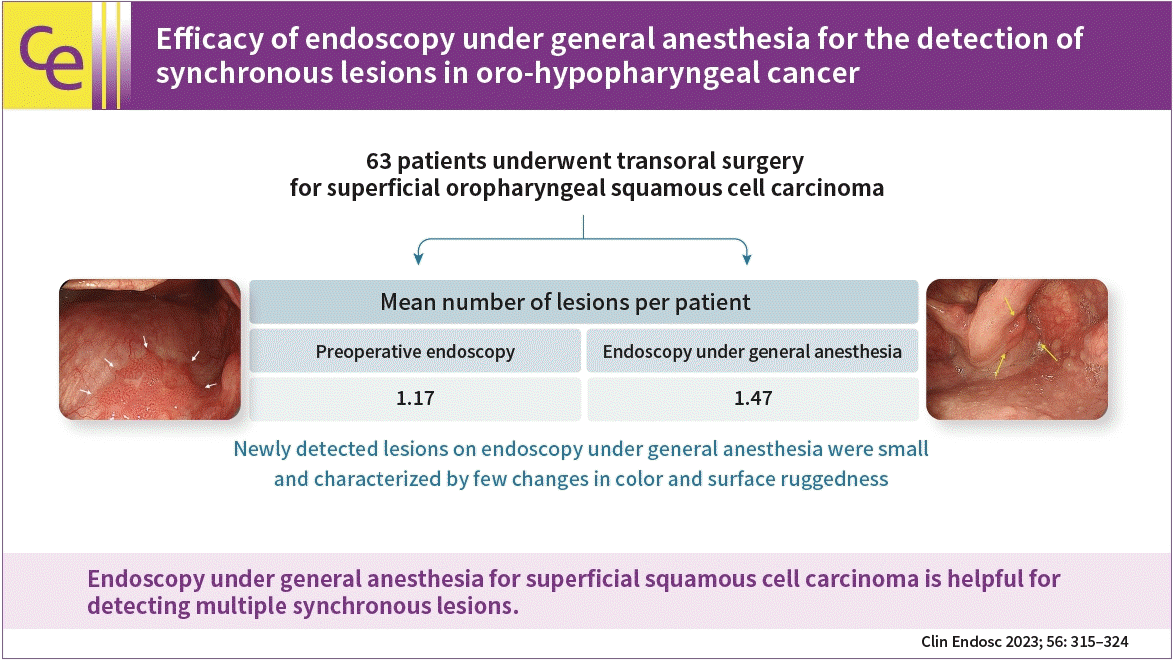
Efficacy of endoscopy under general anesthesia for the detection of synchronous lesions in oral hypopharyngeal cancer
Article information
Abstract
Background/Aims
Image-enhanced endoscopy can detect superficial oro-hypopharyngeal squamous cell carcinoma; however, reliable endoscopy of the pharyngeal region is challenging. Endoscopy under general anesthesia during transoral surgery occasionally reveals multiple synchronous lesions that remained undetected on preoperative endoscopy. Therefore, we aimed to determine the lesion detection capability of endoscopy under general anesthesia for superficial oro-hypopharyngeal squamous cell carcinoma.
Methods
This retrospective study included 63 patients who underwent transoral surgery for superficial oropharyngeal squamous cell carcinoma between April 2005 and December 2020. The primary endpoint was to compare the lesion detection capabilities of preoperative endoscopy and endoscopy under general anesthesia. Other endpoints included the comparison of clinicopathological findings between lesions detected using preoperative endoscopy and those newly detected using endoscopy under general anesthesia.
Results
Fifty-eight patients (85 lesions) were analyzed. The mean number of lesions per patient detected was 1.17 for preoperative endoscopy and 1.47 for endoscopy under general anesthesia. Endoscopy under general anesthesia helped detect more lesions than preoperative endoscopy did (p<0.001). The lesions that were newly detected on endoscopy under general anesthesia were small and characterized by few changes in color and surface ruggedness.
Conclusions
Endoscopy under general anesthesia for superficial squamous cell carcinoma is helpful for detecting multiple synchronous lesions.
