Potassium-competitive acid blocker-associated gastric mucosal lesions
Article information
Abstract
Since the introduction of vonoprazan, a potassium-competitive acid blocker (P-CAB), it has been demonstrated to reversibly inhibit gastric acid secretion by engaging in potassium-competitive ionic binding to H+/K+-ATPase. In contrast, proton pump inhibitors (PPIs) achieve H+/K+-ATPase inhibition through covalent binding to cysteine residues of the proton pump. Reported cases have indicated an emerging trend of P-CAB-related gastropathies, similar to those associated with PPIs, as well as unique gastropathies specific to P-CAB use, such as the identification of web-like mucus. Pathologically, parietal cell profusions, which show a positively correlated with hypergastrinemia, have a higher incidence in P-CAB users compared to PPI users. Thus, this review aims to summarize the endoscopic and pathological findings reported to date concerning P-CAB-related gastric mucosal lesions. Additionally, it seeks to discuss the differences between the PPIs and P-CABs in terms of the formation and frequency of associated gastropathies. This review highlights the evident differences in the mechanism of action and potency of acid inhibition between P-CABs and PPIs, notably contributing to differences in the formation and frequency of associated gastropathies. It emphasizes the necessity to distinguish between P-CAB-related and PPI-related gastropathies in the clinical setting.
INTRODUCTION
Proton pump inhibitors (PPIs) have been used worldwide to treat acid-related disorders, such as gastroesophageal reflux disease and peptic ulcer disease, as well as for preventing gastroduodenal mucosal damage induced by nonsteroidal anti-inflammatory drugs.1 Recently, vonoprazan, a potassium-competitive acid blocker (P-CAB), has been developed and approved for clinical use in Japan.2 It acts by reversibly inhibiting gastric acid output through K+-competitive ionic binding to H+/K+-ATPase,3 distinguishing itself from PPIs that inhibit the gastric H+/K+-ATPase via covalent binding to cysteine residues of the proton pump, thereby impeding acid output. Notably, PPIs exhibit certain characteristics, including: (1) a delayed onset of acid inhibition; (2) a short blood half-life resulting in insufficient inhibition of acid secretion throughout the day and night; and (3) intragastric pH achieved with a PPI that depends on the CYP2C19 genotype status.4 In contrast, the P-CAB is characterized by its ability to rapidly suppress acid secretion and provide sustained acid-suppressing effect from the first day of administration. Thus, since its introduction, the frequency of P-CAB use has increased. A comparative study, assessing the 24-hour pH ≥4 holding time between the P-CAB and PPIs, demonstrated a more potent acid-inhibitory property for the P-CAB than for PPIs.5 Moreover, although both PPIs and P-CAB induce a low acid state in the stomach, prompting gastrin secretion from antral G cells,6 a comparative study on long-term administration revealed notably higher serum gastrin levels in individuals receiving P-CAB (median, 870 pg/mL; range, 120–3,000 pg/mL) compared to those receiving PPIs (median, 440 pg/mL; range, 87–2,000 pg/mL).7 Thus, P-CAB demonstrates greater potency in inhibiting acid secretion corresponding with elevated serum gastrin levels compared to PPIs.
PPI-induced hypergastrinemia has been reported to result in histopathological changes, including parietal cell protrusion (PCP) into the gland lumen and cystic dilation of the gastric fundic gland. These changes are associated with characteristic endoscopic findings known as PPI-related gastropathies.8-13 Recent reports have highlighted similar and novel gastropathies associated with P-CABs, suggesting the importance of recognizing these P-CAB-related gastropathies in clinical setting. Thus, this review aimed to summarize the endoscopic and pathological findings reported to date on P-CAB-related gastric mucosal lesions (Table 1) and to discuss the differences between PPIs and P-CABs in the formation and frequency of associated gastropathies.
