Radiation Proctitis and Management Strategies
Article information
Abstract
Radiotherapy (RT) is a treatment modality that uses high-energy rays or radioactive agents to generate ionizing radiation against rapidly dividing cells. The main objective of using radiation in cancer therapy is to impair or halt the division of the tumor cells. Over the past few decades, advancements in technology, the introduction of newer methods of RT, and a better understanding of the pathophysiology of cancers have enabled physicians to deliver doses of radiation that match the exact dimensions of the tumor for greater efficacy, with minimal exposure of the surrounding tissues. However, RT has numerous complications, the most common being radiation proctitis (RP). It is characterized by damage to the rectal epithelium by secondary ionizing radiation. Based on the onset of signs and symptoms, post-radiotherapy RP can be classified as acute or chronic, each with varying levels of severity and complication rates. The treatment options available for RP are limited, with most of the data on treatment available from case reports or small studies. Here, we describe the types of RT used in modern-day medicine and radiation-mediated tissue injury. We have primarily focused on the classification, epidemiology, pathogenesis, clinical features, treatment strategies, complications, and prognosis of RP.
INTRODUCTION
Radiation therapy (RT) is a treatment modality based on the utilization of high-energy rays or radioactive agents to generate ionizing radiation against rapidly dividing cells. The main objective of RT for the treatment of cancer is to impair or halt tumor cell division [1]. The discovery of X-rays in 1895 by Wilhelm Conrad Röntgen sparked great interest within the scientific community to study the physiological effects of radiation and utilize it in the treatment of cancers [2-4]. Over the past few decades, with advancement in technology, the introduction of newer methods of RT, and a better understanding of the pathophysiology of cancers has enabled physicians to deliver doses of radiation that match the exact dimensions of the tumor for greater efficacy, with minimal exposure of surrounding tissues. However, radiation proctitis (RP) is one of the most common complications of radiation to the pelvis. It is characterized by damage to the rectal epithelium by secondary ionizing radiation therapy. Based on the time from initiation of RT to the development of the presenting signs and symptoms, RP can be classified into two subtypes, acute and chronic. Acute RP is usually self-limiting with minimal complications; however, chronic RP tends to be more severe and is often associated with numerous complications. The diagnosis can be established via direct visualization through rigid or flexible sigmoidoscopy and microscopic evaluation; however, the procedure should always be performed by a highly experienced gastroenterologist or colorectal surgeon because the friable mucosa is highly prone to perforation. The treatment options available for RP are limited, with most of the data on treatment strategies available from case reports or small studies. In this review of the literature, we describe the types of RT and their utilization in modern-day medicine. We have also discussed the classification, epidemiology, pathogenesis, clinical manifestations, tools to establish the diagnosis, treatment strategies, complications, and prognosis of RP. Furthermore, we highlight the need for additional large, multicenter prospective studies to determine the burden of RP, to better understand the pathogenic mechanisms, identify additional risk factors that increase the incidence of RP, and compare different treatment modalities.
HISTORY OF RADIOTHERAPY
RT is a treatment modality based on the utilization of high-energy rays or radioactive agents, with the intent to impair or halt the division of tumor cells [1]. Before the advent of ionizing radiation, there were limited therapeutic options available for the management of cancers; however, the discovery of X-rays in 1895 by Wilhelm Conrad Röntgen revolutionized the treatment of cancers [2]. After a year of its discovery, and before its physiological effect on tissues were completely understood, X-rays were already being used in treating a patient with breast cancer by Emil Herman Grubbe [3]. Within the scientific community, the discovery of ionizing radiation sparked an immense interest towards studying the phenomenon of radioactivity, and determining its physiological effects on tissues and organ systems while also exploring other potential natural sources of radiation. Over the last century, RT alone, or combined with other treatment modalities, has served an essential role in managing numerous cancers [5].
