International Digestive Endoscopy Network consensus on the management of antithrombotic agents in patients undergoing gastrointestinal endoscopy
Article information
Abstract
Antithrombotic agents, including antiplatelet agents and anticoagulants, are widely used in Korea because of the increasing incidence of cardiocerebrovascular disease and the aging population. The management of patients using antithrombotic agents during endoscopic procedures is an important clinical challenge. The clinical practice guidelines for this issue, developed by the Korean Society of Gastrointestinal Endoscopy, were published in 2020. However, new evidence on the use of dual antiplatelet therapy and direct anticoagulant management has emerged, and revised guidelines have been issued in the United States and Europe. Accordingly, the previous guidelines were revised. Cardiologists were part of the group that developed the guideline, and the recommendations went through a consensus-reaching process among international experts. This guideline presents 14 recommendations made based on the Grading of Recommendations, Assessment, Development, and Evaluation methodology and was reviewed by multidisciplinary experts. These guidelines provide useful information that can assist endoscopists in the management of patients receiving antithrombotic agents who require diagnostic and elective therapeutic endoscopy. It will be revised as necessary to cover changes in technology, evidence, or other aspects of clinical practice.
INTRODUCTION
Antithrombotic agents, such as vitamin K antagonists (warfarin), direct oral anticoagulants (DOACs; apixaban, dabigatran, edoxaban, and rivaroxaban), P2Y12 receptor inhibitors (clopidogrel, prasugrel, and ticagrelor), and acetylsalicylic acid, are widely used in clinical practice for the primary and secondary prevention of cardiocerebrovascular disease.1 The number of patients with cardiocerebrovascular disease and the use of antiplatelet drugs for secondary prevention have increased because of the aging population. DOACs are used to prevent stroke in patients with atrial fibrillation and are increasingly prescribed from year to year.2
Recent developments in endoscopic equipment and technology have improved the performance of various endoscopic procedures for diagnostic and therapeutic purposes.3,4 Accordingly, the frequency of adverse events, such as bleeding, may also increase. Particularly, the risk of bleeding is higher when therapeutic procedures are performed on patients being administered antithrombotic drugs.5 Whether endoscopic procedures can be performed safely and effectively on patients receiving antithrombotic drugs remains a concern for endoscopists. In such cases, the patient’s thrombotic risk, morbidity, characteristics of the antithrombotic agent used, and bleeding risk during the endoscopic procedure should be considered when determining the appropriate management of antithrombotic agents during the procedure.
Clinical practice guidelines (CPGs) have been developed by gastroenterology and endoscopy societies in the USA, Europe, Japan, and the Asia-Pacific region.6-10 The Korean Society of Gastrointestinal Endoscopy (KSGE) published practice guidelines for gastrointestinal endoscopy in 2020.11 Since then, the latest evidence on the use of antithrombotic drugs and large-scale cohort studies on the use of DOAC have been published. Therefore, it was necessary to revise the previous Korean guidelines. At the time of revision, an “International Digestive Endoscopy Network (IDEN) consensus” was developed based on the consensus of local and international experts of the IDEN. Gastroenterologists, cardiologists, and neurologists developed the revised guidelines, and 36 multidisciplinary experts, including six international expert panels, reviewed and voted on the recommendations. These guidelines have been endorsed by the Korean Neurological Association and the Korean Society of Cardiology. The guidelines categorize thrombotic risk in patients using antiplatelet drugs and anticoagulants and the bleeding risk associated with various endoscopic procedures. Recommendations are provided for the management of antithrombotic agents based on these risks. This revision is updated based on the current evidence and provides a detailed management schedule for DOACs. However, because these guidelines do not cover all individual patients and situations, it is essential to consider patient characteristics and use a multidisciplinary approach in clinical practice.
METHODS
Purpose and scope of the clinical practice guideline
This CPG aimed to provide information on the management of antithrombotic agents during the periendoscopic period based on a comprehensive review of current evidence and CPGs on bleeding and thromboembolic adverse events associated with endoscopic procedures in patients receiving antithrombotic agents. This CPG is for adult patients being administered antithrombotic agents for the primary or secondary prevention of cardiocerebrovascular disease and those who undergo diagnostic or elective therapeutic endoscopic procedures, excluding emergency endoscopic procedures such as endoscopic hemostasis. The target readership of this CPG is gastroenterologists who perform endoscopic procedures in primary, secondary, and tertiary health care institutions. This CPG is intended to assist gastroenterologists in making timely decisions regarding appropriate treatment with antithrombotic agents before and after endoscopic procedures. Furthermore, it aims to serve as a guide for resident physicians and healthcare workers and provide practical information for patients and the general public.
Organization of the clinical practice guideline committee and the development process
The CPG committee convened in April 2022 and included the president (Oh Young Lee), vice president (Jong-Jae Park), and executive committee members of the KSGE. Members of the CPG committee established a strategy for the development of the CPG, appointed a director of the project, and reviewed and approved the project budget. They reviewed the suggested recommendations and ensured the editorial independence and participation of all parties involved in the development process. To develop the CPG, Kee Don Choi, a board-certified gastroenterologist and member of the KSGE, was appointed director of the CPG development committee. Eight other gastroenterologists participated as members of the CPG development committee. An expert in CPG development methodology (Miyoung Choi) from the National Evidence-Based Healthcare Collaborating Agency collaborated with the committee to develop the guidelines. Cardiologists and neurologists were also involved in the guideline’s development.
The development committee revised the guidelines published in 2020 according to the methods suggested in the Cochrane handbook and the handbook published by the National Evidence-Based Healthcare Collaborating Agency.11,12 Briefly, partial revisions were made after reviewing the previous version of the Korean guidelines, guidelines from other countries published after 2020, and the latest literature on the use of antithrombotic agents during endoscopy. Additional literature was searched for 14 key questions, as in the previous guidelines published in August 2022. Based on the results of the selected studies, recommendations were made according to the Grading of Recommendations, Assessment, Development, and Evaluation (GRADE) methodology.13 The development committee held a total of 13 meetings on May 9, 2022. The development committee also held a workshop with four cardiologists to reach an agreement on cardiovascular risk stratification on November 11, 2022. In April 2023, the CPG committee and international experts at the IDEN reviewed the draft of the recommendations and participated in the first round of voting.
