasound (EUS) has changed from a diagnostic to a therapeutic one. Especially, with the development of the linear device in the 1990s, endosonographically controlled interventional techniques are becoming increasingly common. A rapidly growing area is endovascular therapy [1]. EUS provides real-time and high-resolution imaging of mediastinal and abdominal vascular structures from the gastrointestinal (GI) tract, creating an opportunity for precise vascular access and therapy. Major vessels such as the aorta, portal vein (PV), hepatic vein, mesenteric vessels, and atypical vascular shunts can be identified easily, and even smaller vessels such as the gastro-duodenal artery and renal arteries can all be recognized and confidently traced. With the help of EUS, vascular malformations can be diagnosed. Although rare, visceral pseudoaneurysms (Fig. 1 and Supplementary Video 1) [2] can occur as an adverse event of abdominal surgery or as a serious complication of pancreatitis.
EUS guidance offers an attractive minimally-invasive, alternate access route and opportunity for therapeutic intervention and is less invasive than surgery or radiology.
ESOPHAGEAL VARICEAL BLEEDING
Portal hypertension (PH) that can eventuate in variceal bleeding is a serious complication of liver cirrhosis. Variceal bleeding rate is estimated at 5%–15% and has a mortality rate of 15% [3]. Since the 1990s, the endoscopic band ligation is considered the procedure of choice for the treatment of acute bleeding and eradication of varices [4]. However, bleeding recurrence rates of 15%–65% are reported due to failure to treat the perforating veins and collateral vessels feeding the esophageal varices [5,6]. Here, EUS can be useful. Lahoti et al. proved in a small pilot study that EUS-guided sclerotherapy is effective by injecting sodium morrhuate into the perforating vessels [7]. A mean of 2.2 sessions was needed for the complete eradication of the varices, and after 15 months, no rebleeding or adverse events were reported [7]. A randomized controlled trial compared endoscopic sclerotherapy versus EUS-guided sclerotherapy. Both were safe, and there was no statistical difference in the number of sclerosants and number of sessions to achieve the eradication or recurrence of varices. However, recurrence was significantly associated with the presence of collateral vessels (p=0.003), which was higher in the endoscopic group (33.3% vs. 0%, p=0.004) at 22 months follow-up [7]. So far, no studies have compared standard endoscopic therapies versus the EUS-guided deployment of coils and cyanoacrylate injection in order to prove the benefit of obliterating periesophageal collateral veins and large perforating veins for the eradication of esophageal varices.
GASTRIC VARICES
The prevalence of gastric varices (GVs) on an index gastroscopy in patients with PH is 20%. Bleeding from GVs is less common than from esophageal varices, but they have a higher mortality rate (up to 20%) [7].
GVs are located in the deep submucosal layer and may appear similar to the prominent mucosal gastric folds. EUS helps with the detection of fundal varices by sixfold (Supplementary Video 2) [2,8].
The standard treatment for GVs is obliteration with cyanoacrylate [9]. Although rare, cyanoacrylate injection has been related to some adverse events, the most notably feared being systemic embolization in up to 2% to 3% of cases [10]. Delivery of cyanoacrylate under EUS guidance offers benefits such as the identification of the afferent feeder vessel, precise administration of glue into the varix lumen, and treatment control to confirm vessel obliteration with the assistance of Doppler. This potentially reduces the quantity of cyanoacrylate required to attain the obliteration of GV and thus decreases the risk of embolization [11]. Alternatively, vascular coils can be applied under EUS guidance [12]. The majority of coils used in interventional radiology can be loaded into a 19 gauge (0.035-inch coil) or a 22 gauge (0.018-inch coil) EUS-guided fine needle aspiration (EUS-FNA) needle (Supplementary Video 3) [2]. Deploying sufficient coils within the varix lumen was technically difficult, becoming a limitation of this technique. Combining coiling and cyanoacrylate is a novel hybrid approach that may offer the advantages of each [13,14], as demonstrated in Supplementary Video 4 [2]. The coils serve as a scaffold to retain the glue within the varix and hence reduce the risk of glue embolization. With this method, less cyanoacrylate is required to obtain the obliteration of the varices. In the largest study with 152 patients, Bhat et al. reached a complete obliteration rate of 93%, and recurrent bleeding occurred in 16% [14].