EXTENT OF UTILIZATION OF RADIOTHERAPY
From a treatment perspective, literature reports that RT is an important curative treatment modality for locoregional tumors, and is used in at least two-thirds of cancer treatment regimens, particularly in western countries [6]. It is important to glance at the statistics regarding patients with cancer to understand the extent of utilization of RT in clinical practice. As of January 2019, more than 16.9 million Americans (8.1 million males and 8.8 million females) were alive with a history of cancer [7,8]. This number has been projected to increase to 22.1 million by January 2030 [8]. Furthermore, the percentage of cancer survivors who will receive RT has been projected to increase from 24% in 2000 to 29% by the end of 2020, and it is further estimated to decline slightly to 28% by 2030 [9]. This makes up a sizable population of patients who have received or will receive RT. Additionally, over the past few decades, with substantial technological advancement, the introduction of newer methods for RT, and a better understanding of the pathophysiology of cancers on a molecular level, has enabled physicians to deliver doses of radiation that match the exact dimensions of the tumor [10]. This prevents excessive and unnecessary radiation exposure to normal tissues, thereby limiting tissue injury and increasing the efficacy of RT [10].
TYPES OF RADIOTHERAPY
RT to the pelvis is an important component of the treatment regimens available for pelvic cancers. It can be administered as adjuvant or neoadjuvant RT. Higher doses of radiation may be required for treating rectal cancers as they usually show resistance to low-dose RT [11]. The modes through which RT can be delivered include external beam radiation and brachytherapy [11-16].
External beam radiation
It is administered via an external photon generator, with various sources, including gamma rays, electron beams, and X-rays, with a four-beam approach. External beam radiation usually results in significant exposure to the surrounding tissues. However, newer methods of external beam radiation, such as three-dimensional conformal radiation therapy and intensity-modulated radiation therapy, allow for the utilization of higher doses of radiation to targeted tissues with significantly less exposure to normal tissues. This leads to significantly less radiation damage to normal tissues.
Brachytherapy
It consists of two methods of delivering radiation in a highly site-specific manner, intending to minimize damage to the surrounding tissues. The most common method is implanting radioactive pellets, usually iodine 125 or palladium 103, into targeted tissues, with a gradual release over time. An alternative method involves the use of hollow catheters progressively filled with increasing amounts of radioactive pellets over time. Compared with external beam radiation, brachytherapy has shown decreased rates of both acute (6% vs. 43%) and chronic (2% vs. 21%) complications.
However, as with any treatment modality, there are side effects and complications. With the use of higher doses of radiation for the treatment of cancers, RP is one of the most common complications [17]. Physicians are beginning to encounter more cases of RP due to the increasing number of cancer survivors and the fact that most of these patients have undergone RT as a part of their treatment regimens.
RADIATION PROCTITIS AND ITS CLASSIFICATION
RP is often a misleading term because it implies a continuous chronic inflammatory process in the rectum; however, it continues to be widely used in the literature. Radiation proctopathy is the correct terminology for radiation-induced damage to the rectum. It is characterized by damage to the rectal epithelium secondary to ionizing radiation therapy. The degree of RP is variable and is dependent on both radiation and patient-associated risk factors [18]. Based on the time from initiation of RT to the development of the presenting signs and symptoms, it can be classified into two main subtypes.
Acute radiation proctitis
It is characterized by the involvement of only the superficial mucosa of the rectum [19]. It is a highly dose-dependent phenomenon and can occur almost immediately or within 3 months of initiation of RT [20].
Chronic radiation proctitis
It is a more complex clinical entity characterized by the involvement of the full thickness of the rectal mucosa along with fibrosis and obliterative arteritis [21]. It usually has a delayed onset [22,23]. The first symptoms may often be seen at 9–14 months following radiation exposure but can occur at any time post-radiation for up to 30 years [22,23].
