AbstractUpper gastrointestinal postsurgical leaks are life-threatening conditions with high mortality rates and are one of the most feared complications of surgery. Leaks are challenging to manage and often require radiological, endoscopic, or surgical intervention. Steady advancements in interventional endoscopy in recent decades have allowed the development of new endoscopic devices and techniques that provide a more effective and minimally invasive therapeutic option compared to surgery. Since there is no consensus regarding the most appropriate therapeutic approach for managing postsurgical leaks, this review aimed to summarize the best available current data. Our discussion specifically focuses on leak diagnosis, treatment aims, comparative endoscopic technique outcomes, and combined multimodality approach efficacy.
INTRODUCTIONLeaks, perforations, and fistulas, though often used interchangeably, are different types of transmural defects and are associated with different endoscopic closure rates.1 Most of the literature so far has evaluated the efficacy of endoscopic therapy for transmural defects in general rather than for leaks alone.2
Leaks are defined as abnormal communications between the intraluminal and extraluminal compartments, usually owing to a defect in the integrity of the gastrointestinal wall. Upper gastrointestinal (UGI) postsurgical leaks (PSL) have increased in prevalence in recent years3 and are the strongest independent risk factor for postoperative mortality.4
Management of PSL is often challenging and may require radiological, endoscopic, or surgical intervention.5 Traditionally, either rescue surgery or a watch-and-wait strategy followed by surgery if symptoms persist have been the preferred therapeutic approaches. Recently, endoscopy has been emerging as a first-line therapeutic approach and is associated with lower morbidity and better quality of life compared to surgery.6 Steady advancements in interventional endoscopy in recent decades have allowed the development of new endoscopic devices and techniques that provide a more minimally invasive and effective therapeutic option for PSL than surgery. There are multiple endoscopic surgical options available which can be used as solo therapy or in combination with other surgical techniques.
Currently, there is no consensus regarding the most appropriate therapeutic approach for the management of PSL. Due to the continued widespread use of a watch-and-wait strategy in clinically stable patients, leaks are often referred late for endoscopic treatment. Late referral is unfortunately associated with worse endoscopic outcomes.7 Even when diagnosed early, endoscopic management remains complex and often requires multiple endoscopic treatments spanning several months. In this review, we aimed to summarize the best available data on the treatment of UGI with PSL.
POSTSURGICAL LEAKSLeaks may occur immediately after surgery or, more commonly, after several weeks. Acute leaks are commonly attributed to technical issues such as anastomotic tension, stapler malfunction, or suture or staple line seepage. More delayed leaks can result from poor healing, usually due to ischemia at the staple-line or anastomosis.8,9 Several risk factors for leaks have been identified, including age, male gender, need for emergency surgery, smoking, alcohol abuse, American Society of Anaesthesiologists score, body mass index (BMI) >30 kg/m2, BMI <18.5 kg/m2, malnutrition (albumin <3 g/dL), prolonged operative time, anemia, intraoperative blood loss, diabetes, hypertension, renal failure, cardiovascular disease, steroid use, or atherosclerotic calcification of the aorta and the arteries supplying the gastric tube.10-12 Identifying preoperative risk factors can raise clinical suspicion for early leak diagnosis.
The clinical presentation can range from asymptomatic leaks (diagnosed on incidental imaging) to sepsis-related multi-organ failure. Common initial clinical signs include fever and intra-thoracic or intra-abdominal abscesses.10 Chronic leaks have a more insidious presentation. Inspection of surgical drains (if present) helps in the early identification of a surgical leak.13 Although fluoroscopy with a water-soluble contrast medium and computed tomography (CT) with oral contrast are the best imaging modalities for diagnosis, they are prone to false-negative results. CT scan findings include free or contained extraluminal gas, fluid, or contrast material in the mediastinum or abdomen, or visualization of a transmural defect.14 In addition, CT scans allow inspection of regions beyond the esophagogastric lumen. Endoscopy is a reliable diagnostic modality,15 although its diagnostic value seems to be lower for cervical anastomotic leaks.16 Endoscopic examination is crucial to help identify leaks in uncertain cases and to obtain additional critical information such as the extent of tissue disruption, loss of tissue viability, and the presence of downstream strictures that may perpetuate the leak.2,15 The combination of CT and endoscopy is emerging as the gold standard to diagnose PSL as both mucosal integrity and perianastomotic conditions can be examined.10
PSL management is based on several factors, the most important of which include patient stability and time from surgery.5 Spontaneous closure with conservative and radiological interventions is highly variable, with reported rates ranging from 16% to 46%.17,18 Complex and larger leaks are unlikely to heal spontaneously. Factors that predispose patients to delayed or absent spontaneous leak closure include older age (>65 years), malnutrition, high-output drainage, associated malignancy, previous radiation therapy, immunosuppression, sepsis, diabetes, renal failure, and chemotherapy.19,20
AIMS OF TREATMENTThe aim of PSL therapy is to reestablish digestive tract continuity, prevent or treat infections, reduce risk of further contamination, drain fluid collections, and provide nutritional support.21 Determining optimal therapy requires the careful examination of a patientŌĆÖs clinical status, leak characteristics (site, length, time, and presence of necrosis), and a review of all available technical options and surgical expertise.