Selection of the key questions
The development committee reviewed the key questions in the previous guidelines and guidelines from other countries.7-9,11 After internal discussion, we retained 14 key questions, as in the previous version. Key questions were posed using the population, intervention, comparison, and outcome (PICO) process, and those to be included in the CPG were derived. P (population) represents patients who have undergone diagnostic or elective therapeutic endoscopic procedures while taking antithrombotic agents; I (intervention) represents the interruption or replacement of antithrombotic agents during the periendoscopic period; C (comparison) includes the comparison group, which continues to use antithrombotic agents before and after endoscopic procedures; and O (outcome) represents the risk of adverse events, such as bleeding and thromboembolism, associated with endoscopic procedures.
Literature search and selection of existing guidelines for adaptation
In August 2022, a literature search of the Ovid Medline, Embase, Cochrane Library, and KoreaMed databases was performed based on the key questions. The search words included a combination of terms related to endoscopic procedures (“en¬doscopy” OR “esophagogastroduodenoscopy” OR “colonoscopy” OR “endosonography” OR “endoscopic retrograde cholangiopancreatography” OR “enteroscopy” OR “biopsy” OR “stent” OR “argon plasma coagulation” OR “papillary balloon dilation” OR “sphincterotomy” OR “fine needle aspiration” OR “percutaneous endoscopic gastrostomy” OR “percutaneous endoscopic jejunostomy” OR “tumor ablation” OR “ampullectomy” OR “cystogastrotomy” OR “pneumatic dilation” OR “polypectomy” OR “endoscopic mucosal resection” OR “endoscopic sub¬mucosal dissection”) and terms related to antithrombotic agents (“antiplatelet” OR “platelet aggregation inhibitor” OR “aspirin” OR “acetylsalicylic acid” OR “thienopyridine” OR “clopidogrel” OR “prasugrel” OR “ticagrelor” OR “ticlopidine” OR “cilostazol” OR “triflusal” OR “anticoagulants” OR “warfarin” OR “coumadin” OR “heparin” OR “low molecular weight hep-arin” OR “enoxaparin” OR “dalteparin” OR “nadroparin” OR “non-vitamin K antagonist oral anticoagulant” OR “novel oral anticoagulant” OR “direct oral anticoagulant” OR “dabigatran” OR “apixaban” OR “rivaroxaban” OR “edoxaban” OR “bridge therapy”).
Two members were assigned to each key question, and studies were independently selected according to the established criteria. The literature selection process was conducted using the Preferred Reporting Items for Systematic Reviews and Meta-Analyses flow diagram.14 First, studies that did not meet the inclusion criteria were excluded by reviewing titles and abstracts. If studies were not eliminated during this process, the decision to eliminate or select them was finalized after reviewing the entire study. In cases of disagreement between the two members, the study selection was determined by consensus. If consensus was not reached, the committee leader made the final decision. The exclusion criteria for the latest literature were as follows: (1) studies not involving humans; (2) studies not involving patients relevant to the key questions; (3) studies not conducting interventions and comparative interventions related to the key questions; (4) studies presented only as abstracts, case reports, or reviews; and (5) studies that did not provide the original text. If there was an overlap of study populations between studies, those with smaller sizes were excluded.
Risk of bias assessment, summary of evidence, and grade of recommendation
The validity of selected studies was assessed using consistent, systematic methods. Randomized comparative studies were evaluated using the Cochrane risk of bias,15 whereas non-randomized studies were evaluated using the Risk of Bias Assessment Tool for Non-randomized Studies 2.0.16 Systematic reviews were evaluated using A Measurement Tool to Assess Systematic Reviews.17 The summary of the evidence was determined using the GRADE method.13 Randomized comparative studies were considered to provide a high level of evidence, whereas observational studies were considered to provide a low level of evidence. However, the quality levels of the studies were upgraded or downgraded based on factors affecting their quality. The level of evidence was graded as high, moderate, low, or very low.
The grade of recommendation was classified as strong or conditional, depending on the balance between the benefit and harm of the recommendation, the quality of evidence, values, and preferences. A strong recommendation is applicable to most patients because it has more positive than negative effects, is supported by high-quality evidence, and is highly valuable and strongly preferred over other interventions.1 A conditional recommendation is also beneficial for many patients, although it has relatively fewer positive effects and/or weak-quality evidence. For conditional recommendations, an alternative intervention may be chosen depending on the values and preferences of the physicians and patients.
Review and approval of the guidelines
A draft was created and reviewed by the CPG development committee to ensure the completeness of the guidelines. For a consensus on recommendations by experts, local and international experts, members of the development and CPG committees, neurologists, and cardiologists voted online by e-mail. A revised draft based on the first round of voting was presented at the “IDEN 2023 conference,” in which international gastroenterologists from across the country gathered on June 9, 2023. The final draft of the guidelines was revised based on discussions during this meeting.
Provision of clinical practice guidelines and plans for future updates
For the wide provision and distribution of this CPG, the guidelines will be co-published in Clinical Endoscopy (the official journal of the KSGE) and the Korean Journal of Gastroenterology (the official journal of the Korean Society of Gastroenterology). It will be posted on the KSGE website and registered with the Korean Medical Guidelines Information Center. As the rapid distribution of the CPG to endoscopists through da¬tabases is expected to be difficult, the KSGE will distribute free guidelines through various channels, including email, and will actively promote it at academic conferences, seminars, and workshops. The CPG will be revised to account for changes in technology, new data, or other aspects of clinical practice in the future.
Limitations
The most critical limitation of the CPG is the lack of local evidence in Korea. Evidence from foreign countries cannot be directly applied to the development of guidelines for the Korean population because the risks of adverse events associated with endoscopic procedures and thromboembolism caused by withholding antithrombotic agents differ between countries. This CPG is not intended to provide absolute treatment standards in real clinical practice but to help physicians make evidence-based clinical decisions regarding the management of antithrombotic agents before and after endoscopic procedures. Therefore, the treatment for each patient should be determined by a physician, considering the various clinical factors of the individual patient. This CPG cannot be used as a basis for health insurance, to restrict physicians’ practices, or for the legal judgment of physical practice.