INTRODUCTION
Detailed endoscopic observation of the pharyngeal region is difficult because of its anatomically complicated structure and the functional characteristics that cause swallowing and elicit the gag reflex. Consequently, oro-hypopharyngeal carcinoma is often detected at advanced stages, and its prognosis is poor.1-3 In addition, the synchronous or metachronous occurrence of oro-hypopharyngeal carcinoma is observed frequently.4
It is well established that multiple cancers can occur in the upper aerodigestive tract of patients with oro-hypopharyngeal carcinoma. This phenomenon has been explained by the concept of “field cancerization”,5 in which repeated exposure to carcinogens leads to an accumulation of genetic alterations, ultimately resulting in the development of multiple and independent cancers.
The standard therapy for resectable oro-hypopharyngeal carcinoma is laryngopharyngectomy with pharyngeal reconstruction, which causes loss of natural speech and aphagia.6 Although alternative therapies, such as radiotherapy or chemoradiotherapy, allow for the preservation of the organs, they can cause serious functional disorders, including dysphagia due to severe mucositis and dry mouth that results from radiation injury. These therapies often adversely affect the quality of life of patients.7 Thus, the early detection and treatment of pharyngeal carcinoma are extremely important.
In recent years, the development of image-enhanced endoscopy, such as narrow-band imaging (NBI), and advances in endoscopic devices have enabled the detection of superficial oro-hypopharyngeal squamous cell carcinoma (SCC).8,9 In addition, transoral surgery (TOS), such as transoral video-assisted surgery, endoscopic laryngopharyngeal surgery, endoscopic mucosal resection, and endoscopic submucosal dissection (ESD), can be performed as a minimally invasive treatment for superficial cancer. TOS has been reported to be effective and safe.10-12
Endoscopy of the pharyngeal region is difficult to perform sufficiently and consistently because of its aforementioned anatomical or functional characteristics. In fact, when endoscopy is performed under general anesthesia (GA) during TOS, multiple synchronous lesions that were undetectable using preoperative endoscopy can be observed using white-light endoscopy (WLE) or NBI. However, no studies have reported the capability of endoscopy under GA to detect superficial oropharyngeal SCC. Thus, this study aimed to determine the capability of endoscopy under GA to detect superficial oro-hypopharyngeal SCC lesions.
METHODS
Patients
The subjects were consecutive patients who underwent an initial TOS for oropharyngeal lesions at our hospital between April 2005 and December 2020. The analyses included patients with superficial oro-hypopharyngeal SCC who met the inclusion criteria and did not meet the exclusion criteria.
1) Inclusion criteria
(1) Patients with lesions histopathologically diagnosed as SCC. (2) Patients with lesions with a depth not exceeding the subepithelial layer according to histopathological examination. (3) Patients who underwent endoscopy (WLE and NBI) before surgery. (4) Patients who underwent endoscopy (WLE and NBI) during surgery under GA.
2) Exclusion criteria
(1) History of chemotherapy, radiotherapy, or surgical resection for cancer in the head and neck region. (2) Patients with insufficient results from preoperative endoscopy.
Endoscopic devices
Preoperative endoscopy was performed using an upper gastrointestinal magnifying endoscope (GIF-Q240Z, GIF-H260Z, or GIF-H290Z; Olympus Co. Ltd.). During the resection, endoscopy was performed using an upper gastrointestinal endoscope (GIF-Q260J; Olympus Co. Ltd.). The endoscopic systems used were EVIS LUCERA CV260/CLV-260 and EVIS LUCERA ELITE CV-290/CLV-290SL (Olympus Co. Ltd.). During endoscopy, a black soft hood (MAJ-1989 for Q240Z, H290Z, or MAJ-1990 for H260Z; Olympus Co. Ltd.) was attached to the tip of the endoscope to maintain a consistent distance between the tip of the endoscope lens and the mucosal surface.