Recently, Saxena et al. reported a small case series of EUS-guided coil injection combined with the hemostatic absorbable gelatin sponge for the treatment of bleeding GVs [2]. This technique could theoretically minimize embolic complications, but further randomized studies are warranted.
RECTAL VARICES
Rectal varices occur in 44%–89% of patients with cirrhosis and PH, and they are a significant cause of lower GI bleeding [15,16]. Bleeding can happen from varices that are visible endoscopically but also from endoscopically inevident rectal varices [17]. In the latter, EUS can help to identify them. To date, there is a deficit of specific consensus with regard to the handling of rectal varices. Most used are sclerotherapy, cyanoacrylate glue, and, in particular, band ligation. However, even here, the recurrence rates of bleeding are up to 33% [18].
Different EUS-guided approaches have been described. Sharma et al. used EUS-guided glue injection as a feasible alternative in rectal varices bleeding [19]. Mukkada et al. illustrated in their case report EUS-guided coiling for a recurrent rectal varice bleeding [20]. Weilert et al. combined coils and glue in their series [21].
NON-VARICEAL GASTROINTESTINAL BLEEDING
Patients with non-variceal GI bleeding unsuccessfully treated with endoscopic means, or that are inapt for surgical, radiologic, or even endoscopic interventions, could benefit from EUS-guided hemostatic interventions too. In the management and treatment of Dieulafoy lesions, duodenal ulcers, Rouxen-Y gastric bypass, malignant gastric or esophageal lesions, pancreatic pseudoaneurysms, or gastrointestinal stromal tumors (GISTs), high success rates have been reported in several case series and studies [22-24]. EUS-guided angiotherapy was successfully applied in an elderly patient with a recurrent GI bleeding of a gastric GIST refractory to endoscopic over-thescope placement (Fig. 2 and Supplementary Video 5) [2].
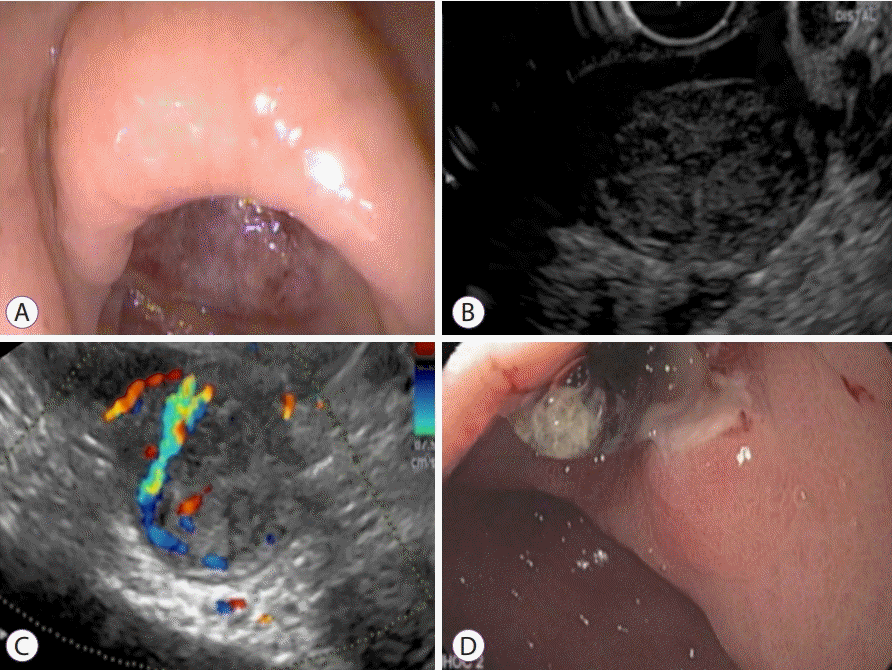
Gastrointestinal stromal tumor (GIST). (A) Endoscopic image of a large ulcer in the antrum of the stomach. (B) Endoscopic ultrasound demonstrates a hypoechoic mass arising from the muscularis propria at the level of the antral ulcer, suggestive of a GIST. (C) Color Doppler of the GIST reveals a vessel feeding into the tumor, the likely cause of recurrent bleeding. (D) Endoscopic appearance of the ulcer post endoscopic ultrasound-guided injection of cyanoacrylate into the feeding vessel. The ulcer has reduced considerably in size. Reproduced from Saxena et al.[2] under the permission of Spring Publishing.