Editorial independence and conflict of interest
This CPG was selected as a KSGE project and received financial support from the KSGE. However, the KSGE did not affect the CPG development process, and none of the members involved in the CPG development had potential conflicts of interest.
BLEEDING RISK OF ENDOSCOPIC PROCEDURES
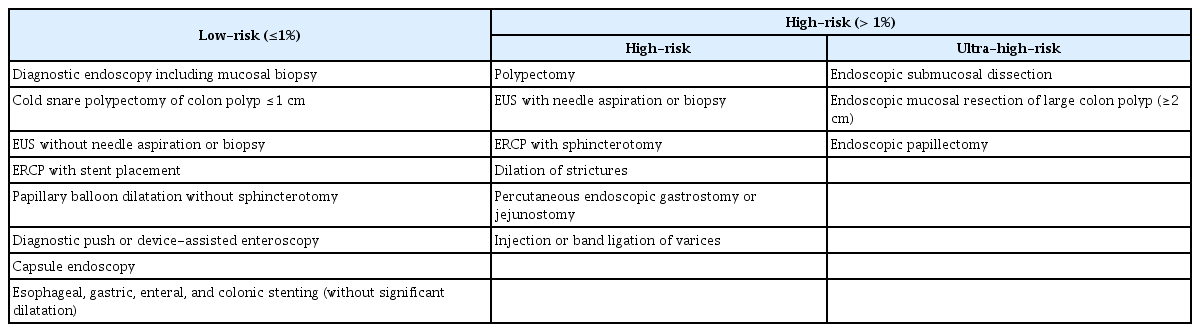
In this version of the guidelines, we categorize endoscopic procedures into low- and high-risk procedures (Table 1). The classification of bleeding risk was based on a previous version of this guideline and guidelines from different academic societies and associations.6-11 Low-risk endoscopic procedures were defined as those in which the risk of postprocedural bleeding (PPB) was expected to be ≤1%. Among high-risk endoscopic procedures, endoscopic mucosal resection (EMR) for large colon polyps (≥2 cm), endoscopic submucosal dissection (ESD), and endoscopic papillectomy, which have a higher bleeding risk than other high-risk endoscopic procedures, were further categorized as ultra-high-risk endoscopic procedures as per the previous versions of this guideline, the Asian Pacific Association of Gastroenterology (APAGE)/Asian Pacific Society for Digestive Endoscopy (APSDE) guideline, and the British Society of Gastroenterology (BSG)/European Society of Gastrointestinal Endoscopy (ESGE) guideline.8,9,11 According to a review and meta-analysis, the bleeding rate associated with papillectomy is 20% to 25%. Therefore, we categorized endoscopic papillectomy as an ultra-high-risk procedure.18-20 Regarding colon polypectomies, the PPB rate after cold snare polypectomy (CSP) for colon polyps less than 1 cm in size is less than 1% regardless of the morphology of the colon polyp, and the delayed PPB rate, even when warfarin is used, is reported to be less than 1%.20-25 It would be useful to separately classify CSP for these lesions as a low-risk procedure because polyps less than 1 cm comprise 70% to 90% of detected polyps during colonoscopy.26
THROMBOTIC RISK OF PATIENTS BEING ADMINISTERED ANTITHROMBOTIC AGENTS
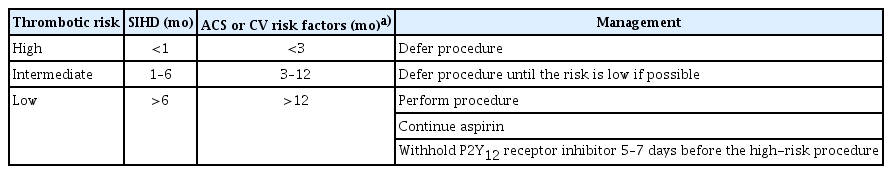
Patients who underwent stent insertion for coronary artery disease required dual antiplatelet therapy (DAPT), including aspirin plus a P2Y12 inhibitor, during the recommended period. Decisions regarding the discontinuation of antiplatelet agents and the timing of high-risk endoscopic procedures should be made after a comprehensive consideration of atherothrombotic events, bleeding, and clinical problems that could occur secondary to delayed procedures. A cardiologist must be consulted for the interruption of P2Y12 receptor inhibitor therapy before performing elective non-cardiac surgery. The guidelines developed by the American Heart Association in 2016 recommend delaying surgery for 6 months after the insertion of a drug-eluting stent and for 30 days after the insertion of a bare-metal stent.27 However, in recent large-scale case-control studies, the prevalence of major adverse cardiac events (MACE) was 7.2% to 11.6% when surgery was performed within 4 to 6 weeks of coronary stent implantation.28-30 Notably, a case-control study involving 9,391 patients showed that the type of stent used was not associated with the risk of MACE.30 Rather, the risk of MACE was related to the patient’s medical history (history of acute coronary syndrome [ACS] and stent thrombosis) and underlying risk factors, such as congestive heart failure, chronic kidney disease, and diabetes mellitus. Based on these results, the guidelines for DAPT in coronary artery disease developed by the European Society of Cardiology in 2017 recommend delaying surgery by 4 weeks after stent implantation, regardless of the type of stent used.31 Furthermore, when surgery is scheduled between 4 weeks and 6 months after stent insertion, it should be deferred, if possible, and the decision to perform surgery should be made after considering the risks and benefits specific to the patient.31 However, this period can be extended to 12 months for patients with a history of ACS or other clinical risk factors. Recommendations regarding the timing of high-risk endoscopic procedures in patients who have undergone coronary stent insertion are shown in Table 2.