Endoscopic findings suggestive of SCC
The following endoscopic findings were used to diagnose superficial oro-hypopharyngeal SCC. (1) SCC was suspected on WLE when mucosal lesions showed changes in color (reddish and whitish), vascular network pattern (increased vascular density and loss of visible vascular pattern), gloss (roughness and minute ruggedness), and surface structure (elevation and depression) (Fig. 1A, B). (2) SCC was suspected on NBI/magnified NBI (M-NBI) when mucosal lesions showed a well-demarcated brownish area and an irregular microvascular pattern (Fig. 1C, D).4,8 (3) SCC was suspected based on iodine staining when mucosal lesions showed an iodine-unstained area and a pink color sign (Fig. 1E, F).12,13
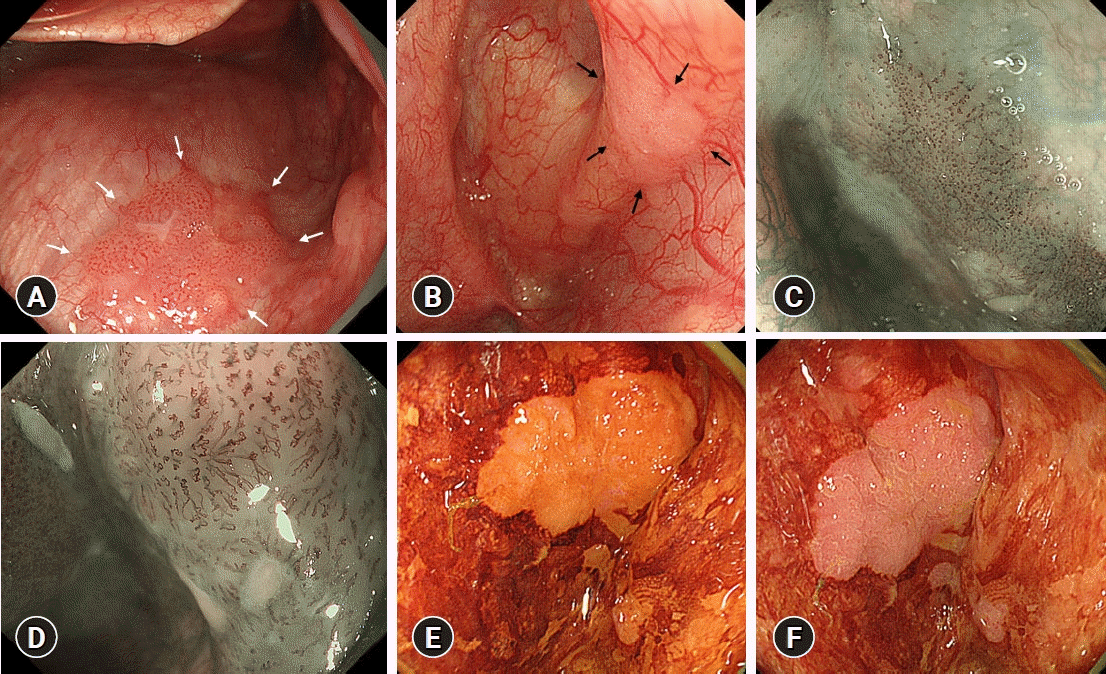
Endoscopic findings suggestive of squamous cell carcinoma. (A) A reddish, slightly rugged lesion showing increased vascular density on conventional white-light endoscopy (white arrows). (B) A whitish lesion with rough mucosa and no visible vascular pattern on conventional white-light endoscopy (black arrows). (C) A lesion with a well-demarcated brownish area on narrow-band imaging. (D) A lesion with an irregular microvascular pattern on magnified narrow-band imaging. (E) A lesion showing an iodine-unstained area on iodine staining. (F) A lesion showing a pink color sign on iodine staining.
Preoperative endoscopy
We dissolved 20,000 units of the mucolytic agent pronase (Pronase MS; Kaken Pharmaceutical Co., Ltd.), 1 g of sodium bicarbonate, and 10 mL of the antifoaming agent 2% dimethylpolysiloxane (dimeticone oral solution, Baros antifoaming oral solution 2%; Horii Pharmaceutical Ind., Ltd.) in 100 mL of tap water. The patients drank the solution 30 min before endoscopy. Pharyngeal anesthesia was induced using xylocaine viscous (2% xylocaine viscous 5 mL; Aspen Japan K.K.).
During preoperative endoscopy, the patients were awake or under conscious sedation with 2 to 4 mg of midazolam (10 mg/2 mL; Fuji Pharma Co., Ltd.) or 5 to 10 mg of diazepam (5 mg/mL; Takeda Pharmaceutical Co., Ltd.). The sedative was adjusted based on the decision of the endoscopist or according to the patient’s preferences. Although deep sedation was not applied as it can cause difficulties when performing pharyngeal region examinations, its use was at the physician’s discretion.