So far, there are no comparative studies for the evaluation of EUS-guided interventions for the management of non-variceal refractory GI bleeding, but the feasibility and safety demonstrated in these reports are encouraging.
ENDOSCOPIC ULTRASOUND-GUIDED PORTAL VENOUS ANGIOGRAPHY AND PORTAL PRESSURE GRADIENT MEASUREMENT
Measurement of PH is an important diagnostic tool in order to determine the stage, possible operability, and evolvement of cirrhosis in individual patients [25]. Commonly, the definition of PH is an indirect measurement of the hepatic venous pressure gradient and is done invasively via jugular or femoral vein access. It is technically demanding and correlates badly with the real portal pressure in patients with presinusoidal PH [26].
Endosonographically, the PV can be identified from the duodenum and the stomach, always close to the transducer tip, making this an ideal target for vascular access. The first EUS-guided PV punctures were conducted on porcine models in 2004 [27]. All the following EUS-guided portal venous angiography studies revealed no evidence of adverse events such as tissue damage, bleeding, or infections [28-30]. The first human prospective study of portal pressure gradient (PPG) measurement in human patients with cirrhosis was reported by Huang and colleagues in 2017 using a 25 G FNA and a novel manometer (Cook Medical, Bloomington, IN, USA) [31]. They achieved a 100% procedural success rate without any complications. PPG had a perfect correlation with the presence of varices, portal hypertensive gastropathy, and thrombocytopenia. This proved the feasibility, efficacy, and accuracy of EUS-guided portal pressure measurement. Furthermore, it showed the potential of this new technique, giving for the first time the accurate measurements of presinusoidal PH, as hepatic vein pressure gradient is not reliable in this setting.
ENDOSCOPIC ULTRASOUND-GUIDED CREATION OF AN INTRAHEPATIC PORTOSYSTEMIC SHUNT
Transjugular intrahepatic portosystemic shunt (TIPS) is an effective treatment for PH and its associated problems. In 2009, Buscaglia et al. reported the first EUS-guided creation of an intrahepatic portosystemic shunt in a pig [32]. In 2017, a group from Boston successfully deployed a EUS-guided lumen-apposing metal stent for the creation of a TIPS in five porcine models [33]. However, whether the EUS-guided creation of an intrahepatic portosystemic shunt may become a useful alternative to conventional TIPS is questionable [34].
ENDOSCOPIC ULTRASOUND-GUIDED FINE NEEDLE ASPIRATION OF PORTAL VEIN THROMBOSIS FOR THE STAGING OF HEPATOCELLULAR CARCINOMA
In hepatocellular carcinoma (HCC), PV thrombosis is a common problem due to venous stasis and endothelial injury or due to the invasion of the HCC into vessels. If the PV is invaded, liver transplantation or curative resection is often contraindicated [35]. As a result, a definite diagnosis or exclusion of tumor thrombus becomes critically important and thus a tissue diagnosis is often warranted. Transabdominal ultrasound puncture is limited by the potential of sample contamination and the risk of serious biliary or vascular injury. The safety and suitability of EUS-FNA of the PV thrombus has been described by a number of case series [36,37].