Decisions to continue or discontinue anticoagulants in patients undergoing endoscopic procedures should consider both the bleeding risk associated with endoscopic procedures and the risk of thromboembolism associated with withholding anticoagulants. The risk of thromboembolism, which may increase because of the discontinuation of anticoagulants, is closely related to the underlying disease that requires the use of anticoagulants.32,33 The American College of Chest Physician guidelines categorize patients into three groups based on the risk of thromboembolism: (1) low-risk (<4% per year risk of arterial thromboembolism [ATE] or <4% per month risk of venous thromboembolism [VTE]), (2) moderate-risk (4% to 10% per year risk of ATE or 4% to 10% per month risk of VTE), and (3) high-risk (>10% per year risk of ATE or >10% per month risk of VTE).34 Based on recent studies and previously developed guidelines regarding the management of antithrombotic agents before and after endoscopic procedures, we summarized high-risk patients for whom there was a high risk of thromboembolism when anticoagulants were withheld and who required heparin bridging therapy (Table 3).
RECOMMENDATIONS FOR THE MANAGEMENT OF ANTITHROMBOTIC AGENTS
Statement 1-1. We do not recommend the discontinuation of aspirin before endoscopic procedures for patients being administered aspirin (strength of recommendation: strong; level of evidence: moderate).
The bleeding risk associated with diagnostic endoscopy, including mucosal biopsy, is ≤0.5%, even when antiplatelet agents such as aspirin or clopidogrel are used.35-39 A prospective study reported the bleeding rate after upper gastrointestinal endoscopy, including mucosal biopsy, performed without withholding antiplatelet agents before the procedure. Bleeding rates in the aspirin-only and clopidogrel-only groups were 0.4% and 0.0%, respectively.39 Therefore, we recommend that aspirin should not be discontinued during low- or high-risk procedures, as recommended by previous guidelines.
Statement 1-2. For ultra-high-risk endoscopic procedures, withholding aspirin before the procedure could be considered, depending on the risk of bleeding in patients with low thrombotic risk (strength of recommendation: conditional; level of evidence: low).
Considering procedures with the highest bleeding risk, including EMR for large lesions, ESD, and endoscopic papillectomy, studies have shown varying results regarding whether aspirin use increases bleeding.
ESD is associated with a higher risk of bleeding than EMR.40-42 Delayed bleeding rates after gastric ESD have been reported to be 1.3% to 11.9%. Research findings on the bleeding risk after gastric ESD with aspirin are inconsistent, with some studies reporting an increased bleeding risk if aspirin was not stopped before the procedure.43,44 In contrast, other studies have reported no increased risk of bleeding with continued aspirin use.45-50 Meta-analysis of these studies showed that continuous use of aspirin increased post-ESD bleeding compared with interruption (risk ratio [RR], 1.63; 95% confidence interval [CI], 1.13–2.36), as shown in Figure 1.43-50 The bleeding rate in the continuation group who underwent gastric ESD was 10.8% (95% CI, 8.5%–13.1%). A recent retrospective multicenter study in patients who underwent ESD for early gastric cancer also showed that among aspirin users (n=665), the continuation group had significantly more cases of post-ESD bleeding (odds ratio [OR], 2.79; 95% CI, 1.77–4.37).44

Postendoscopic submucosal dissection bleeding in aspirin users comparing the interruption and continuation groups. M-H, Mantel–Haenszel; CI, confidence inter¬val.
A recent prospective study evaluated the safety of continued antiplatelet therapy in patients who received antiplatelet agents and underwent EMR for colorectal polyps. There was no difference in the major PPB rate between the withholding and continuing groups among aspirin users (2.0% vs. 4.2%, p=0.30); however, the PPB rate was significantly higher in the continuing group than in the withholding group among clopidogrel users (18.2% vs. 0%, p=0.02).51 Polyp size is a known risk factor for delayed bleeding after a colorectal polypectomy. There is a high risk of bleeding after EMR for colorectal polyps ≥2 cm in size, and aspirin use is associated with an increased risk of bleeding. A retrospective study showed that discontinuation of aspirin was an independent protective factor for PPB (hazard ratio [HR], 0.13; 95% CI, 0.03–0.75; p=0.022), especially when the polyp was ≥12 mm.52
A large retrospective cohort study including consecutive patients undergoing colonoscopic polypectomy reported that thromboembolic events occurred in two out of 487 patients (0.41%) who continued aspirin during the procedures and two out of 568 patients (0.35%) who stopped aspirin before the procedure.53 Considering the rate of thromboembolic events in aspirin users, aspirin may be discontinued during ultra-high-risk procedures. However, the decision on whether to withhold aspirin before ultrahigh-risk procedures should be based on the risk of thromboembolism and bleeding, ideally after consultation with a cardiologist or neurologist.
Statement 2. We recommend continuing P2Y12 receptor inhibitors for low-risk endoscopic procedures in patients using a single antiplatelet agent for secondary prevention (strength of recommendation: strong; level of evidence: low).
P2Y12 receptor inhibitors (clopidogrel, prasugrel, and ticagrelor) are frequently used for DAPT, along with aspirin, in patients with ACS and after coronary stent placement. After 6 to 12 months of DAPT, a single antiplatelet agent, usually aspirin, is administered for a prolonged period of time. However, in several recent randomized controlled trials (RCTs), P2Y12 receptor inhibitor monotherapy after approximately 1 to 3 months of DAPT, compared with prolonged DAPT (≥12 months), resulted in similar rates of all-cause mortality, major cardiac events, and fewer bleeding events.54,55 Clopidogrel monotherapy is also recommended in patients with symptomatic peripheral vascular disease and may be used following an ischemic cerebrovascular accident.56,57 Patients can be considered to have a high-to-moderate cardiovascular risk, even if they are receiving P2Y12 inhibitor monotherapy, within 6 months after percutaneous coronary intervention and within 12 months after ACS. Therefore, it is necessary to consult a cardiologist regarding the discontinuation of P2Y12 receptor inhibitors in these cases. However, in patients with a low cardiovascular risk, discontinuation of P2Y12 receptor inhibitors 5 to 7 days before the procedure can be considered if a high-risk endoscopic procedure is required.