P-CAB-ASSOCIATED GASTRIC MUCOSAL LESIONS
Fundic gland polyps
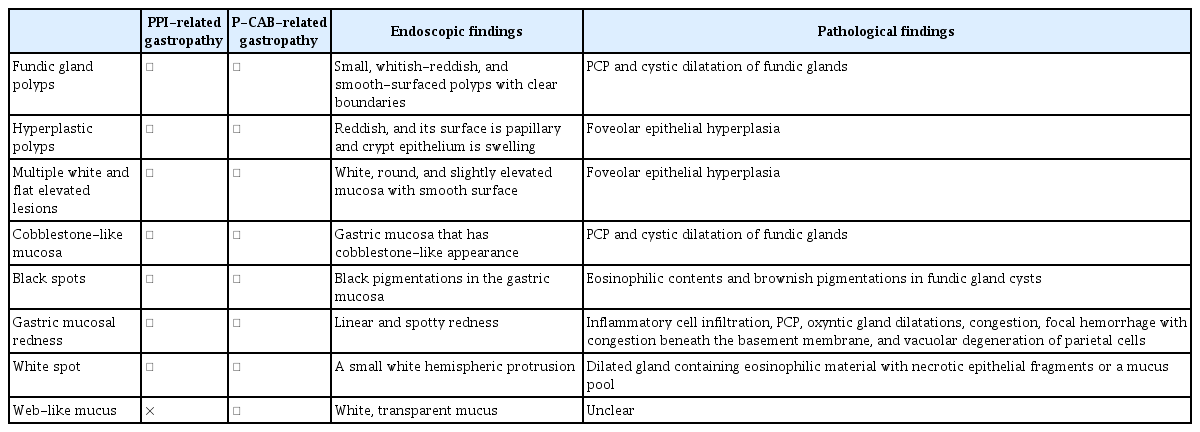
Fundic gland polyps (FGPs) occur in the gastric corpus and fundus, characterized by their small size (<1 cm), whitish-reddish appearance, and smooth-surfaced texture with clear boundaries (Fig. 1A). A meta-analysis of 12 studies established a notable association between the use of PPIs and an increased prevalence of FGPs (odds ratio [OR], 2.46; 95% confidence interval [CI], 1.42–4.27). This association was observed with long-term PPI use (PPI use ≥6 months: OR, 4.71; 95% CI, 2.22–9.99; PPI use ≥12 months: OR, 5.32; 95% CI, 2.58–10.99).14 Notably, FGPs may develop and increase in frequency with long-term PPI administration, showing a tendency to decrease following PPI discontinuation. Equally noteworthy are two recent case reports documenting the regression of FGPs following P-CAB discontinuation.15,16 Of the two case reports, Iwamuro et al.15 reported a case wherein FGPs regressed after transitioning from P-CAB to a histamine-2 (H2) blocker. The other case report by Nishimura et al.16 discussed the notable decrease and regression of FGPs subsequent to P-CAB discontinuation in a patient with anemia, with a concomitant reduction in serum gastrin levels compared to that before drug discontinuation. Moreover, Haruma et al.17 conducted a multicenter prospective study (VISION trial) and reported a noteworthy increase in the prevalence of FGPs from 43.7% to 66.1% over a three-year period following the initiation of P-CAB therapy. Additionally, Shinozaki et al.18 found a notably higher prevalence of FGPs among patients receiving P-CAB for more than one year compared to that in non-P-CAB users. Their association with P-CAB use was further validated through multivariate analysis (OR, 2.20; 95% CI, 1.41–3.44).
Endoscopic findings of potassium-competitive acid blocker-related gastric mucosal lesions. (A) Fundic gland polyps. (B) Hyperplastic polyps. (C, D) Multiple white and flat elevated lesions. (E) Cobblestone-like mucosa. (F) Black spots. Written informed consent was obtained from the patients for publication of the case report as part of the review and any accompanying images included.