SURGICAL TREATMENTThe choice of surgical option for PSL depends mostly on the leakage site and the presence or absence of necrosis. It is usually limited to patients with severe sepsis, with an uncontained leak (allowing irrigation and drainage of intra-abdominal collections), with defects not amenable to endoscopic closure, or after failed endoscopic treatment.3,21 Reported outcomes of salvage surgical procedures is often prone to selection bias, as patients are generally sicker or have failed multiple previous therapies. Despite the high morbidity and mortality of salvage surgical therapy,22 it should not be ignored if deemed appropriate, for fear of complications or a poor outcome.
ENDOSCOPIC TREATMENT AND OUTCOMESThe available endoscopic approaches range from primary and secondary closure techniques using endoluminal suturing devices, over-the-scope clips (OTSCs), fibrin glue, diversion with stents, endoscopic internal drainage (EID) using nasocystic drains or double-pigtail stents, endoscopic vacuum therapy (EVT), and septotomy with or without pneumatic dilation.
A summary of the best available evidence per technique and between techniques is summarized in Tables 1 and 2, respectively.23-37
Evaluation per technique1) StentsStents are the endoscopic treatment of choice for oncologic and bariatric leaks, with ample supporting evidence (Fig. 1). Reported clinical success rates range from 48% to 100%.38-42 Multiple endoscopic sessions using multiple stents, as well as use of other adjunctive therapies, may be necessary to achieve leak closure.38,39,43,44 Van Halsema and van Hooft45 reported a clinical success rate of 81.4% for PSL in a 247-patient cohort. Based on three systematic reviews of endoscopic stents,23-25 clinical success in oncologic leaks and perforations ranged from 81% to 87%, with no significant differences among stent types. Repeat endoscopic intervention was needed in 17% to 25% of patients, and 7% to 13% required further surgical intervention. Median stent indwell time ranged from 5 to 10 weeks.45 Even though clinical success rates between stents are comparable, self-expandable metal stent (SEMS) have been reported to have higher technical success rates, reduced risk of migration and subsequent need for stent repositioning, as well as lower risk of perforation compared to self-expandable plastic stents.24 Regarding bariatric leaks, leak closure and adverse event rates range from 65% to 100% and 14% to 86%, respectively, with migration being the most frequent adverse event reported with rates of 5% to 67%.38-42 A recent meta-analysis26 reported an 89% leak closure rate with stent migration occurring in 23% of cases, with the higher success rate possibly due to the more frequent use of stents designed to treat post-bariatric leaks. Recent reports using bariatric stents have shown similar success rates without statistically significant differences in migration rates when compared with conventional stents,40,46,47 with a potentially higher risk of perforation and chest pain.48
Delayed stent placement,49 persistent leakage after initial stent placement,50 proximal esophageal leaks, stents traversing the gastroesophageal junction, larger leak defects, and distal conduit leaks51 are associated with a higher chance of oncologic leak treatment failure. A recent study suggested that stents should not be used for leaks extending more than 30% of the luminal circumference.3 Leaks larger than 1 cm52 and delayed stenting can also affect endoscopic outcomes in bariatric leaks.38,53,54
Though SEMS complications are fairly common (20% to 72%),7 most are usually mild and can be managed conservatively. Stent migration is the most common adverse event, however, in rare cases, severe bleeding and perforation may occur.23-25,40,53 Migration rates are higher with fully covered (FC) SEMS than with partially covered SEMS.55 However, fixation techniques such as OTSCs or suturing may reduce its occurrence56,57 without the difficulties associated with PC-SEMS removal.58 Other methods to reduce the risk of stent migration include the Shim technique59 as well as using wider diameter stents.55,60
2) Over-the-scope-clipsSeveral studies have reported on the effectiveness and safety of OTSCs with most focusing on all types of transmural defects. Haito-Chavez et al.61 reported a 73% success rate in 30 anastomotic leak cases. In contrast, Baron et al.62 and Honegger et al.63 reported success rates below 33% for post-esophagectomy leaks, potentially due to the relatively narrow diameter of the esophageal lumen. A recent systematic review of 1,517 cases found a 66% OTSC success rate in a subset of 97 anastomotic leak cases.27 Another systematic review of anastomotic leaks reported a 73% clinical success rate.28 In the context of post-bariatric leaks, a closure rate of 67.1% was reported.26 The number of endoscopic sessions ranged from 2 to 7.64