As mentioned in Statement 1-1, the bleeding risk associated with diagnostic endoscopy is low even when an antiplatelet agent is used.38,58 In a prospective Japanese study involving patients being administered antiplatelet agents, delayed bleeding did not occur after upper gastrointestinal endoscopy or colonoscopy, including mucosal biopsy.59 These results support the recommendation that antiplatelet agents should not be discontinued before performing low-risk endoscopic procedures.
Statement 3. We suggest withholding P2Y12 receptor inhibitors for 5–7 days (5 days for clopidogrel and ticagrelor and 7 days for prasugrel) before high-risk endoscopic procedures in patients using a single P2Y12 receptor inhibitor for secondary prevention (strength of recommendation: conditional; level of evidence: very low).
Two RCTs investigated the safety of continued clopidogrel use in patients undergoing colon polypectomy. Chan et al.60 randomly assigned 216 patients receiving clopidogrel, with or without concomitant aspirin, into two groups that either continued the medication or received a placebo. The majority of the polyps were ≤10 mm (83.8%), and the largest polyp was 20 mm in size. The rate of immediate bleeding was slightly higher in the clopidogrel group (8.5%) than in the placebo group (5.5%); however, the difference was not statistically significant. The incidence of delayed bleeding was similar in both groups (3.8% in the clopidogrel group vs. 3.6% in the placebo group; p=0.945), and there was no significant difference in serious atherothrombotic events. Ket et al.61 compared the continuous use of clopidogrel and the temporary replacement of clopidogrel with aspirin. This study randomized 107 patients with 276 polyps ≤10 mm in size into two groups. Intraprocedural bleeding requiring clipping frequently occurred in the clopidogrel group. Conversely, PPB was more common in the temporary replacement group. Thromboembolic complications occurred in one patient in each group, but the difference was not statistically significant. Another small observational study showed that, in clopidogrel users, there was no difference in delayed bleeding between the continuation (0/13) and discontinuation (1/11; p=0.45) groups.62 Although colon polypectomy is usually classified as a high-risk procedure, the immediate and delayed PPB rates for hot snare or CSP for polyps ≤10 mm in size were 2% to 5% and 0.1% to 0.9%, respectively.21,63,64 In two RCTs and one observational study, there was no difference in delayed bleeding when P2Y12 receptor inhibitors were continued. Considering that the delayed bleeding rate after polypectomy for polyps ≤1 cm is low, P2Y12 receptor inhibitors do not need to be discontinued during polypectomy. Delayed bleeding in patients receiving antiplatelet agents is less common after CSP than after conventional polypectomy.23 Therefore, CSP is preferred to minimize PPB in patients using antiplatelet agents. Meticulous hemostasis, including clip placement, should be considered because of a slight increase in the risk of immediate bleeding.
For other high-risk procedures, there have been three observational studies on gastric ESD, colon ESD, and endoscopic sphincterotomy (EST). Kono et al.65 reported the outcome of gastric ESDs for 1,020 lesions, of which a single antiplatelet agent was used in 135 patients. Among the patients using a single antiplatelet agent, 113 discontinued the antiplatelet agent before the procedure, and 22 continued the treatment. The delayed bleeding rate in the discontinuation group was 4.4% (5/113), which was not significantly different from that in the continuation group (4.5%, 1/22). Arimoto et al.66 reported the outcome of 919 colon ESDs, out of which a single antiplatelet agent was administered in 136 cases. Of these, 110 lesions were treated after discontinuation, and 26 were treated while continuing the agent. There was no significant difference in the bleeding rate between the two groups (4.5% in the discontinuation group vs. 0% in the continuation group, p=0.27). Prophylactic clipping was frequently performed in 35.0% (9/26) of the patients in the continuation group and 13.6% (15/110) in the discontinuation group (p=0.01). However, aspirin was the most commonly used antiplatelet agent in this study, and P2Y12 receptor inhibitors were used in only 19.7% of the patients (23/117). A nationwide database study reported the EST bleeding rate in patients treated with antiplatelet agents.67 Severe bleeding after EST occurred in 0.6% (3/462) of patients in the continuation group and 1.3% (43/3,376) of patients in the discontinuation group, with no significant differences between the groups. The proportions of aspirin and P2Y12 receptor inhibitor users in the continuation group were 76.6% (354/462) and 17.3% (80/462), respectively. Three studies reported no significant difference in the incidence of severe postoperative bleeding between the continuation and discontinuation groups. However, these studies were retrospective and had limitations in that differences in the risk factors between patients who discontinued and those who continued antiplatelet agents were not adjusted for. The use of preventive measures, such as the use of endoclips, differed between the groups. Furthermore, the antiplatelet agents used, including aspirin, cilostazol, and P2Y12 receptor inhibitors, and the bleeding risk associated with each agent were not described. Therefore, it is difficult to accurately determine the risk associated with the continuous use of P2Y12 receptor inhibitors based on these results and P2Y12 receptor inhibitors should be discontinued 5 to 7 days before high-risk endoscopic procedures considering the incidence of severe postoperative bleeding, except for colon polypectomy for polyps of less than 1 cm in size. This recommendation applies to patients with a low cardiovascular risk. If a high-risk endoscopic procedure cannot be delayed in a patient with moderate to high cardiovascular risk, it can be performed with a single antiplatelet agent while ensuring meticulous hemostasis and instituting preventive measures, such as endoclip application.
Statement 4. We suggest resuming P2Y12 receptor inhibitors after adequate hemostasis, considering the onset time, the potency of the medication, and the risk of bleeding and cardiovascular events (strength of recommendation: conditional; level of evidence: very low).
Currently, there are no data supporting the ideal timing of resuming P2Y12 receptor inhibitor administration after high-risk endoscopic procedures. Therefore, consulting a cardiologist or neurologist regarding the duration of discontinuation and the timing of resumption will be helpful. Considering that clopidogrel usually requires 3 to 5 days after the resumption of its administration to exert its full effect, it should be resumed as soon as possible if adequate hemostasis is achieved during the procedure and there is no evidence of bleeding after the procedure.68 However, because the onset time of prasugrel or ticagrelor is fast and their antiplatelet potency is greater than that of clopidogrel, the timing of restarting these antiplatelet agents should be determined after considering these characteristics.67 Given that the resumption of P2Y12 receptor inhibitor therapy after high-risk endoscopic procedures may increase the risk of delayed bleeding, patient education and close monitoring are warranted.