EPIDEMIOLOGY OF RADIATION PROCTITIS
Due to variability and lack of consensus among physicians in the definition and reporting of RP, it has been challenging to establish the exact incidence rate. In literature, the incidence rate of chronic RP is estimated to range from 2%–20% [21]. The incidence of RP in patients treated with brachytherapy alone is estimated to range from 8%-3% vs. 21% when used in combination with other therapies [24]. Literature also reports a variable prevalence of chronic RP, ranging from as low as 5%–20% to as high as 47% in patients treated with RT for cervical cancers [25,26]. Furthermore, a retrospective study by Willet revealed that patients with inflammatory bowel disease (IBD) are at increased risk of developing RP along with other complications from external beam radiation, and the reported incidence rate of the complications was found to be 46% at 32 months [27]. Additionally, malignancies associated with human immunodeficiency virus (HIV)/acquired immunodeficiency syndrome (AIDS), such as cervical cancer, anal cancer, and lymphomas, are on the rise and may require RT directed towards the rectum [11]. No comprehensive data currently exists on the incidence and prevalence of RP in these patients; however, studies have reported increased toxicity and decreased tolerance to radiation in patients with HIV/AIDS and low CD4 counts [28,29]. Hence, we strongly advocate for additional large prospective, multicenter studies to investigate the exact epidemiology of RP and also to determine the radiation dose adjustments required in proinflammatory and immunocompromised states.
RISK FACTORS FOR RADIATION-MEDIATED TISSUE INJURY
Several risk factors have been identified and associated with gastrointestinal (GI) injury secondary to RT. They can be subdivided into patient-associated or radiation-associated risk factors.
Radiation-associated risk factors
GI injury secondary to RT is highly contingent on the modality through which radiation is delivered, the dose of radiation, the total duration of radiation, the area of exposure, and the intensity of radiation [18,30]. A radiation dose of <45 Gy is associated with minimal long-term effects, whereas more complications have been reported in patients receiving doses between 45 Gy and 70 Gy, although these are of lesser intensity compared with higher doses of radiation [31,32]. Patients receiving doses >70 Gy sustain longstanding injury to surrounding tissues [31,32]. The mode of delivery of radiation also plays a considerable role, and literature reports fewer complications in patients receiving brachytherapy as compared to external beam radiation [11]. Additionally, combinations of chemotherapy with RT have also been shown to increase the risk of intestinal toxicity [33,34].
Patient-associated risk factors
Certain patient-specific characteristics have also been linked to an increased risk of intestinal radiation injury. Conditions like hypertension, diabetes mellitus, atherosclerosis, and smoking are presumed to increase intestinal ischemia and vascular injury post radiation and may also hinder tissue repair [11]. Furthermore, patients aged <60 years have also been reported to have an increased risk of radiation injury [35]. However, it is unclear whether these patients demonstrate an increased radiation-induced inflammatory response or if they are more likely to notice and report clinical symptoms compared with an older demographic [35]. As discussed earlier, patients with IBD have a greater risk of radiation-induced damage to the GI tract [27]. Literature also reports that patients with collagen vascular diseases such as scleroderma, systemic lupus erythematosus, rheumatoid arthritis, and polymyositis are more prone to radiation-induced injury to the GI tract because of their low radiation tolerance [36,37].
PATHOGENESIS OF RADIATION PROCTITIS
The pathogenesis of RP is complex and yet to be fully understood. To better comprehend the pathogenic process, it is divided into two sections.
Mechanism of radiation-mediated tissue damage
It is essential to first understand the mechanism by which ionizing radiation causes damage to tissues at the cellular level. The damage to tissues secondary to RT is widespread; however, in essence, both direct and indirect mechanisms of tissue damage via radiation target cellular deoxyribonucleic acid (DNA), thereby inhibiting transcription and preventing cellular replication [38]. Through the direct mechanism, ionizing radiation directly damages the DNA or cell membrane. It can induce double-stranded DNA breaks, cause inter- and intra-strand cross-linkages, or mutations of the DNA and can compromise the rigidity of the phospholipid bilayer and the electrical gradient of the cell membrane [39]. The indirect mechanism involves the generation of free radicals from the ionization of water molecules, leading to oxidative stress injuries [40]. However, as this radiation-induced damage is in process, DNA repair mechanisms are activated to fix the DNA strands. At low doses of radiation, the repair mechanisms are successful and lead to the resolution of DNA injuries [41]. However, at higher doses, the ionizing radiation can overwhelm the DNA repair mechanism, leading to apoptosis of the cell or inhibition of mitosis [39]. It is also important to note that cells with high rates of mitosis, such as stem cells and cancers, are most affected by RT.