Endoscopic observation of the pharyngeal region was performed in the following sequence using WLE and/or NBI: soft palate, palatine uvula, left and right arches of the palate, side and posterior walls of the oropharynx, lingual surface of the epiglottis, vocal folds, and pyriform sinuses. We attempted to observe the pharyngeal region as appropriate using patient vocalization to improve the visibility of the pharyngeal region (Fig. 2A).
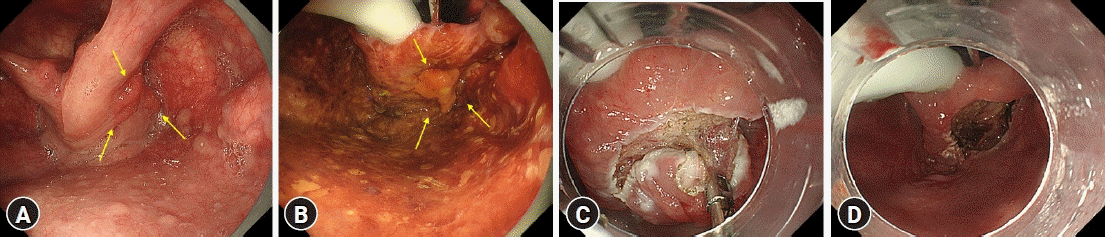
Endoscopic findings under general anesthesia of endoscopic submucosal dissection for superficial oro-hypopharyngeal squamous cell carcinoma. (A) Preoperative endoscopy using patient vocalization. Superficial pharyngeal squamous cell carcinoma (arrows) in the right pyriform sinus. (B) Endoscopy under general anesthesia. Superficial pharyngeal squamous cell carcinoma (arrows) in the right pyriform sinus (iodine staining). (C) Subepithelial dissection was carried out with traction using curved and cupped forceps. (D) En bloc resection was achieved.
During the preoperative endoscopy procedures, iodine staining was not performed. Only WLE and/or NBI were performed to detect the lesions. Biopsy was performed on lesions diagnosed as SCC based on the aforementioned diagnostic criteria.
Indications for TOS
TOS for superficial oro-hypopharyngeal SCC was indicated when all of the following criteria were met: (1) Lesions were histopathologically diagnosed as SCC or strongly suspected to be SCC endoscopically/histopathologically. (2) Lesions were free of lymph node metastasis on computed tomography/magnetic resonance imaging/positron emission tomography. (3) Lesions were determined to have a tumor wall at a depth not exceeding the subepithelial layer.
Endoscopy under GA
Endoscopy was performed under GA with intratracheal intubation, with the patient in the head-hanging position. Using a curved rigid laryngoscope (Nagashima Medical Instruments Co., Ltd.), the larynx was exposed to secure sufficient visual fields and working spaces.
Next, WLE, NBI, and M-NBI using endoscopes were performed for lesions identified as treatment targets by preoperative endoscopy. Next, other parts of the oro-hypopharyngeal region were examined using WLE, NBI, and M-NBI to confirm the presence or absence of other multiple synchronous lesions. In addition, iodine staining was performed to more accurately identify the lesions targeted for treatment and determine the extent of the lesions (Fig. 2B). When lesions suspected to be multiple synchronous lesions were detected in the process described above, we simultaneously resected lesions that were diagnosed as SCC with high confidence based on the aforementioned diagnostic criteria; we also performed biopsy in lesions diagnosed as SCC with low confidence.
Procedure of TOS
Resection was performed using ESD (Fig. 2C, D). The high-frequency generator used was VIO300D (ERBE). A Flush Knife N-S device was used for ESD (DK2618J; Fujifilm Medical Corporation). Lesions with small diameters (<10 mm) were resected using endoscopic mucosal resection with a cap-fitted endoscope.14
Handling of resected specimens and histopathological diagnosis
The resected specimen was spread on a flat rubber plate with the mucosal side facing upward, and the entire margin of the specimen was fixed with stainless steel pins. The specimen was then immersed in 20% buffered formalin solution and fixed overnight. The fixed specimens were cut into 2 to 3-mm thick sections. The sections were embedded in paraffin and sliced to a thickness of 5 µm to prepare the histopathological specimens.
Histopathological diagnosis was performed according to the general rules for clinical studies on head and neck cancer15 issued by the Japan Society for Head and Neck Cancer. Tumor types were classified into slightly elevated, flat, and slightly depressed types. The depth of tumor invasion was classified by the depth of the intraepithelial and subepithelial layers. In addition, tumor thickness was measured as the distance from the tumor surface to the deepest point at which the tumor cells existed.