Statement 5. For patients on dual antiplatelet therapy (aspirin and clopidogrel), we suggest continuing both antiplatelet agents before low-risk endoscopic procedures (strength of recommendation: conditional; level of evidence: very low).
Patients with coronary stents receiving DAPT are at a risk of developing stent thrombosis, which has an approximately 40% risk of acute myocardial infarction or death if both antiplatelet agents are discontinued.8 In a large US registry, the median time to stent thrombosis was as short as 7 days when both antiplatelet agents were withheld, whereas the median time was prolonged to 122 days when one antiplatelet agent was continued.68,69 In a retrospective cohort study of patients who underwent colon polypectomy in Hong Kong, thrombotic events occurred in 10% of patients (even within seven days) when both agents were discontinued.53 Therefore, discontinuing both antithrombotic agents in patients with coronary artery stents can increase cardiovascular complications and should be avoided, if possible.
Both antiplatelet agents can be administered during low-risk procedures. In a Japanese prospective study that analyzed 48 upper gastrointestinal endoscopies and 12 colonoscopies in 60 patients, including a total of 101 biopsies, there was no significant bleeding for 2 weeks after endoscopy (0/101; 95% CI, 0%–3.6%).38 Furthermore, visual inspection revealed that the time until the bleeding stops after biopsy did not differ between patients taking a single antiplatelet agent and those on DAPT (2.4±1.4 and 2.1±2.1 minutes, respectively).39 There were two RCTs of CSP for colon polyps ≤1 cm. Won et al.70 reported a similar rate of clinically significant delayed bleeding among 87 patients who were randomized to continue DAPT and aspirin after CSP for colon polyps less than 1 cm in size (1/42 [2.4%] with DAPT and 0/45 with aspirin use). No thromboembolic events were observed in either of the groups.
Statement 6. For patients on dual antiplatelet therapy, we recommend withholding the P2Y12 receptor inhibitor for 5–7 days (5 days for clopidogrel and ticagrelor and 7 days for prasugrel) before high-risk endoscopic procedures while continuing aspirin during the procedure (strength of recommendation: strong; level of evidence: very low).
Statement 7. We suggest resuming the P2Y12 receptor inhibitor after adequate hemostasis is secured, considering the onset time, the potency of the medication, and the risk of bleeding and cardiovascular events (strength of recommendation: conditional; level of evidence: very low).
We identified one RCT and six observational studies of high-risk procedures in patients using DAPT. One RCT on colon polypectomy (≤2 cm) showed no significant differences between patients who continued DAPT or aspirin use.60 In this study, among 170 patients undergoing DAPT, 86 maintained DAPT and 84 used only aspirin during colon polypectomy. The incidence of immediate bleeding was slightly higher in those that continued DAPT (8/84 [9.4%] vs. 3/86 [3.5%], p=0.110); however, the delayed bleeding rate did not differ between the groups (4/84 [4.8%] in those that continued DAPT and 4/86 [4.7%] in aspirin users, p=0.958). However, this study included a relatively small number of patients, and the bleeding rate in the aspirin group was higher than that in previous reports that included aspirin users. Therefore, further research on this topic is necessary. Observational studies on gastric ESD, another high-risk procedure, have reported a high bleeding rate among patients who continued DAPT during the procedure. A meta-analysis of six studies on gastric ESD showed higher delayed bleeding rates in patients who continued DAPT than in single antiplatelet users (RR, 2.45; 95% CI, 1.75–3.42), as shown in (Figure 2).5,48,50,65,71,72 The pooled delayed bleeding rate after gastric ESD in the patients who continued DAPT was 22.7% (95% CI, 17.7%–28.5%). Considering the high delayed bleeding rate in patients who continued DAPT, the short-term discontinuation of P2Y12 inhibitors is recommended for patients undergoing high-risk procedures.

Postendoscopic submucosal dissection bleeding comparing the dual antiplatelet therapy and single antiplatelet therapy groups. DAPT, dual antiplatelet therapy; APA, antiplatelet agent; M-H, Mantel–Haenszel; CI, confidence interval.
Statement 8. We do not recommend withholding warfarin before low-risk endoscopic procedures (strength of recommendation: conditional; level of evidence: low).
To update the evidence for the previous KSGE guidelines, we performed a literature search and identified eight retrospective and prospective cohort studies.53,59,73-78 Various low-risk endoscopic procedures, such as double-balloon enteroscopy,73,78 diagnostic endoscopy,53,75 endoscopic papillary large balloon dilatation,76 or endoscopic biopsy,59,77 were evaluated to determine whether warfarin could be continued or discontinued before the procedures. All included studies indicated that the overall rate of early or delayed hemorrhage did not differ between the warfarin interruption and non-interruption groups. However, the temporary interruption of antithrombotic therapy during the procedure was associated with a significantly higher risk of thromboembolic events.53 Considering the significant sequelae of thromboembolisms, warfarin therapy should be continued whenever possible. However, because the bleeding risk increases when the international normalized ratio exceeds the therapeutic range, it should be ensured that the international normalized ratio remains within the therapeutic range during the periendoscopic period of low-risk endoscopic procedures.8
Statement 9. We suggest withholding warfarin 3–5 days before high-risk endoscopic procedures. Heparin bridging therapy is recommended only in patients with high thromboembolic risk (strength of recommendation: conditional; level of evidence: low).
Statement 10. We suggest resuming warfarin as soon as possible once adequate hemostasis has been secured (strength of recommendation: conditional; level of evidence: low).