Tissue response to radiation and pathogenesis of radiation proctitis
The mucosa of the GI tract is highly proliferative. As per the literature, enterocytes have the highest turnover rate of any fixed cell in the body [42]. Hence, the rapidly dividing mucosal stem cells present within the crypts of Lieberkühn are highly susceptible to radiation injury. Initiation of RP occurs via radiation-induced damage to the mucosa, followed by late indolent connective tissue growth and remodeling, and subsequently tissue response to the ongoing ischemia [43]. Damage to the rapidly dividing intestinal crypt stem cells in the radiation field leads to their depletion, resulting in crypt involution, mucosal injury, and exposure of the underlying lamina propria to luminal bacteria [20]. An acute inflammatory response may be generated after exposure to the bacteria, often involving T-lymphocytes, macrophages, and neutrophils [20]. Additional damage to the extracellular matrix, mucosa, and submucosa of the bowel wall may be secondary to the production of enzymes and reactive oxygen species [44,45]. On gross visualization, early radiation injury will show edema, mucosal hyperemia, and ulceration of the tissue. Histological changes of early radiation damage may be seen within a few hours of RT, followed by infiltration of leukocytes and crypt abscess formation in 2–4 weeks [39]. Subsequently, progressive occlusive vasculitis with foam cell invasion of the intima and hyaline thickening of the media of arterioles may be seen, which contributes to obliterative endarteritis, leading to full-thickness ischemia of the bowel wall [20,46]. After cessation of RT, the acute inflammatory process subsides, and intestinal crypt cells start to regenerate. Animal models suggest that migration and engraftment of stem cells from the bone marrow may be responsible for the repair of damaged crypts [47,48]. However, in some patients, for reasons unknown, the inflammatory process may exaggerate, leading to ulceration of the mucosa followed by fibrosis and the development of chronic inflammatory changes [20]. Additionally, radiation can directly damage the vascular and endothelial cells leading to full-thickness bowel ischemia; hence, it plays a major role in the pathogenesis of RP [49,50].
CLINICAL MANIFESTATIONS OF RADIATION PROCTITIS
The clinical presentation varies for both acute and chronic RP. Not only does the time of the onset of symptoms from the initiation of RT vary between the two, but patients with chronic RP have more severe symptoms. Patients with acute RP may present with diarrhea, nausea, cramps, tenesmus, urgency, mucus discharge, and minor bleeding in approximately 20% of the cases, which may interrupt treatment [51]. Patients with chronic RP may have all the symptoms of acute RP and additional symptoms secondary to full-thickness bowel ischemia and fibrotic changes, such as features of malabsorption, severe bleeding, the formation of strictures, perforations, fistulas, and bowel obstruction [52]. Involvement of the anal sphincter may lead to fecal incontinence [18]. Conditions that affect microvascular circulation, such as diabetes and peripheral arterial disease, also increase the risk of chronic RP [31,32]. For patients who present with symptoms of chronic RP several years after RT, it is essential to rule out recurrence of malignancy. Other conditions sharing common symptoms with chronic RP, such as parasitic infections, Clostridium difficile infection, sexually transmitted diseases associated with proctitis such as gonorrhea, herpes simplex virus infection, cytomegalovirus infection, medication-induced colitis, and chronic bowel ischemia, should also be ruled out. Results of physical examination of patients with RP may be unremarkable. However, in some patients, a digital examination may reveal anorectal stenosis, while in others, the examination may be extremely painful due to extensive involvement of the GI tract [18]. To better understand and assess the extent of rectal toxicity associated with RT, numerous grading criteria have been developed. A commonly used classification system is the Radiation Therapy Oncology Group (RTOG) and the European Organization for Research and Treatment of Cancer (EORTC) scoring system [53]. The RTOG scoring system describes acute toxicity, whereas the EORTC scoring system classifies chronic toxicity. However, in patients with chronic toxicity, their accuracy and validity have been challenged [54]. Furthermore, the National Cancer Institute of the National Institutes of Health have published standardized definitions for adverse events, known as the Common Terminology Criteria for Adverse Events (CTCAE) grading system to describe the severity of toxicity in patients receiving cancer therapy [55]. For patients with proctitis, the CTCAE grading system is summarized in Table 1 [55].
