Preventive effect of tacrolimus on patients with post-endoscopic retrograde cholangiopancreatography pancreatitis
Article information
Abstract
Background/Aims
In patients undergoing endoscopic retrograde cholangiopancreatography (ERCP), calcineurin activates zymogen, which results in pancreatitis. In this study, we aimed to determine the efficacy of tacrolimus, a calcineurin inhibitor, in preventing post-ERCP pancreatitis (PEP).
Methods
This was a prospective pilot study in which patients who underwent ERCP received tacrolimus (4 mg in two divided doses); this was the Tac group. A contemporaneous cohort of patients was included as a control group. All patients were followed-up for PEP. PEP was characterized by worsening abdominal pain with an acute onset, elevated pancreatic enzymes, and a duration of hospital stay of more than 48 hours. Serum tacrolimus levels were measured immediately before the procedure in the Tac group.
Results
There were no differences in the baseline characteristics between the Tac group (n=48) and the control group (n=51). Only four out of 48 patients (8.3%) had PEP in the Tac group compared to eight out of 51 patients (15.7%) who had PEP in the control group. The mean trough tacrolimus level in patients who developed PEP was significantly lower (p<0.05).
Conclusions
Oral tacrolimus at a cumulative dose of 4 mg safely prevents PEP. Further randomized controlled studies are warranted to establish the role of tacrolimus in this context.
INTRODUCTION
Endoscopic retrograde cholangiopancreatography (ERCP) is a common procedure performed for the nonoperative management of a variety of biliopancreatic disorders. Despite vast advances in techniques, relative safety, and clinical efficacy, post-ERCP pancreatitis (PEP) remains one of the most serious and frequent complications of this procedure, with an incidence ranging from 2%–10% depending on the patient population and outcome definitions.1-3 Although majority of PEP cases are mild, a significant number of patients may develop severe pancreatitis that necessitates management in the intensive care unit and prolonged hospital stay.1,4
Since PEP continues to be a serious problem, there is an ongoing interest in identifying reliable prophylactic measures for PEP or in reducing its severity. Multiple prospective trials employing multivariate analysis have identified an array of patient- and procedure-related risk factors that are associated with an increased risk of PEP.5-12 The exact mechanisms by which these factors cause PEP is not well understood and may include mechanical factors from instrumentation, chemical, or hydrostatic injury related to contrast instillation, and thermal injury from electrocoagulation/electrocautery incision during therapeutic interventions.8,13
Commonly employed preventive measures include pharmacological measures, such as intravenous somatostatin,14 gabexate,15 octreotide,16 and rectal nonsteroidal anti-inflammatory drugs (NSAIDs),17-21 or interventional strategies, such as wire-guided cannulation22 and prophylactic pancreatic duct (PD) stent placement.23,24 The cascade of events leading to the development of PEP involves three major successive steps: (1) inciting events, (2) zymogen activation, and (3) inflammation due to a variety of cytokine pathways.13 Current prevention strategies aim to either reduce the impact of inciting events by employing interventional modifications or by inhibiting the inflammatory end-products of zymogen activation through pharmacological agents, such as NSAIDs.
Zymogen activation in acinar cells is a critical step in initiating the inflammatory cascade that results in pancreatitis. Therefore, an effective means of inhibiting zymogen activation via an external stimuli can potentially arrest the inflammatory cascade, thereby preventing the development of PEP. In a novel animal study conducted by Orabi et al.,25 the authors found that early events in zymogen activation leading to PEP are induced through the calcium-activated phosphatase calcineurin. In addition, PEP is dependent on acinar cell calcineurin in vivo. The same investigators also showed that PEP does not occur in calcineurin-knockout mice globally. These elegant animal studies suggest that calcineurin is critical in zymogen activation, and its inhibition can potentially arrest the progression of events that lead to PEP. In support of these preliminary findings, a large multi-center study involving patients who had undergone ERCP after liver transplantation showed that the consumption of tacrolimus, a calcineurin inhibitor, reduced the risk of PEP by 79%.26 Tacrolimus is primarily used as an immunosuppressant in transplant recipients. It is a macrolide produced by Streptomyces tsukubaensis. It binds to an intracellular protein, FKBP-12. A complex consisting of tacrolimus-FKBP-12, calcium, calmodulin, and calcineurin is subsequently formed, which inhibits the phosphatase activity of calcineurin.27
Building on this emerging body of evidence, an interventional pilot study was conducted to explore the feasibility, safety, and preliminary efficacy of oral tacrolimus (cumulative dose of 4 mg) in the prevention of PEP by comparing two prospective cohorts.