One multicenter, parallel, non-inferiority RCT79 and 26 retrospective or prospective cohort studies59,62,65,74,80-100 were identified from the literature search. Various high-risk endoscopic procedures, such as colorectal EMR,59,62,74,79-86,88,89,91,93-96,100 ESD,74,79,90,96 gastric ESD,65,74,79,96,98 EST,74,79,92 esophageal ESD,74,79 duodenal EMR,79 PEG,74,99 endoscopic ultrasound-guided fine needle aspiration,74,87 and endoscopic ultrasound-guided biliary drainage97 were evaluated. Most studies focused on the risks and benefits of heparin bridging therapy before the procedure.65,74,79-81,84,85,88,93,94,96,100 Heparin bridging therapy is performed to reduce the risk of thromboembolism associated with the temporary cessation of warfarin therapy. However, procedure-related hemorrhage is significant when warfarin is continued or when heparin bridging therapy is performed during high-risk endoscopic procedures. Several studies commonly recommend not using heparin bridging therapy because of its associated PPB risk.65,74,79-81,84,85,88,93,94,96,100 Some studies have advocated for continuing warfarin therapy before and after therapeutic procedures.59,62,82,83,86,87,89,90,92,95,97-99 However, most of these studies were conducted in Japan, and their retrospective nature hampered changes in previous statements.59,82,83,86,89,90,92,95,97,98 Considering that temporary interruption of anticoagulation therapy during procedures was associated with a significantly higher risk of thromboembolic events53 and continuing antithrombotic therapy was associated with a significantly higher risk of procedure-related bleeding,91 we still need to stratify the patients’ thromboembolic risk, and heparin bridging therapy is recommended for only patients with a high thromboembolic risk.
Statement 11. We suggest omitting the morning dose of DOACs on the day of a low-risk endoscopic procedure (strength of recommendation: conditional; level of evidence: very low).
Statement 12. We suggest resuming DOACs once adequate hemostasis has been secured after a low-risk endoscopic procedure (strength of recommendation: conditional; level of evidence: very low).
DOACs include thrombin (dabigatran) and factor Xa (rivaroxaban, apixaban, and edoxaban) inhibitors. Unlike warfarin, these drugs have a rapid onset of action, and full anticoagulant activity is established within 3 hours of the first dose.8
For low-risk procedures, we identified six studies with adequate control groups. There was no difference in bleeding complications between the continuous DOAC use group and patients who did not use anticoagulants (0/19 in the continuous DOAC use group and 0/263 in the no medications group)101 or patients who temporarily stopped DOAC use (0/18 in the continuous DOAC use group and 0/4 in the cessation of medication group) among patients undergoing endoscopic biopsy.102 However, these studies included only a small number of patients who used DOACs. One prospective observational study enrolled patients who received DOACs and underwent CSP for colon polyps ≤ 10 mm in size.103 In one group, DOACs were not discontinued, whereas in the other group, DOACs were withheld only on the day of the procedure. Delayed bleeding after CSP occurred in 4/27 patients (8.5%) in the DOAC-continued group versus 0/66 (0%) in the group that omitted DOAC on the day of the procedure (p<0.01). A prospective cohort study assessed the effectiveness and safety of the recommendations of the BSG/ESGE guidelines.104 The BSG/ESGE guidelines recommend omitting the morning dose of DOACs on the day of the procedure and resuming the drug the same evening.8 For low-risk procedures, intraprocedural bleeding occurred in 1/105 (0.9%) patients in the group that skipped the morning dose and 2/50 (4.0%) patients in the group that continued the medication until the day of the procedure. Although the difference was not statistically significant due to the small sample size, the group that skipped the morning dose showed a lower bleeding rate. Regarding the time of resumption, there was no difference in the delayed bleeding rate between the group starting on the same day (1/188 [0.5%]) and the group starting later (1/139 [0.7%]). Only one of 327 patients undergoing low-risk procedures (0.3%; 95% CI, 0.01–0.9) experienced thromboembolic events two days after a procedure. Therefore, omitting the morning dose of DOACs is suggested before low-risk procedures, and restarting as soon as possible after the procedure is recommended. Decisions regarding resumption should be made on the basis of the risks of the procedure and the securing of adequate hemostasis. DOACs have a rapid onset of action, with a peak effect occurring 1 to 3 hours after intake.34 In the Perioperative Anticoagulation Use for Surgery Evaluation (PAUSE) study, DOACs were resumed one day after a procedure with a low risk of bleeding, provided that hemostasis was secured.105
Statement 13. We recommend withholding DOACs for more than 48 hours before a high-risk endoscopic procedure (strength of recommendation: strong; level of evidence: low).
Statement 14. We suggest resuming DOACs within 2–3 days after high-risk endoscopic procedures once adequate hemostasis has been secured (strength of recommendation: conditional; level of evidence: very low).
Four retrospective cohort studies on high-risk procedures have been conducted.84,104,106,107 In a retrospective study of 73 patients using DOACs who underwent colon polypectomy, PPB occurred in 16.0% (8/50) of patients who continued DOACs during the procedures.84 However, no PPB was observed in patients who discontinued DOACs for >24 hours (0/4) before the procedure. Another retrospective study reviewed 728 patients who received anticoagulants and underwent ESD for gastric neoplasms at 25 institutions in Japan.106 Delayed bleeding occurred in 11.2% (23/206) of the patients who discontinued DOACs one or two days before ESD, which was significantly lower than that in patients who continued DOACs (35.7%, 5/14). Masuda et al. reported the post-EST bleeding rates in patients using DOACs.107 The post-EST bleeding rate was significantly lower in patients who discontinued DOACs for more than one day (1 of 25 [4%]) than in those who were administered DOACs within one day (5 of 17 [29%]) of the procedure. Therefore, discontinuation of DOACs 1 to 2 days before the procedure decreases postprocedural bleeding rates in high-risk procedures. In the PAUSE study, a protocol of taking the last DOAC dose 3 days before the high-risk procedure and restarting 1 to 2 days after the procedure was adopted.105 In the cohort using this protocol, the risk of major bleeding within 1 month was 0.88% to 2.96%, and the risk of thromboembolic events such as stroke was 0.16% to 0.60%. Therefore, we recommend withholding DOACs for more than 48 hours before high-risk procedures and restarting them within 2 to 3 days after the procedure, according to the bleeding risks. Because the half-life of DOAC is approximately 12 hours, we predict that DOAC levels will be almost undetectable after 48 hours. However, DOAC metabolism is also affected by renal function. In particular, approximately 80% of dabigatran is eliminated by the kidneys, and its elimination is affected by a decline in renal function. Therefore, special attention should be paid to DOAC management in patients with impaired renal function. As shown in Figure 3, the last dabigatran dose should be administered five days before high-risk procedures in patients with renal insufficiency (creatinine clearance<50 mL/min). Approximately 50% of edoxaban is excreted from the kidneys; therefore, it is necessary to extend the duration of discontinuation if the renal function deteriorates. The protocols for periendoscopic DOAC management are summarized in Figure 3.