3) SutureThe largest study evaluating endoscopic sutures included 122 patients, of which 15 had anastomotic leaks, with a clinical success rate of 27% in leak closure.29 The tissue status and suture feasibility of the wall defect layers were the main outcome predictor. A case series of full-thickness endoscopic suturing of post-sleeve gastrectomy leaks suggested that suturing alone may be sufficient to treat small acute leaks; however, larger leaks would likely require adjunctive therapies such as SEMS placement.67
4) Tissue sealantsThe reported success rate of tissue sealants is highly variable, ranging from 55.7% to 96.8%,68-70 with complication rates reaching 12.5%.26 The efficacy of glue sealants as a primary treatment of PSL is controversial71 since they are usually adjunctive to other primary treatments such as stents and clips.69,72 Reported outcomes are difficult to interpret and prone to bias. Sealants might be more suitable for small leaks (<15 mm), leaks without concurrent infection,73 or residual small collections after the use of other techniques.72 Complete leak closure might require the adjunctive use of vicryl plugs or multiple sealant applications (one to nine sessions repeated every two to three days).26,68
5) Endoscopic vacuum therapyEVT is typically performed using polyurethane sponges (Fig. 2). Macroporous low-density sponges are commonly used because of their greater debriding capacity and stronger contraction under negative pressure, which leads to a more pronounced wound cavity shrinkage (macro-deformation). Permeable films have significant advantages as connection materials compared to polyurethane foam-based drains depending on the clinical indication. These ŌĆ£open-pore film drains,ŌĆØ in which the perforated area of the drain is directly wrapped with an open-pored film, are easier to place due to their smaller diameter and are less adherent to the wound cavity, allowing easier removal.74
The reported clinical success rate of EVT varies widely, ranging from 66.7% to 100%. Schorsch et al.75 and Laukoetter et al.76 reported leak closure rates of 95.2% and 92.3% in 21 and 39 patients, respectively. Median treatment durations were 11 (range, 4ŌĆō46) and 20 days (range, 3ŌĆō104), respectively. Bludau et al.77 reported a healing rate of 77.9% in a cohort of 59 patients. In several studies, additional therapies such as OTSCs or stents were placed after EVT. A recent systematic review of oncologic leaks reported 79.5% and 90% clinical success rates for esophagectomy and gastrectomy leaks, respectively, with stenosis rates reaching 15.9% and 9.2%, respectively.30 Neoadjuvant treatment, rescue application, and intraluminal leak location have all been associated with a higher risk of EVT failure.78
Adverse event rates range from 4.1% to 12.0%, with the majority being minor such as limited bleeding upon sponge exchange, sponge dislodgement, discomfort from repeated procedures, or stricture formation after EVT therapy.73,79 Rarely, major events like significant bleeding can occur.76,80,81
A recently developed technique combining EVT with luminal stenting (VACStent; VAC Stent GmbH) allows oral enteral feeding, continuous drainage, and wound healing.82,83 This technique is only suitable for intraluminal EVT because of the cylindrical shape of the polyurethane foam. The available evidence for this new system is limited to small case series.84
6) Endoscopic internal drainageThe largest study evaluating EID (Fig. 3) as a first-line approach for sleeve gastrectomy leaks (n=617) reported an overall clinical success rate of 84.7%, median treatment duration of 80 days (interquartile range, 29ŌĆō128 days), and a complication rate of 4.5%.31 Complications were managed conservatively in approximately half of the cases. Donatelli et al.85 reported the use of double pigtails as a first-line approach in 67 patients achieving a 78% leak closure rate. Bouchard et al.86 reported EID outcomes in 33 patients post-sleeve gastrectomy or gastric bypass with persistent fluid collections (despite previous endoscopic treatment in 19 patients), with a 78.8% clinical success rate after a mean of 115 days (range, 23ŌĆō773). Gonzalez et al.87 reported the outcomes in 44 patients with sleeve gastrectomy leaks, either as first-line treatment (n=22) or after prior therapy (n=22). The efficacy was comparable between the groups (86% vs. 82%, respectively), with a median 3.0ŌĆō6.0 vs. 4.5┬▒2.4 number of endoscopic sessions. Healing time from endoscopy was 46 days excluding follow-up.87 EID for oncologic leaks has not been widely studied. Recently, Hallit et al.32 and Donatelli et al.88 reported success rates of 100% in 38 and five patients, respectively.