METHODS
Study design
This was a prospective interventional pilot study performed from January 1, 2018 to December 31, 2018. Patients aged >18 years in whom therapeutic ERCP was indicated during the study period were considered for the study. Patients with a prior history of acute or chronic pancreatitis, coagulopathy, past history of allergy to tacrolimus, or a medically unfit status for the procedure were excluded. In addition, patients with a history of sphincterotomy and surgically altered anatomy, such as gastrojejunostomy, were excluded from the study. Detailed informed consent was obtained from all patients. The rationale, benefit, and safety of the intervention were thoroughly explained to all patients. This study was approved by the institutional ethics committee after due diligence. Patient demographics, such as age, sex, comorbidities, and indications for ERCP, were recorded in a pre-designed proforma. Patients who consented to receive the drug were included in the Tac group, whereas the remaining patients were included in the control group. Patients in the tacrolimus group received two doses of 2 mg oral tacrolimus (PanGraf tacrolimus capsules; Panacea Biotech, New Delhi, India) at 8 PM on the preceding day and at 8 AM on the day of ERCP. The procedure was performed within 2 hours of the morning dose, and a blood sample for assessment of serum tacrolimus levels was drawn at the time of ERCP. Tacrolimus was estimated using chromatography techniques at the same laboratory for all samples. Patients in the control group underwent the procedure in accordance with the institutional protocol.
Institutional protocol and technical considerations for the procedure
All ERCP procedures were performed by a single experienced endoscopist in the dedicated therapeutic endoscopy laboratory under general anesthesia. The choice of anesthetic agent was left to the discretion of a designated anesthesiologist who was present throughout the duration of all procedures. A standard adult ERCP scope (TJF Q180V; Olympus, Tokyo, Japan) with an outer diameter of 13.7 mm and a channel diameter of 4.2 mm was used. All procedures were performed in an endoscopy suite under fluoroscopic guidance (FlexaVision; Shimadzu, Kyoto, Japan). Biliary cannulation was attempted using a wire-guided cannulation technique in all cases. The procedure for the biliary cannulation was in accordance with the hospital protocol. When selective biliary cannulation was unsuccessful, needle-knife sphincterotomy was performed using a needle-knife sphincterotome (G24885; Cook Medical Inc., North Carolina, USA). Difficult biliary cannulation was characterized by at least two of the following: more than 10 attempts at cannulation, changing accessories for cannulation, more than 10 minutes of cannulation, or the need for a pre-cut sphincterotomy in cases of failed cannulation. All patients with difficult biliary cannulation and/or inadvertent PD contrast instillation received rectal NSAIDs, 100 mg of diclofenac suppository in particular, after the procedure. Prophylactic PD stenting (single pigtail, plastic stent, 5 Fr diameter) was performed in addition to rectal NSAIDs in all patients who had inadvertent PD cannulation or inadvertent contrast instillation into the PD. Technical details of the ERCP procedure, such as the time taken for cannulation, number of attempts at cannulation, inadvertent PD cannulation, contrast injection, and sphincterotomy, which were known to enhance the risk of PEP, were meticulously recorded by the endoscopist immediately after each procedure.