Gold standard
Histopathological diagnosis of resected specimens was used as the gold standard.
Endpoints
The primary endpoint was the comparison of the number of superficial oro-hypopharyngeal SCC lesions detected per patient using WLE and NBI/M-NBI between preoperative endoscopy and endoscopy under GA. Therefore, the number of lesions detected using iodine staining during endoscopy under GA was not included in the comparison.
The other endpoints were comparisons among the clinicopathological findings (tumor location, disease type, color, tumor diameter, invasion depth, and tumor thickness) between superficial oro-hypopharyngeal SCC lesions detected by preoperative endoscopy and superficial oro-hypopharyngeal SCC lesions newly detected by endoscopy under GA.
Statistical analysis
Statistical analyses were performed using IBM SPSS software ver. 21.0 (IBM Corp.). All continuous variables are presented as the mean (95% confidence interval [CI]). A paired t-test was performed to compare the number of superficial oro-hypopharyngeal SCC lesions detected per patient between preoperative endoscopy and endoscopy performed under GA. To compare the clinicopathological findings between superficial oro-hypopharyngeal SCC lesions detected using preoperative endoscopy and superficial oro-hypopharyngeal SCC lesions detected using endoscopy under GA, t-tests and Fisher exact tests were performed. The statistical significance was set at p<0.05.
Ethical considerations
This study was approved by the institutional review board of Fukuoka University (approval number: C21-04-002). This study was exempt from obtaining informed consent because it was a retrospective study of recorded data obtained by standard clinical practice, that is, preoperative and intraoperative endoscopy of patients with superficial oro-hypopharyngeal SCC.
RESULTS
Clinicopathological characteristics of the study patients and lesions
Between April 2005 and December 2020, 63 consecutive patients (91 lesions) underwent an initial TOS for oropharyngeal lesions at our hospital. Of these, 58 patients (85 lesions) were included in the analyses after excluding two patients who were histopathologically diagnosed with noncancerous lesions (two lesions), one patient who was histopathologically diagnosed with cancer invading the muscularis propria (one lesion), and two patients who had previously been treated with chemoradiotherapy (three lesions) (Fig. 3).
During the preoperative endoscopy, 42 patients underwent conscious sedation with midazolam or diazepam. The remaining 16 patients did not receive sedation.
Table 1 shows the clinicopathological characteristics of the patients and their lesions. The tumor locations were the oropharynx in 14 lesions and hypopharynx in 71 lesions. Regarding macroscopic type, 34 lesions were classified as slightly elevated, 40 as flat, and 11 as slightly depressed. The mean tumor diameter (95% CI) was 15.4 mm (13.1–17.7 mm). Histopathological examination revealed that the depth of the tumor wall reached the intraepithelial layer in 60 lesions and the subepithelial layer in 25. The mean tumor thickness (95% CI) was 407.2 μm (315.7–498.6 μm).
The number of superficial oro-hypopharyngeal SCC lesions detected per patient by WLE/NBI between preoperative endoscopy and endoscopy under GA
The mean number of superficial oro-hypopharyngeal SCC lesions detected per patient by WLE/NBI was 1.17 (95% CI, 1.05–1.29) using preoperative endoscopy and 1.47 (95% CI, 1.29–1.64) using endoscopy under GA. The mean number of superficial oro-hypopharyngeal SCC lesions detected per patient was significantly higher when using endoscopy under GA than when using preoperative endoscopy (p<0.001, paired t-test) (Table 2).

Comparison of the number of superficial oro-hypopharyngeal squamous cell carcinoma lesions detected per patient by white light endoscopy/narrow-band imaging between preoperative endoscopy and endoscopy under general anesthesia (n=58)
In addition, all superficial oropharyngeal SCC lesions detected on preoperative endoscopy were identified on endoscopy under GA. During endoscopy under GA, WLE/NBI failed to detect three superficial oro-hypopharyngeal SCC lesions, all of which were detected by iodine staining.
Clinicopathological findings of superficial oro-hypopharyngeal SCC lesions detected by WLE/NBI on preoperative endoscopy and newly detected by endoscopy under GA
Table 3 shows the clinicopathological findings of 68 lesions detected by WLE/NBI on preoperative endoscopy and 14 lesions newly detected by endoscopy under GA.