Adverse events such as discomfort, ulceration, dysphagia, and splenic hematoma are rare.86 When combined with surgical cleansing in patients presenting with severe sepsis, EID allows early surgical drainage removal and a reduction in chronic fistula tract formation.89 Longer delays between diagnosis and treatment, larger leaks, sepsis, presence of gastrobronchial fistula, and previous OTSC deployment are risk factors for treatment failure.33
7) Endoscopic septotomyEndoscopic septotomy may be used as a first-line or salvage therapy, with clinical success rates ranging from 70% to 85%.89-91 Baretta et al.92 reported their experience with endoscopic septotomy in 27 patients with post-bariatric leaks. After one to six endoscopic sessions, all patients achieved leak resolution with a mean healing time of 18 days. More than half of the patients underwent additional dilatation of the angularis incisura stenosis. Complications included perforation and bleeding.93
Comparison between techniquesThere are a limited number of studies comparing efficacy of different endoscopic modalities for the management of leaks. Farnik et al.34 retrospectively compared FC-SEMS and OTSCs and reported leak closure rates of 69% and 31%, respectively; clinical success after primary intervention was 40% for FC-SEMS and 70% for OTSCs. However, defects treated with FC-SEMS were larger than those treated with OTSC (12.6 mm vs. 7.1 mm). Manta et al.94 primarily utilized OTSCs or combined OTSCs with SEMS, with leak closure rate of 81% to 85%. Lorenzo et al.33 reported better outcomes with EID than with a combination of stents, tissue sealants, and OTSCs (86% vs. 64%, p=0.55) in 100 patients with post-sleeve gastrectomy leaks.
Recently, the outcomes of SEMS placement were compared with those of EVT for PSL treatment in several meta-analyses. EVT was associated with a higher leak closure rate (16%ŌĆō21% higher), a lower mortality rate (10%ŌĆō12% lower),30,35,36 fewer adverse events,35 and shorter treatment duration,35,36 with no difference in the length of hospital stays.30,35 These results have not been replicated in all studies. Berlth et al.,95 in a large cohort of 111 patients with post-esophagectomy leaks, reported a closure rate of 85.7% for EVT vs. 72.4% for SEMS. This difference was not statistically significant.
Recently, EID has also been compared with stent placement and EVT. Hallit et al.32 reported higher closure rates with EID than with stent placement for the treatment of oncological PSL (95% vs. 67%, p=0.002). The success rate increased to 100% and 77% after using adjunctive therapies (OTSC and crossover to EID, respectively). In univariate analysis, only primary EID use was associated with treatment success. Jung et al.37 compared EID and low-negative pressure EVT for oncological PSL and reported better overall success rates (100% vs. 85.2%, p=0.03) and primary success rates (91.4% vs. 74.1%, p=0.09) with EID. EVT had a shorter treatment duration but required more sessions. However, the two cohorts of patients were not treated uniformly since each institution performed only one type of endoscopic treatment. Low negative pressure applied during EVT could have affected its efficacy.