Hyperplastic polyps
A hyperplastic polyp (HP) is identifiable as a reddish lesion with a papillary surface, often involving swelling of the crypt epithelium (Fig. 1B). In a study by Hongo et al.,19 it was reported that HP was present in 8.9% of long-term PPI users and that new instances of HP occurred in 5.9% and 22.2% of patients with FGP and HP, respectively. HP has been observed to develop and increase in frequency with long-term PPI administration, a trend that decreases following PPI discontinuation. In line with these observations, two recent case reports underscore the regression of HP following P-CAB discontinuation.15,20 Of the two case reports, Iwamuro et al.15 detailed a case of HP regression after transitioning from the P-CAB to an H2 blocker, while Goto et al.20 reported a case where HP notably reduced after switching from P-CAB to an H2 blocker in a patient with severe anemia, subsequently improving the anemia condition. Results from the VISION trial17 highlighted a notable increase in the prevalence rate of HPs from 3.7% to 14.7% over a three-year period in patients initiating P-CAB treatment. Additionally, Shinozaki et al.18 reported a notably higher prevalence of HP among patients receiving P-CAB for more than one year compared to that of non-P-CAB users. The association of HP with P-CAB use was reaffirmed through multivariate analysis (OR, 3.37; 95% CI, 1.44–7.90).
Multiple white and flat elevated lesions
Multiple white and flat elevated lesions (MWFELs) are characterized by white, round, and slightly elevated mucosa with a smooth surface, appearing circumscribed and sharply marginated (Fig. 1C, D).21 These lesions are distributed in various locations in the upper corpus and, histologically, represent hyperplastic changes in the foveolar epithelium with normal fundic glands.22 In an observational study by Hatano et al.,21 MWFELs were notably associated with PPI use (OR, 3.58; 95% CI, 1.94–6.61) and Helicobacter pylori eradication therapy. The VISION trial17 demonstrated an increase in the prevalence of MWFELs from 2.2% to 6.4% over a three-year period following the initiation of P-CAB treatment. Moreover, Shinozaki et al.23 reported a notably higher prevalence of MWFELs among patients receiving P-CAB for more than one year compared to that in non-P-CAB users, with its association with P-CAB use reaffirmed through multivariate analysis (OR, 1.77; 95% CI, 1.14–2.74).
Cobblestone-like mucosa
Cobblestone-like mucosa (CLM) is characterized by gastric mucosa displaying a cobblestone-like appearance, endoscopically detected as multiple smooth, elevated lesions in the corpus under white-light imaging (Fig. 1E). Histologically, CLM is identified by PCP and cystic dilatation of the fundic glands, with these changes typically observed in non-atrophic mucosa with abundant fundic glands.24 In an observational study by Hatano et al.,21 CLM demonstrated a notable association with PPI use (OR, 4.57; 95% CI, 2.34–9.96). Notably, Miyamoto et al.25 reported a case of CLM exhibiting similar endoscopic and pathological findings after six months of P-CAB use. The VISION trial17 reported a notable increase in the prevalence of CLMs from 3.0% to 14.7% over a three-year period in patients initiating P-CAB treatment. Additionally, Shinozaki et al.18 reported a notably higher prevalence of CLM in patients using P-CAB for more than one year compared to that in non-P-CAB users, with its association shown with P-CAB use confirmed through multivariate analysis (OR, 2.55; 95% CI, 1.03–6.32).
Black spots
Black spots (BS), characterized as black pigmentations within the gastric mucosa observed during conventional endoscopy, are exclusively identified in the fundic gland region (Fig. 1F). Histologically, BS consists of eosinophilic contents and brownish pigmentations within fundic gland cysts.26 Kiso et al.27 reported the frequency of BS to be 5.7% among PPI users and 3.2% among non-PPI users. An observational study by Hatano et al.21 demonstrated a notable association between BS and PPI use (OR, 2.94; 95% CI, 1.66–5.21) and H. pylori eradication therapy (OR, 3.01; 95% CI, 1.73–5.24). Notably, the VISION trial17 indicated an increase in the prevalence of BS from 0.7% to 5.5% over a three-year period following the initiation of P-CAB treatment. However, the components of BS and its mechanism of formation remain unknown.