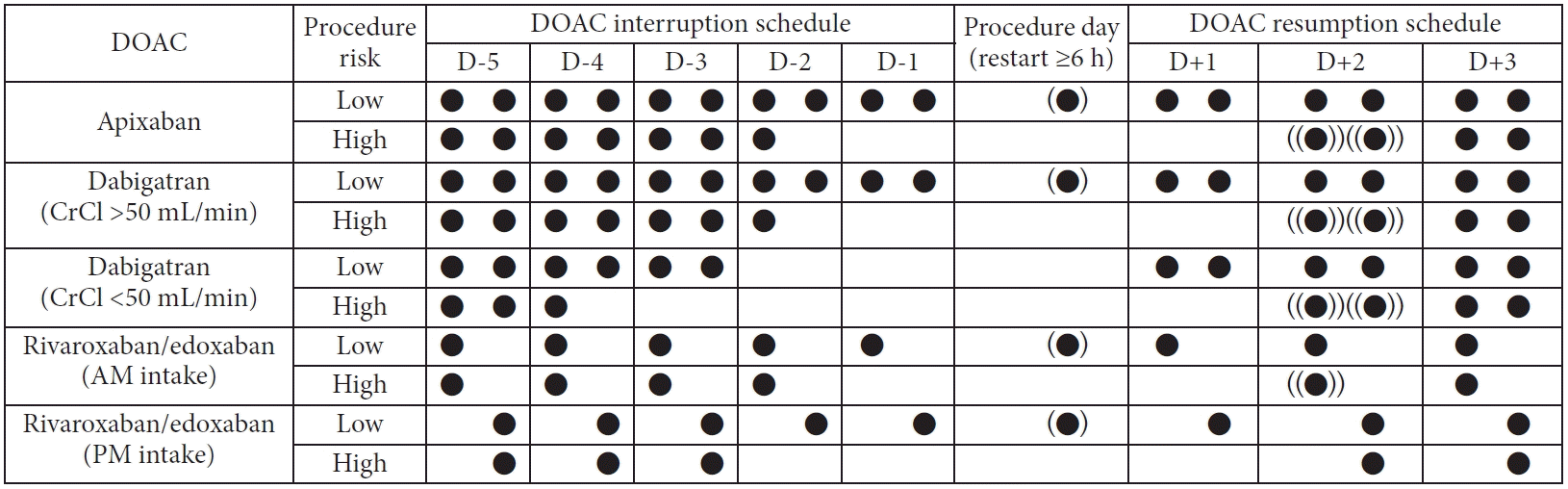
Suggested protocol for perioperative direct oral anticoagulant (DOAC) management. (●) means that DOACs can be administered on the same day if procedures that damage the mucosa (biopsy, cold snare polypectomy, etc.) were not performed. ((●)) means that DOACs can be administered two days after high-risk procedures if there are no risk factors for postprocedural bleeding and no symptoms or signs of postoperative bleeding. CrCl, creatinine clearance; AM, ante meridiem; PM, post meridiem.
There is no evidence supporting the use of heparin bridging therapy in patients receiving DOACs. Neither APAGE/APSDE nor BSG/ESGE recommend heparin bridging therapy during the discontinuation of DOACs because of their rapid onset of action.8,9 The Korean Heart Rhythm Society also does not recommend heparin bridging therapy during the temporary cessation of DOACs because their anticoagulation effect is predictable.108
CONCLUSION AND FUTURE DIRECTIONS
The aging population is experiencing an increase in the incidence of cardiocerebrovascular diseases. The risk of bleeding varies with the endoscopic procedure, and the use of antithrombotic agents can further increase the risk of serious clinical events. To determine whether and when to withhold the use of antithrombotic agents before endoscopic procedures, the risk of thromboembolism caused by withholding antithrombotic agents and the bleeding risk associated with endoscopic procedures should be considered simultaneously. These guidelines should improve the safety and effectiveness of endoscopic procedures by minimizing adverse events, such as bleeding and thromboembolism, in patients using antithrombotic agents. However, owing to the lack of well-designed RCTs, most recommendations are conditional and based on expert opinion and consensus. Therefore, well-designed, large-scale studies on this issue are required. Furthermore, some studies have shown that thrombosis and bleeding tendencies differ between Western and Asian populations.109 However, there is insufficient evidence to suggest that antithrombotic drugs should be managed differently during endoscopic procedures. Therefore, it is necessary to determine whether antithrombotic drugs should be administered to Asian patients while paying more attention to bleeding.
Notes
Conflicts of Interest
Rungsun Rerknimitr is an associate editor of Clinical Endoscopy, Geun Am Song is a member of editorial board of Clinical Endoscopy, and Oh Young Lee is an associate editor of Clinical Endoscopy. The other authors have no potential conflicts of interest.
Funding
None.
Acknowledgments
We thank the executive members of the Korean Society of Gastrointestinal Endoscopy, the Korean Society of Cardiology, the Korean Neurological Association, the Korean Society of Gastroenterology, the Korean College of Helicobacter and Upper Gastrointestinal Research, the Korean Association for the Study of Intestinal Disease, and the Korean Pancreatobiliary Association for reviewing the manuscript and providing expert opinions.
Author Contributions
Conceptualization: GAS, OYL; Formal analysis: SJK, CHT, CSB, CMS; Investigation: CSB, CMS; Methodology: MC; Supervision: YHJ, KDC, KNS, JHH, YS, PWYC, RR, CK, VVK; Writing–original draft: SJK, CHT, Writing–review & editing: GAS, OYL, CSB, CMS, MC, YHJ, JHH, YS, PWYC, RR, CK, VVK, KDC, KNS.