Outcome measures and study procedure
All patients were closely followed-up for complications related to ERCP, such as bleeding, infections, and PEP. Follow-ups were conducted by an independent investigator who was blinded to the group allocation. Serum amylase and lipase levels were measured 12 and 24 hours post-ERCP. PEP was defined severe abdominal pain with an acute onset or worsening that necessitated an unplanned extended hospital stay of a minimum of 48 hours and an elevated serum amylase/serum lipase level of ≥3 times the upper limit of normal. Pancreatitis was classified as mild, moderate, or severe according to the established guidelines.28 The rate of PEP was compared between the two groups. All known confounders, such as age, sex, past history, difficult biliary cannulation, PD injection, rectal NSAIDs, and prophylactic PD stenting, were also compared between the two groups. To assess the dosing and pharmacokinetics of the drug, serum trough levels of tacrolimus were measured at the time of the procedure and were correlated with the outcome measures at the end of the study.
Statistical analyses
Statistical analyses were performed using the IBM SPSS ver. 20 (IBM Corp., Armonk, NY, USA). Quantitative data are reported as mean±standard deviation, while categorical data are reported as numbers and percentages. The incidence of PEP was compared between the two groups using the chi-squared test or Fisher exact test. Independent two-sample t-test and Mann-Whitney U-test were used for parametric and nonparametric variables, respectively, and categorical variables were analyzed using the chi-square test or Fisher exact test in the univariate analysis. Statistical significance was set at p<0.05.
Ethical statements
This study was reviewed and approved by the Amrita Institute of Medical Sciences Institutional Review Board (IRB No: DM/MCh/2015/33). All the patients signed an informed consent prior to the enrollment into the study. A contemporaneous cohort of patients who did not receive the drug were studied as controls.
RESULTS
Baseline characteristics of the study population
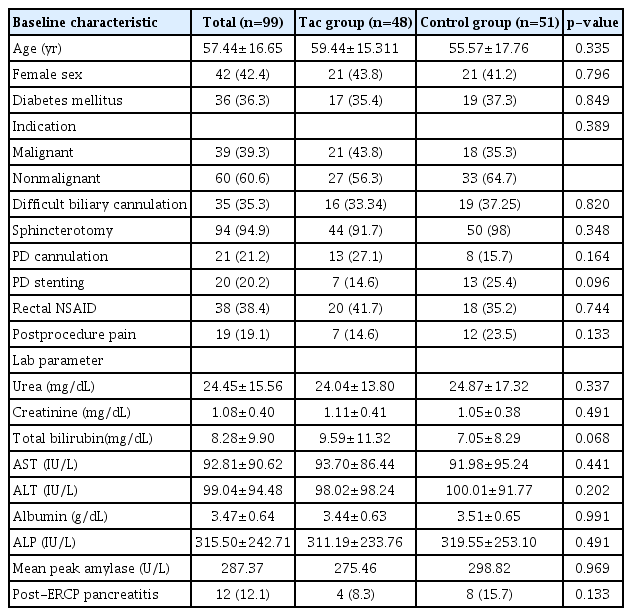
A total of 99 patients were included in the study, of which 48 patients were included in the Tac group, while 51 patients were included in the control group. Table 1 presents the baseline patient characteristics of the participants in this study. The mean age of the population was 57.44±16.65 years, with a male to female ratio of 1.35. The indications for ERCP were predominantly nonmalignant (n=60, 60.6%), including choledocholithiasis with or without cholangitis and biliary strictures. Malignant indications (n=39, 39.3%) included cholangiocarcinoma, periampullary cancer, and secondary malignant biliary strictures. A major comorbidity observed was diabetes mellitus, which was seen in 36 patients (36.3%) with a mean HbA1c of 7.8±4.8 gm/dL. Sphincterotomy was performed in 94 patients (94.9%) and biliary cannulation was deemed difficult in 39 patients (39.3%). Inadvertent PD cannulation and contrast instillation were performed in 21 patients (21.2%). All patients with difficult biliary cannulation and inadvertent PD contrast injection received prophylactic rectal NSAIDs (n=38, 38.4%). Prophylactic PD stenting was performed in 20 patients (20.2%). Overall, PEP was observed in 12 patients (12.1%), and all of them had mild pancreatitis with no mortality within 30 days after ERCP.