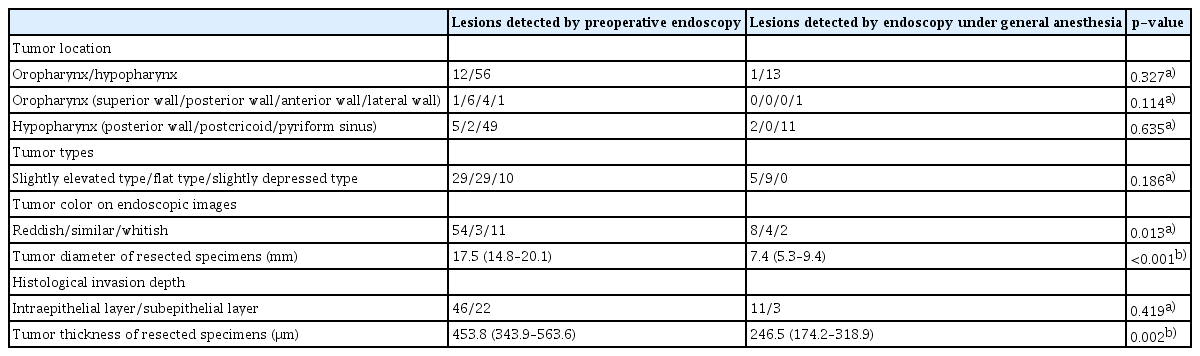
Comparison of the clinicopathological findings of superficial oro-hypopharyngeal squamous cell carcinoma lesions detected by preoperative endoscopy and newly detected by endoscopy under general anesthesia
Regarding the color of lesions, the percentage of lesions with a similar color to that of the surrounding non-neoplastic mucosa was significantly higher in the lesions newly detected by endoscopy under GA (4/14, 28.6%; 95% CI, 11.3–55.0%) than in the lesions detected by preoperative endoscopy (3/68, 4.4%; 95% CI, 1.0–12.7%) (p=0.013). The mean tumor diameter was 7.4 mm (95% CI, 5.3–9.4 mm) in lesions newly detected by endoscopy under GA and 17.5 mm (95% CI, 14.8–20.1 mm) in lesions detected by preoperative endoscopy. The tumor diameter of lesions newly detected by endoscopy under GA was significantly smaller than that of lesions detected by preoperative endoscopy (p<0.001). In addition, the mean tumor thickness was 246.5 μm (95% CI, 174.2–318.9 μm) in the lesions newly detected by endoscopy under GA and 453.8 μm (95% CI, 343.9–563.6 μm) in the lesions detected by preoperative endoscopy. The tumor thickness of the lesions newly detected by endoscopy under GA was smaller than that of the lesions detected by preoperative endoscopy (p=0.002).
DISCUSSION
The results of this study showed that the number of superficial oro-hypopharyngeal SCC lesions detected per patient on endoscopy using WLE and NBI/M-NBI under GA was significantly larger than the number of lesions detected per patient on preoperative endoscopy. This study revealed that endoscopy under GA was more likely to detect multiple synchronous lesions that remained undetected by preoperative endoscopy.
NBI has been reported to be effective for the detection of superficial SCC in the pharyngeal region, and it has been reported to be effective and widely used in daily clinical practice. However, no studies have compared the capability of endoscopy under GA to detect superficial oro-hypopharyngeal SCC with that of endoscopy performed with conventional sedatives. This study reported for the first time that endoscopy under GA had a superior capability to detect superficial SCC in the pharyngeal region.
Studies on the long-term prognosis following endoscopic resection of superficial oro-hypopharyngeal SCC have reported that many patients develop multiple metachronous lesions (16–26.6%).9,16-18 This phenomenon is known as “field cancerization,” proposed by Slaughter et al.5
Although there have been no reports on the time taken for superficial oro-hypopharyngeal SCC to progress to an advanced cancer stage, it has been reported that continuous surveillance, including endoscopy using NBI, is effective for the early detection of multiple metachronous lesions in patients who have undergone TOS of superficial oro-hypopharyngeal SCC.17,19 However, there have been reports of cases where patients could not undergo TOS because of the risk of stricture and pulmonary functional impairment, which were due to the presence of lesions close to the larynx or previous treatments with radiotherapy or surgery; there have also been cases where surgical resection was required because the lesions were spread over a wide area.17,19 As described above, although continuous surveillance is effective for early detection of multiple metachronous lesions, minimally invasive procedures such as TOS cannot be performed in some patients with metachronous lesions detected by surveillance. Thus, when TOS is performed under GA for superficial oro-hypopharyngeal SCC, detailed endoscopic surveillance of multiple synchronous lesions in areas other than the sites of the treatment target lesions allows for the early detection of multiple synchronous lesions and the simultaneous resection of these lesions. In fact, lesions newly detected by endoscopy under GA were successfully resected endoscopically, as shown in this study.