Gastric mucosal redness
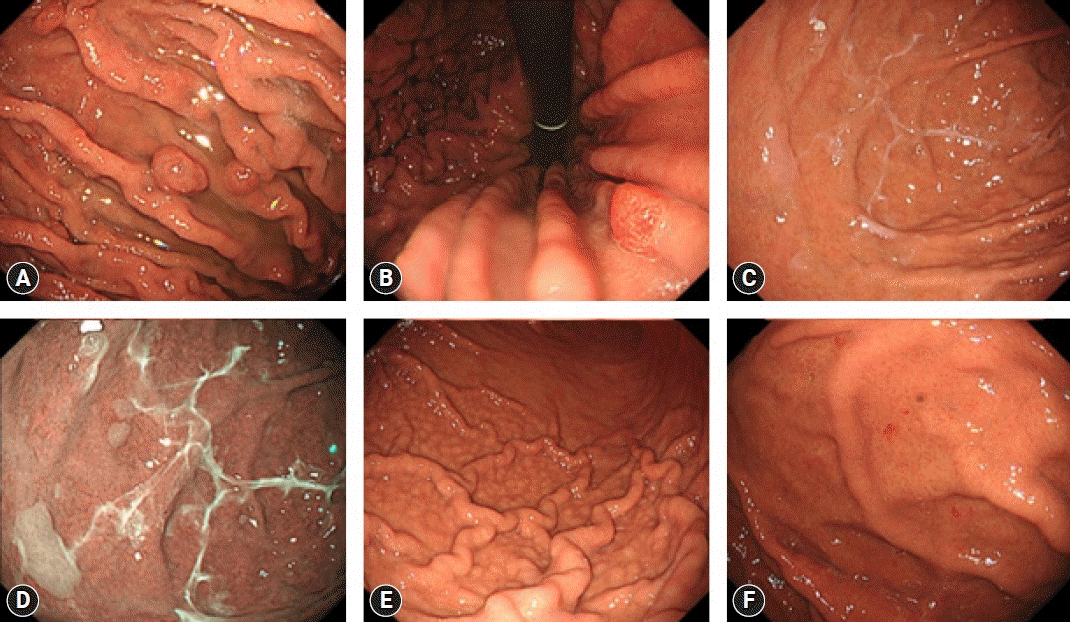
Gastric mucosal redness (GMR) is characterized by linear and spotty redness in the gastric body and was initially reported as a P-CAB-related gastropathy (Fig. 2A–C). Kubo et al.28 reported four cases of GMR emerging after the initiation of P-CAB treatment and subsequently disappearing upon its discontinuation. Histologically, GMR is characterized by the presence of inflammatory cell infiltration, PCP, oxyntic gland dilatation, congestion, focal hemorrhage with congestion beneath the basement membrane, and vacuolar degeneration of parietal cells.29 The mechanism of GMR formation is suspected to involve hypergastrinemia, given that serum gastrin levels are elevated in patients with GMR. Notably, Shinozaki et al.23 reported a higher prevalence of GMR in patients receiving the P-CAB and PPIs for over one year compared to that in non-P-CAB/non-PPI users, with its association with P-CAB use (OR, 3.42; 95% CI, 1.48–7.90) and PPI use (OR, 2.67; 95% CI, 1.14–2.74) confirmed through multivariate analysis.
Endoscopic findings of potassium-competitive acid blocker-related gastric mucosal lesions. (A, B, C) Gastric mucosal redness observed on white-light imaging (WLI) was highlighted on texture and color enhancement imaging (TXI) mode 1 and narrow-band imaging (NBI), respectively. (D, E, F) White spots observed on WLI were highlighted on NBI. Magnifying endoscopy with NBI showed the mucosal surface of white spots to be rich in microscopic vessels. (G, H, I) Web-like mucus observed on WLI was highlighted on TXI mode 1 and NBI, respectively. Written informed consent was obtained from the patients for publication of the case report as part of the review and any accompanying images included.