ENDOSCOPIC ULTRASOUNDGUIDED PORTAL VEIN SAMPLING OF CIRCULATING TUMOR CELLS IN PANCREATOBILIARY MALIGNANCIES
Circulating tumor cells (CTCs) shed from the original tumor, and then dissemination to distant sites occurs mainly via the hematogenous systems while preserving its similar characteristics. The CTCs have been promising new biomarkers in solid tumors, especially in pancreatic cancer—more than 140 articles have been published in this field in the last twenty years (2000–2020). However, with an estimation of one tumor cell per one billion circulating blood cells, peripheral CTCs are extremely rare [38]. Ting et al. showed that CTCs were found in PV blood samples in 58% of patients undergoing a Whipple procedure for pancreatic cancer [38]. EUS-acquired CTC samples of the PV could be utilized for molecular testing, clinical predication of possible future hepatic metastasis, and drug sensitivity analyses [39].
ENDOSCOPIC ULTRASOUND-GUIDED PORTAL INJECTION CHEMOTHERAPY USING DRUG-ELUTING MICROBEADS
Nowadays, treatment for diffuse hepatic metastases is limited to systemic palliative chemotherapy. Novel approaches have aimed to administer chemotherapy agents in the PV with the intention of reducing systemic side effects and the likelihood of ischemic biliary strictures. EUS-guided portal injection of chemotherapy (EPIC) has been achieved successfully in pigs [40]. EPIC showed increased hepatic concentrations of the chemotherapeutic agent (irinotecan) by 60% and diminished systemic levels by 24% to 32% compared with systemic administration. This approach may prove to be advantageous in a wide variety of clinical conditions and raises hope as a novel treatment for hepatic metastases.
ENDOSCOPIC ULTRASOUND ACCESS TO THE HEART
Given that the heart and pulmonary trunk are in proximity to the esophagus, transesophageal EUS-guided access to the heart has been conducted safely in animal models and humans [41,42]. Moreover, two Spanish groups reported the case studies of the EUS-FNAs of atrial and pericardial tumors [43,44].
CONCLUSIONS
In many consensus guidelines and recommendations papers, the EUS-guided vascular approaches are only sparsely described. However, as this review demonstrates, EUS-guided interventions are secure and feasible techniques. In fact, current studies and data have often proved the superiority of EUS-guided endovascular therapy over the endoscopic technique that urges the implementation of EUS-guided therapy in therapeutical algorithms and guidelines.
Given the wide availability of EUS, with already many applications available and further work ahead, the area of EUS-specific vascular access technologies will continue to expand.
Notes
Conflicts of Interest: Payal Saxena is a consultant for Boston Scientific. The other authors have no financial conflicts of interest.
Author Contributions
Conceptualization: Dominic A. Staudenmann
Formal analysis: DAS
Investigation: DAS
Project administration: DAS
Supervision: Arthur J. Kaffes, Payal Saxena
Validation: DAS, AJK, PS
Visualization: DAS
Writing-original draft: DAS
Writing-review&editing: AJK, PS
Supplementary Materials
Reproduced from Saxena et al.[2] under the permission of Spring Publishing.
Video 1.
Visceral pseudoaneurysm. Endoscopic ultrasound video of a celiac artery aneurysm (https://doi.org/10.5946/ce.2020.222.v001).
Video 2.
Gastric varices. Endoscopic ultrasound identification of fundal varices (https://doi.org/10.5946/ce.2020.222.v002).
Video 3.
Loading coils. Coils being loaded into an endoscopic ultrasound-guided fine needle aspiration needle (https://doi.org/10.5946/ce.2020.222.v003).
Video 4.
Treatment of large gastric varices. Endoscopic ultrasound-guided combined cyanoacrylate injection and coiling (https://doi.org/10.5946/ce.2020.222.v004).
Video 5.
Gastrointestinal stromal tumor. Endoscopic ultrasound-guided cyanoacrylate injection of a feeding vessel of a gastrointestinal stromal tumor causing gastrointestinal bleeding (https://doi.org/10.5946/ce.2020.222.v005).