Baseline characteristics of the Tac group
The Tac group had a mean age of 59.44±15.31 years (range, 16–87 years), with a male to female ratio of 1.28. Biliary cannulation was difficult in 16 patients (33.3%). Postprocedure complications were noted in six patients. Among them, two (4.1%) had postprocedure cholangitis, while the other four (8.3%) had PEP.
Baseline characteristics of the control group
The control group had a mean age of 55.57±17.76 years (range, 13–90 years) with a male to female ratio of 1.42. Biliary cannulation was difficult in 19 patients (37.3%) in the control group. Postprocedure complications were seen in nine patients (17.6%), with one patient having postprocedure cholangitis and the remaining eight patients (15.7%) having PEP.
Outcome analysis
Both the Tac and the control groups were comparable. There were no statistically significant differences in terms of patient demographics, indications, and comorbidities. The incidence of PEP was found to be almost half of that observed in the controls (four out of 48 patients [8.3%] in the Tac group and eight out of 51 patients [15.7%] in the control group, p=0.24). There were no statistically significant differences in the distribution of known confounders, such as difficult biliary cannulation, inadvertent PD cannulation, and sphincterotomy between the two groups, as shown in Table 1. Those who received rectal NSAIDs in the two groups were also comparable (20 patients [41.7%] in the Tac group vs. 18 patients [35.2%] in the control group, p=0.82).
Univariate and multivariate analyses for the predictors of PEP were performed. However, none of the factors included in this study, such as age, sex, indication, rectal NSAIDs, difficult cannulation, PD stent, and PD cannulation, were found to be significantly associated with the incidence of PEP, as seen in Table 2. Seven patients (14.6%) underwent prophylactic PD stenting in the Tac group compared to the 13 patients (25.4%) in the control group. No adverse events due to intake of tacrolimus were observed in this study. All of the patients who had PEP only had mild disease and were thus managed conservatively. There was no difference in the mean hospital stay between the two groups (3.4±2.8 vs. 4.2±3.9 days, p=0.82).
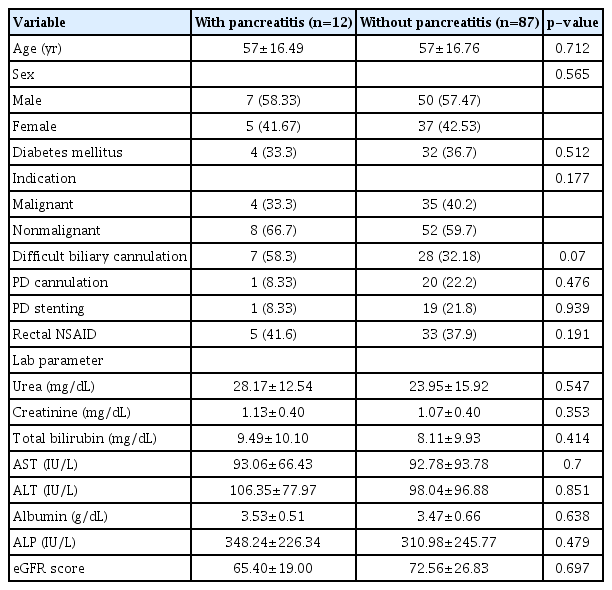
Interestingly, the mean tacrolimus levels were significantly lower in all patients who had PEP as compared to those who did not (3.30±0.44 ng/mL vs. 6.98±3.15 ng/mL, p=0.001), as shown in Figure 1. A receiver operating characteristic curve analysis was performed and the area under the curve of serum tacrolimus levels for the prevention of PEP was found to be 0.918 (p=0.001), as demonstrated in Figure 2. Using the coordinates on the curve, an optimal cutoff of 3.38 ng/mL could prevent PEP with a sensitivity of 86.2% and a specificity of 83.3%. There was considerable variability in the serum tacrolimus levels in the Tac group (median, 6.7 ng/mL; range, 1.8–13.5; variance, 10.2). There were no significant differences (age, sex, renal function tests, liver function tests, estimated glomerular filtration rate) between patients with high serum tacrolimus levels (>6.7 ng/mL) and those with low serum tacrolimus levels (≤6.7 ng/mL) in this study, as shown in Table 3.