DIAGNOSING RADIATION PROCTITIS
The diagnosis of RP can be established via direct visualization through rigid or flexible sigmoidoscopy. The procedure should always be performed by an experienced gastroenterologist or colorectal surgeon, because the friable mucosa is highly prone to perforation. In patients with acute RP, gross visualization of the rectal mucosa may reveal it to be beefy red, edematous, with ulceration or sloughing (Fig. 1) [11,56,57]. A microscopic evaluation may reveal distortion of the microvilli, hyperemia, edema, and ulceration [11,56]. A colonoscopic biopsy is not recommended in acute RP due to the increased risk of bleeding and fistula formation [11]. In cases of chronic RP, grossly, the mucosa may appear pale, noncompliant with telangiectasias, and may also have strictures, ulcerations, fistulas, or heavy bleeding (Fig. 2) [21,58]. Microscopically, there may be intimal fibrosis with focal destruction or distortion of the small arteries or arterioles [21]. In few patients with chronic RP, multiple areas of strictures may be noted, making it challenging to distinguish it from a malignancy [59]. A computed tomography scan can be used to rule out recurrent malignancy, and magnetic resonance imaging should be performed in patients with suspected fistulas. Barium or water-soluble enema studies may also be performed, which can reveal strictures, obstruction, shortening, and narrowing of the rectosigmoid area with loss of normal curvature [18].
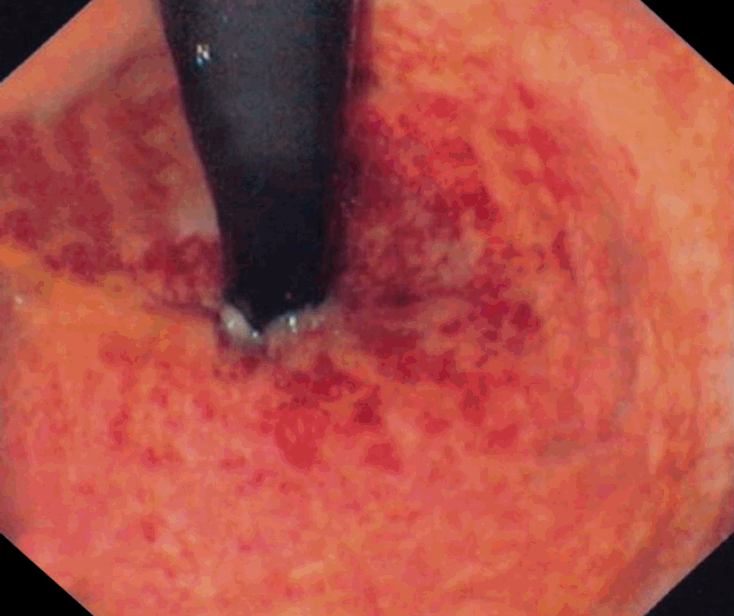
Endoscopic findings of acute radiation proctitis. Rectal mucosa shows erythema, petechiae and bleeding. Adapted from the article of Katsanos KH et al. Ann Gastroenterol 2012;25:65, with permission.