Multimodality approachB├©ge et al.96 assessed multimodal treatment in 27 patients who underwent bariatric PSL. Primary procedures were successful 41% of the time and all patients achieved leak resolution after a mean 4.4 endoscopic sessions and a mean of 86 days. Rodrigues-Pinto et al.55 performed the largest multicenter study, which included 206 patients treated with UGI PSL. Although high overall clinical success (80.1%) and leak resolution rates (83.5%) were achieved, the first endoscopic technique was successful in only 44% of leaks and multimodal therapy was often required (40.8% of the time). Clinical success correlated with the duration of treatment, with leak resolution rates reaching a plateau between the third and fourth endoscopic techniques (approximately 70ŌĆō80%), and a median time to leak closure of 52 days. Only 10% of the leaks successfully closed after 125 days of treatment. A different study also demonstrated a reduced endoscopic resolution rate of sleeve gastrectomy leaks over time, from 76.4% in the first postoperative month to 48.5% after six months.97 This reflects the need to better define endoscopic failure. In a survey study, although there was no definitive definition consensus, persistent inflammation with clinical sepsis and the impossibility of resuming oral feeding were suggested as components of the definition.98 The inability to close the leak over time, especially after four months of treatment, should also prompt consideration of therapeutic alternatives such as surgery.
TREATMENT SELECTIONDespite the increasing effectiveness of EID and EVT in the treatment of PSL, stent placement remains the most widely available and frequently used technique in current practice.55,97,98 The approach to UGI PSL should always be tailored in a patient-specific manner. Leak location, size, chronicity, and associated cavities are the most relevant leak characteristics to consider when deciding the treatment.98 The type of previous surgery should also influence therapeutic decisions: EVT and stent placement (with or without percutaneous/surgical drainage) is a good option in oncologic leaks, whereas EVT and EID are best after bariatric surgery.98 Early referral of leaks is the most important predictor of treatment success.55,97,99,100
Considering most responses to a survey study,98 stent placement is commonly used for acute and small leaks without associated collections (defects up to 3 cm in size), OTSC placement for defects up to 1 cm in size, and endoscopic suturing for defects up to 2 cm in size. In the setting of an associated fluid collection, stents can be considered if external drainage is also performed. Otherwise, EVT and EID are options for acute and chronic leaks, whereas endoscopic septotomy can be performed for leaks lasting more than 4 weeks. Although endoscopic septotomy can be considered for all leak sizes, EVT is ideal for leaks > 2 cm in size.