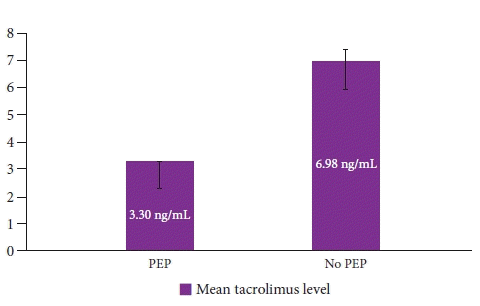
Comparison of the mean trough serum tacrolimus levels between patients with PEP and those without (p=0.001). PEP, post-endoscopic retrograde cholangiopancreatography pancreatitis
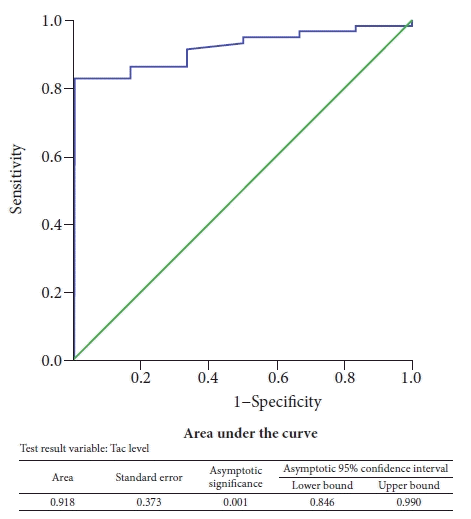
Receiver operating characteristic curve analysis for the efficiency of tacrolimus in preventing post-endoscopic retrograde cholangiopancreatography pancreatitis.
DISCUSSION
Pancreatitis is a known complication of ERCP.2,3,11 The evolution of PEP from the initial pancreatic insult to the eventual local and systemic complications of pancreatitis is a multi-step process dependent on zymogen activation, enzymatic activation, and the release of a variety of mediators that ultimately sets in motion an inflammatory cascade that is self-propagating. Prophylactic measures that have been found useful thus far, are restricted to the interventional measures that soften the initial blow to the pancreas and those that reduce pancreatic injury below the threshold required for zymogen activation. Prophylactic measures also include pharmacological agents that target inflammatory mediators that aid in propagating the inflammatory cascade. However, these measures cannot be collectively exhaustive to eliminate the incidence of PEP. This may be due to two reasons. First, we are currently unable to accurately identify a threshold for zymogen activation that can reliably predict subsequent events in these patients. Second, our knowledge of the gamut of downstream inflammatory mediators is far from complete. Zymogen activation seems to be a point of convergence that can theoretically be permissive to the propagation of inflammation. Therefore, it would be an ideal therapeutic target that can reliably and effectively arrest the inflammatory cascade despite inciting pancreatic injury. Initial promising animal experiments conducted by Orabi et al.25 demonstrated that calcium signaling pathways had a central role, more specifically via the calcineurin receptor, which might activate zymogen and induce the subsequent enzymatic activity that led to inflammation. Moreover, the same investigators showed the absence of PEP in calcineurin-knockout mice. Intraductal infusion of calcineurin inhibitors into PD prevented contrast-induced pancreatitis in mice, as demonstrated by histological findings, along with a decrease in amylase and interleukin 6 levels. In an interesting study by Law et al.,29 the incidence of PEP in liver transplant recipients was found to be substantially lower. Although they found a weak correlation between steroids and the incidence of PEP, subsequent studies failed to demonstrate this benefit.30 More recently, a large retrospective study showed a significant reduction in the risk of PEP with tacrolimus and rectal indomethacin consumption.26 In light of these findings and in conjunction with the study by Orabi et al.,25 it is possible that tacrolimus has a significant impact on the prevention of PEP.