The result that endoscopy under GA for superficial oro-hypopharyngeal SCC was helpful for detecting multiple synchronous lesions may be obvious. Unfortunately, it is not practical to perform endoscopy under GA as a screening test, particularly considering the invasiveness of the procedure performed on patients under GA, the need to procure necessary personnel, and the associated healthcare costs. However, at some institutions, only preoperatively detected lesions can be observed and treated during TOS. Therefore, we would like to emphasize the clinical significance of the results of this study, as the study demonstrated that detailed observation of the oro-hypopharyngeal region was necessary for the detection of multiple synchronous lesions, in addition to preoperatively detected lesions, before patients are treated for already known lesions under GA.
The lesions that were newly detected by endoscopy under GA in the patients included in this study were small and characterized by few changes in color and surface ruggedness. In terms of tumor location, no significant difference was observed between the lesions detected by preoperative endoscopy and those newly detected by endoscopy under GA. In other words, regardless of the tumor location in the oro-hypopharyngeal region, the detection of small, minutely rugged SCCs with colors similar to those of the surrounding mucosa may be beyond the technical limitations of preoperative endoscopy using WLE or NBI.
Regarding sedation, pethidine hydrochloride has been reported to exert a superior sedative effect during endoscopy of the pharyngeal region in patients with esophageal cancer.20 Poor endoscopic observation of the palatine uvula and piriform sinus has been reported in patients receiving midazolam, as sedation depresses consciousness and makes vocalization impossible. Patient vocalization is an important technique to facilitate the observation of the hypopharyngeal region, especially on both sides of the piriform sinus. However, this study observed no difference in lesion detection capability between preoperative endoscopy and endoscopy under GA according to lesion location. Although it seems unlikely that lesion detection capability varies depending on the type of sedative used, further studies are needed to address this issue.
As the conditions for the two endoscopic sessions, such as the endoscopic devices used and the employed observation methods, were presumably similar, the difference in the capability to detect superficial oro-hypopharyngeal SCC between preoperative endoscopy and endoscopy under GA could be attributed to the favorable observation conditions for endoscopy under GA. Under GA, the respiratory status and hemodynamics of the patients were stable, the gag reflex was inhibited, and a sufficient visual field was secured with curved rigid laryngoscopes when the patients were placed in the head-hanging position. These conditions allowed for more detailed endoscopic examination.
This study has several limitations. First, it was a single-center retrospective study. Second, this study included only a small number of patients and lesions. In addition, there was a lack of analysis to minimize confounding factors in this study. Thus, the results obtained in this study need to be verified in a multicenter prospective study with a larger sample size. Third, the sedation applied during the preoperative endoscopy procedures was not standardized. The use of pethidine hydrochloride, which has been reported to be useful for pharyngeal observation, may increase the lesion detection capability of preoperative endoscopy. Thus, studies using preoperative endoscopy under sedation with pethidine hydrochloride are needed to assess the procedure's lesion detection capability.
In conclusion, the capability of endoscopy under GA to detect superficial oropharyngeal SCC lesions is high. Endoscopy under GA was effective in detecting small, flat, multiple synchronous lesions that had not been detected by preoperative endoscopy.
Notes
Conflicts of Interest
The authors have no potential conflicts of interest.
Funding
None.
Acknowledgments
The authors thank the laboratory staff for their excellent technical support.
Author Contributions
Conceptualization: YO, KY, YT; Data curation: YO, YT, SI, TH, TU, SN, AI; Formal analysis: YO, KY, YT; Investigation: YO, YT, SI, KI, AK, KO, TK, MM, AO, HT, SH, SS, RW; Methodology: YO, KY, YT; Project administration: YO, KY, YT; Supervision: KY, YT; Validation: YO, KY, YT; Visualization: YO, YT; Writing–original draft: YO; Writing–review & editing: all authors.