CONCLUSIONSTherapeutic endoscopy for UGI PSL management is safe, effective, and reproducible when a skilled endoscopy team is available. However, endoscopic management should be personalized and multidisciplinary, involving close collaboration among interventional endoscopists, radiologists, and surgeons. There is wide expert variation in the management of these patients, emphasizing the need to identify patients as early as possible and to select the best therapeutic option for each patient. Comparisons between different approaches are difficult because of heterogeneous study populations, the retrospective nature of relevant studies, lack of uniform definitions, and lack of prospective comparative studies. Therefore, it is difficult to establish a standard therapeutic algorithm. Combined treatment with simultaneous or sequential use of several endoscopic methods appears to be optimal for the management of UGI PSL. Future research should focus on assessing the effectiveness of combined therapies rather than focusing on individual endoscopic methods alone.
Fig.┬Ā1.Endoscopic image of a post total gastrectomy leak (A) with an associated collection (B), with surgical drain in place. A fully covered self-expandable metal stent (28/23/28├Ś155 mm) was placed covering the leak (C). Stent was removed 40 days later with leak resolution (D). 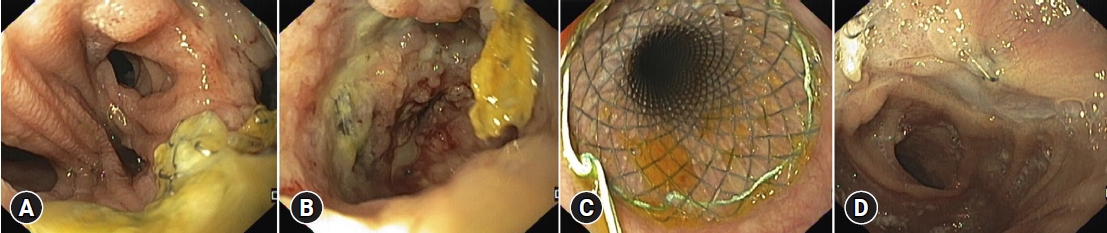
Fig.┬Ā2.Endoscopic image of a post Mckeown esophagectomy leak, with a surgical drain in place and a guidewire placed in the gastric lumen (A). A partially covered self-expandable metal stent (28/23/28├Ś155 mm) was placed covering the leak (B, C), however, leak persisted after stent removal (D). Intraluminal endoscopic vacuum therapy was performed (E) with leak resolution after two sponge exchanges (F). 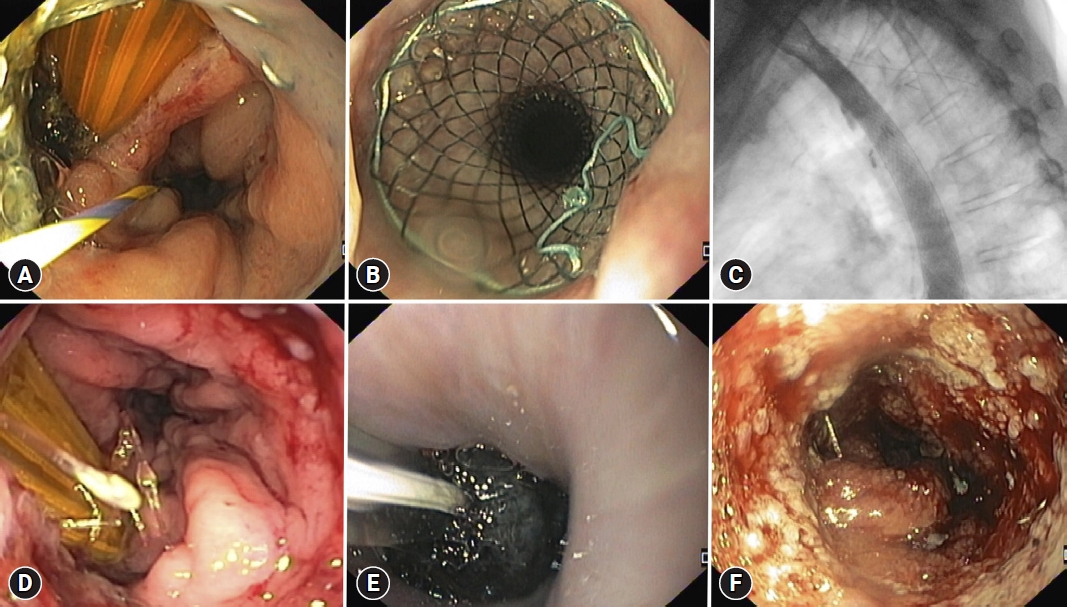
Fig.┬Ā3.Endoscopic image of a post-sleeve gastrectomy leak (A, B), with an associated perigastric collection, visible on fluoroscopy (C). Endoscopic internal drainage of the collection was performed with placement of one double-pigtail plastic stent (7 Fr 4 cm) across the leak orifice (D), with drainage of purulent content (E). Fluoroscopic image of the double-pigtail stent, with one extremity in the perigastric collection and the other in the gastric tube (F). 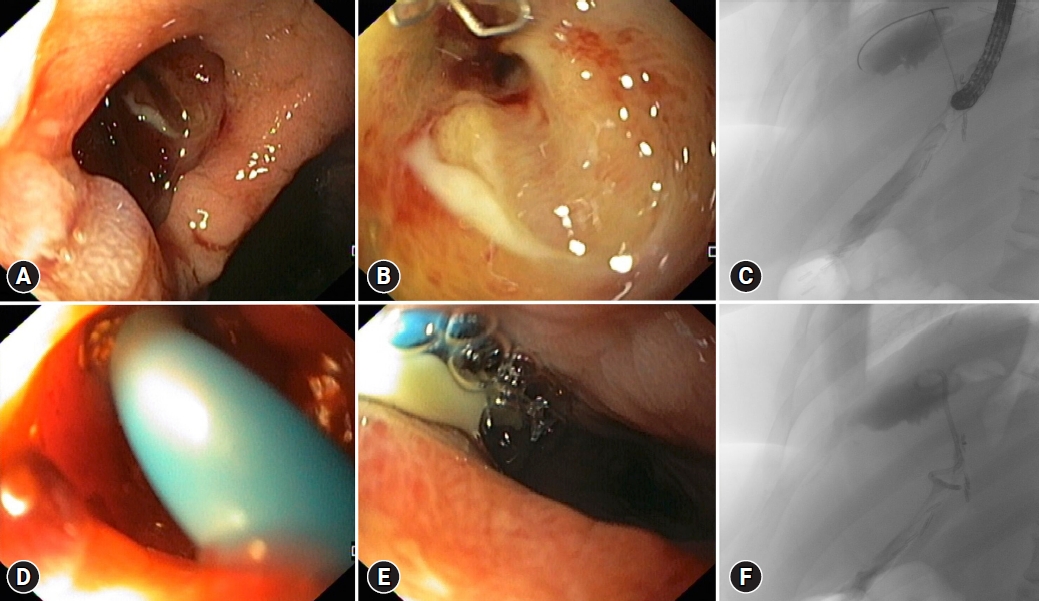
Table┬Ā1.Summary of best evidence of endoscopic treatment for postsurgical leaks per technique
Table┬Ā2.Comparison between endoscopic techniques for treatment of postsurgical leaks
REFERENCES1. Bruce J, Krukowski ZH, Al-Khairy G, et al. Systematic review of the definition and measurement of anastomotic leak after gastrointestinal surgery. Br J Surg 2001;88:1157ŌĆō1168.