TREATMENT STRATEGIES FOR RADIATION PROCTITIS
Unfortunately, there are no large, multicenter, randomized clinical trials evaluating the treatment options for RP; hence, most of the data on treatment have been obtained from case reports and small clinical trials [60,61]. The treatment algorithm for RP is summarized in Fig. 3 [62].
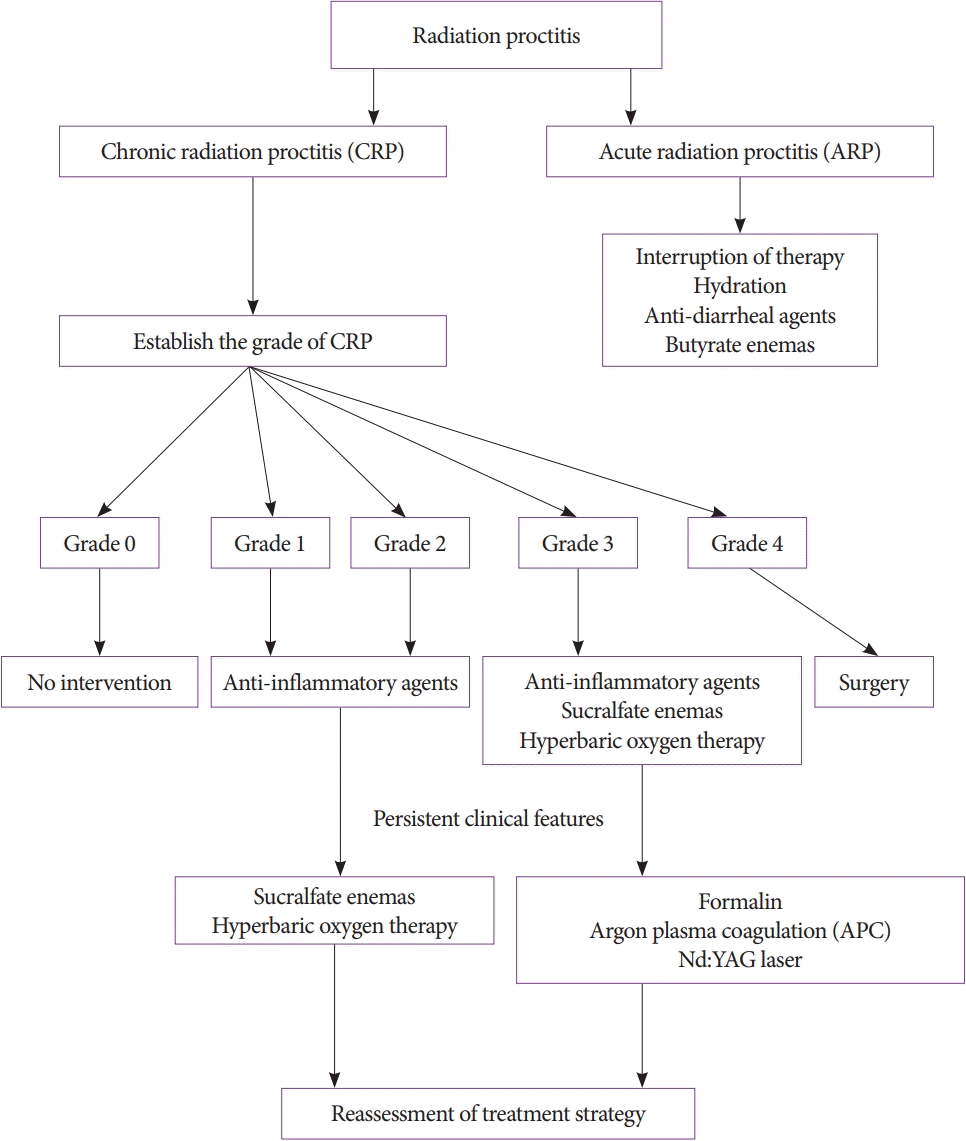
Treatment algorithm for radiation proctitis in accordance with the American Society of Colon and Rectal Surgeons.