White spots
White spots (WS) is characterized by a small, white hemispheric protrusion, with its surface exhibiting regular microscopic vesicles under magnifying narrow-band imaging (Fig. 2D–F). WS was first identified as a P-CAB-related gastropathy.30,31 Histologically, WS is characterized by a dilated gland containing either eosinophilic material with necrotic epithelial fragments or a mucus pool.30-33 Iwamuro et al.34 reported two cases of WS in P-CAB and PPI users. Miwa et al.35,36 reported a case of WS emerging after the initiation of P-CAB treatment and subsequently disappearing after transitioning to a PPI. The findings by Yoshizaki et al.32 revealed a notably higher prevalence of WS in P-CAB used compared to that in non-P-CAB users (4.9% vs 0.2%, p<0.001), with an association of >205 days of P-CAB use (OR, 6.99; 95% CI, 4.60–10.88), confirmed through multivariate analysis. Shinozaki et al.23 also reported a notably higher prevalence of WS in patients receiving a P-CAB and a PPI for more than one year compared to non-P-CAB/non-PPI users, with its association with P-CAB use (OR, 192.69; 95% CI, 60.20–616.77) and PPI use (OR, 23.19; 95% CI, 6.55–82.08) verified through multivariate analysis. Moreover, Nishiyama et al.33 demonstrated a notably larger number of patients with high serum gastrin levels among patients positive for WS (18/31) compared to that in patients negative for WS (5/43) (p<0.001). This suggests an association between WS and hypergastrinemia, implying that P-CAB-related hypergastrinemia may be involved in the formation of WS. Mechanistically, it may be hypothesized that hypergastrinemia induces structural disruption in the area extending from the isthmus to the neck of the gland, leading to the obstruction of mucus outflow from the gland, ultimately resulting in cystic dilation of the gland.
Web-like mucus
Web-like mucus is characterized by white, transparent mucus that proves challenging to remove through water rinsing. This mucus is predominantly observed in the area from the fundus to the greater curvature of the body (Fig. 2G–I).37 The mechanism of its formation has been speculated to be linked to the strong and sustained inhibition of acid secretion by the P-CAB.
Histopathology of the gastric mucosa during P-CAB and PPI therapy
PCP, a pathological finding associated with PPI-related gastropathy, has been reported to be positively correlated with hypergastrinemia.9 The VISION trial provided information on the histopathology of the gastric mucosa during a three-year period of P-CAB maintenance therapy. PCP was observed in 102/109 (93.6%) patients in the P-CAB group, compared to 45/57 (78.9%) patients in the PPI group, based on gastric mucosal biopsies performed three years after the administration of either drug. Moreover, one patient in the P-CAB group developed hyperplastic endocrine cell micro nests, but none developed gastric neuroendocrine tumors.
CONCLUSIONS
The emergence of P-CAB-related gastropathies, shared with PPIs (FGPs, HP, MWFEL, CLM, and BS) and unique to P-CABs (GMR and WS), marks a notable development since the introduction of P-CABs. Additionally, web-like mucus has also been reported as a gastropathy specific to P-CABs. The observed differences in the mechanism of action and potency of acid inhibition between P-CABs and PPIs greatly contribute to the differences in the formation and frequency of associated gastropathies, indicating the need to promote recognition of the differences between P-CAB-related and PPI-related gastropathies in clinical setting. However, it is important to note that the endoscopic findings of the P-CAB-associated gastropathies described here were derived from the users of vonoprazan and that, to date, no similar endoscopic features have been reported in the users of other P-CABs, such as tegoprazan and fexuprazan.
Notes
Conflicts of Interest
The authors have no potential conflicts of interest.
Funding
None.
Author Contributions
Conceptualization: KK; Investigation: all authors; Methodology: all authors; Supervision: KK; Validation: KK, MK; Writing–original draft: KK; Writing–review and editing: all authors.