In our study, the rate of PEP among the Tac group was lower than that in the control group (8.3% vs. 15.7%). Age and sex distributions, along with comorbidities, were comparable between the two groups. The factors known to increase the risk of PEP, such as difficult biliary cannulation with multiple attempts, instrumentation, inadvertent PD cannulation, contrast injection, and sphincterotomy, were equally distributed, with no statistically significant differences between the two groups. More importantly, the number of patients with difficult cannulation who received PD stents and rectal NSAIDs was comparable between the two groups.
We also examined the serum trough Tac levels in patients who developed PEP, which were found to be significantly lower in patients with PEP than in those without (3.3 ng/mL vs. 6.98 ng/mL, p=0.001). The optimal cutoff for trough tacrolimus levels to prevent PEP is unknown. In a retrospective study of liver transplant recipients, a trough tacrolimus level of 2.5 ng/mL was found to lower the odds of PEP by 79%.26 Notably, only two patients (4.6%) had tacrolimus levels lower than 2.5 ng/mL; among these two, one patient developed PEP. The remaining three patients who developed PEP in the Tac group had levels exceeding 2.5 ng/mL. In this study, using the receiver operating characteristic analysis, an optimal cutoff of 3.38 ng/mL was determined which could prevent PEP, with a sensitivity of 86.2% and specificity of 83.3%. However, it should be mentioned that there was considerable variability in the trough tacrolimus levels in this study (range, 1.8–13.5; variance, 10.2). This can be further explained by the fact that oral tacrolimus has been shown to exhibit erratic absorption with varying bioavailability in healthy volunteers.27,31 These observations suggest that the pharmacokinetic properties of oral tacrolimus, such as optimal dosage, ideal trough tacrolimus level, and safety, are important aspects that merit further study.
This study is the first clinical trial to assess the feasibility and safety of oral tacrolimus for the prevention of PEP. The relatively higher rate of PEP, albeit mild in severity and without mortality within 30 days after ERCP, in our patient cohort is an important finding. A relatively higher incidence of PEP was noted by some investigators in large university hospitals and tertiary referral centers. This may be related to the higher proportion of patients with prior unsuccessful attempts at cannulation, difficult cannulation, and complicated disease with multiple comorbidities being referred to these centers.32 The more liberal use of PD stenting and rectal NSAIDs can be a potential intervention for future practice. Although oral tacrolimus at a cumulative dose of 4 mg was found to be feasible and safe, its efficacy in preventing PEP has to be assessed further in a larger sample size. In addition, isolated case reports of acute pancreatitis among renal transplant recipients, which could possibly be attributed to tacrolimus, was described in the literature.33,34 Although rare, this is an important aspect that merits consideration in future studies on the use of tacrolimus in the prevention of PEP.
In conclusion, this pilot study clearly demonstrates that oral tacrolimus at a dose of 4 mg can safely prevent PEP in patients undergoing ERCP. Further randomized studies with larger sample sizes can help establish an ideal trough tacrolimus level that can reliably prevent PEP following ERCP procedures.
Notes
Conflicts of Interest
The authors have no potential conflicts of interest.
Funding
None.
Author Contributions
Conceptualization: HRB, AKK, RPV; Data curation: PKV, PN; Formal analysis: HRB, PKV, PN; Investigation: AKK, RPV; Methodology: HRB, RPV; Project administration: HRB, AKK, RPV; Supervision: HRB, RPV; Validation: HRB, PN; Writing–original draft: HRB; Writing–review & editing: HRB, PKV, PN, AKK, RPV.