Acute RP is often self-limiting and does not increase the risk of developing chronic RP. Approximately 20% of patients undergoing external beam radiation may require a short interruption of therapy. Treatment of patients with acute RP is usually supportive, consisting of hydration, anti-diarrheal agents, and butyrate enemas to promote tissue healing [63].
The American Society of Colon and Rectal Surgeons (ASCRS) has proposed clinical practice guidelines for treating chronic RP. The treatment options available for chronic RP are discussed in the following sections [62].
Medical management
Formalin (formaldehyde 4%–10%) has been used in treating chronic RP over the past few decades [64]. A formalin-soaked gauze can be applied directly to the mucosa via rigid proctoscopy under light sedation, and it brings about chemical cauterization of the ulcers and telangiectasias, thereby achieving hemostasis [65]. Literature reports that after the first application of formalin, the symptoms were resolved in 50% of the patients, and most patients required an average of only two treatment sessions [62]. Sucralfate retention enemas have been found to be moderately effective and may also be used for rectal bleeding from chronic RP [62]. According to the latest guidelines by ASCRS, short-chain fatty acid enemas are not useful; hence they are not recommended for chronic RP [62,66]. Similarly, other treatment modalities, such as ozone therapy, mesalamine, and metronidazole, have not proven to be efficacious and therefore are not recommended by ASCRS. The medical management for chronic RP is summarized in Table 2 [62].
Endoscopic management
ASCRS recommends using argon beam plasma coagulation (APC) as it is a safe and effective modality for treating chronic RP [62]. APC uses an ionized gas, argon, to transmit high-frequency energy to tissues. The literature reports the need for a median of two APC sessions (range: 1–5 sessions) to control rectal hemorrhage, thereby resulting in cessation or a substantial decrease in bleeding in 79%–100% of the patients [62,67]. Patients who develop rectal ulceration post-APC therapy can be treated with mesalamine suppositories and/or glucocorticoid enemas [68]. A complete bowel lavage should be performed prior to APC therapy to evacuate combustible gas and prevent bowel explosion with perforation [69]. Neodymium-doped yttrium aluminum garnet laser can also be used to coagulate the bleeding vessel in these patients [70]. It has shown symptomatic improvement in 78% of the patients; however, it is expensive and not widely available [70]. The American Society for Gastrointestinal Endoscopy has also proposed guidelines for using endoscopic therapy, such as electrocoagulation, heater probe, radiofrequency ablation, and cryoablation, for chronic RP [71]. However, additional studies are still needed to determine their safety and efficacy. Therefore, they may be used on a case-by-case basis and have received a Grade 1C recommendation for use by the ASCRS [62].
Hyperbaric oxygen therapy
It has emerged as an effective treatment modality for non-healing wounds secondary to chronic RP. The theorized mechanism of action of hyperbaric oxygen therapy (HBOT) is based on improved tissue oxygenation, possible angiogenesis, and antibacterial effects [62]. Multiple large studies have proven the substantial benefit of HBOT in patients with chronic RP [72,73]. However, it is expensive, and requires specialized equipment and personnel, and several weeks of therapy; hence, it is not widely available.
Surgical intervention
Surgery is reserved for patients who fail to show improvement in their symptoms following medical or endoscopic management or in patients with severe complications of RP, such as strictures leading to bowel obstruction, perforations, or fistulas [62]. As per the literature, approximately 10% of the patients with RP may require surgical intervention [74]. In severe cases, proctectomy may become necessary; however, there is no universally approved first-line approach for the surgery [75]. Resection of the rectum remains highly controversial as it is challenging to perform a safe anastomosis in the radiation-injured tissue and carries a high risk of anastomotic leakage and mortality from postoperative peritonitis [76]. Diversion of the bowel segment in the form of ileostomy or colostomy has demonstrated significant improvement in the quality of life without further surgical interventions [77]. It reduces bacterial contamination and irritation injury to the irradiated tissues by the fecal stream [78]. This decreases rectal bleeding and promotes healing of the tissue [78]. It may also accelerate the course of fibrosis of the bowel mucosa, thereby preventing the conversion of deep ulcerations into fistulas [78]. Although ostomy may improve the overall quality of life, it has its own set of physical, psychological, and social issues along with complications [79]. Complications vary with the type of ostomy and can be classified based on the time of onset.
Very early complications (days after the procedure)
It is often related to technical issues with the procedure, requiring correction [80].
Early complications (within 3 months of the procedure)
It is often related to suboptimal site selection for ostomy, or patient-related factors, such as age, obesity (higher body mass index), poor nutritional status, diabetes mellitus, higher American Society of Anesthesiologists class, smoking, procedure setting (emergency vs. elective), and underlying malignancy [81,82]. Early complications include stomal bleeding, ischemia, necrosis or retraction, or mucocutaneous separation.
Late complications (more than 3 months after the procedure)
A temporary ostomy is usually reversed in 3 months; hence, late complications are described only for a permanent procedure [83]. Risk factors implicated in the development of late complications include inadequate mobilization of the bowel with a resultant height of stoma <10 mm, inappropriate size of the aperture, duration of the stoma, increased intra-abdominal pressure secondary to conditions like obesity or chronic obstructive pulmonary disease, and emergency surgery [82,84,85]. The most frequently encountered late complications include parastomal hernia, stomal prolapse, and stomal stenosis [81].
Skin complications (any time after the procedure)
They are frequently encountered with an ostomy and are caused due to the breakdown of the skin around the ostomy site [81]. The severity can vary from minor skin trauma, dermatitis, ulceration, and pyoderma gangrenosum, which is commonly seen in patients with Crohn’s disease [81].
PROGNOSIS OF RADIATION PROCTITIS
The prognosis of RP depends on the type and severity of the radiation-induced injury. In the literature, a significant decrease in the health-related quality of life in up to 30% of patients with severe disease have been reported [86]. On the other hand, acute RP is often self-limiting with minimal complications. It is also worth noting that patients with RP are at risk of developing secondary malignancies, the majority of which are colon and rectal cancers [87].
CONCLUSIONS
RP, also referred to as radiation proctopathy, is characterized by damage to the rectal epithelium secondary to ionizing radiation therapy. Based on the initiation of symptoms after RT, it can be classified as acute or chronic RP. Patients with acute RP may present with diarrhea, nausea, cramps, tenesmus, urgency, mucus discharge, and minor bleeding. Chronic RP patients may have all the symptoms of acute RP; however, they tend to be severe, with additional manifestations such as malabsorption, severe bleeding, strictures, perforations, fistulas, fecal incontinence, and bowel obstruction. The diagnosis of RP can be established via direct visualization through rigid or flexible sigmoidoscopy. Acute RP is often self-limiting, and approximately 20% of the patients may require interruption of therapy. However, patients with chronic RP may require extensive therapy based on their grades. Complications of chronic RP include bowel perforation, colitis, severe bleeding, fistula formation, and malignancy secondary to radiation. The overall prognosis of RP depends on the type and severity of the radiation-induced injury.
Notes
Conflicts of Interest: The authors have no potential conflicts of interest.
Funding: None.
Author Contributions
Conceptualization: Dushyant Singh Dahiya, Asim, Kichloo, Faiz Tuma, Michael Stanley Albosta, Farah Wani
Data curation: DSD, AK, FT, MSA, FW
Methodology: DSD, AK, FT, MSA, FW
Writing-original draft: DSD, AK, FT, MSA, FW
Writing-review&editing: DSD, AK, FT, MSA, FW