2. Bemelman WA, Baron TH. Endoscopic management of transmural defects, including leaks, perforations, and fistulae. Gastroenterology 2018;154:1938ŌĆō1946.
3. Messager M, Warlaumont M, Renaud F, et al. Recent improvements in the management of esophageal anastomotic leak after surgery for cancer. Eur J Surg Oncol 2017;43:258ŌĆō269.
4. Buttar NS. Minimally invasive endoscopic approaches to manage postsurgical leaks: time to recognize the finger in the dike. Gastrointest Endosc 2021;93:1300ŌĆō1303.
5. Abou Rached A, Basile M, El Masri H. Gastric leaks post sleeve gastrectomy: review of its prevention and management. World J Gastroenterol 2014;20:13904ŌĆō13910.
6. Cai JX, Schweitzer MA, Kumbhari V. Endoscopic management of bariatric surgery complications. Surg Laparosc Endosc Percutan Tech 2016;26:93ŌĆō101.
7. Cereatti F, Grassia R, Drago A, et al. Endoscopic management of gastrointestinal leaks and fistulae: what option do we have? World J Gastroenterol 2020;26:4198ŌĆō4217.
8. Al-Kurd A, Grinbaum R, Abubeih A, et al. Not all leaks are created equal: a comparison between leaks after sleeve gastrectomy and Roux-en-Y gastric bypass. Obes Surg 2018;28:3775ŌĆō3782.
9. Souto-Rodr├Łguez R, Alvarez-S├Īnchez MV. Endoluminal solutions to bariatric surgery complications: a review with a focus on technical aspects and results. World J Gastrointest Endosc 2017;9:105ŌĆō126.
10. Fabbi M, Hagens ER, van Berge Henegouwen MI, et al. Anastomotic leakage after esophagectomy for esophageal cancer: definitions, diagnostics, and treatment. Dis Esophagus 2021;34:doaa039.
11. Iannelli A, Schneck AS, Ragot E, et al. Laparoscopic sleeve gastrectomy as revisional procedure for failed gastric banding and vertical banded gastroplasty. Obes Surg 2009;19:1216ŌĆō1220.
12. Makuuchi R, Irino T, Tanizawa Y, et al. Esophagojejunal anastomotic leakage following gastrectomy for gastric cancer. Surg Today 2019;49:187ŌĆō196.
13. Kapila S, Rozen WM, Huang T, et al. Determining between chyle leak and anastomotic leak after esophageal reconstruction: the utility of methylene blue dye. Laryngoscope 2012;122:779ŌĆō780.
14. Swinnen J, Eisendrath P, Rigaux J, et al. Self-expandable metal stents for the treatment of benign upper GI leaks and perforations. Gastrointest Endosc 2011;73:890ŌĆō899.
15. Hogan BA, Winter DC, Broe D, et al. Prospective trial comparing contrast swallow, computed tomography and endoscopy to identify anastomotic leak following oesophagogastric surgery. Surg Endosc 2008;22:767ŌĆō771.
16. Nederlof N, de Jonge J, de Vringer T, et al. Does routine endoscopy or contrast swallow study after esophagectomy and gastric tube reconstruction change patient management? J Gastrointest Surg 2017;21:251ŌĆō258.
17. Gonzalez R, Sarr MG, Smith CD, et al. Diagnosis and contemporary management of anastomotic leaks after gastric bypass for obesity. J Am Coll Surg 2007;204:47ŌĆō55.
18. Rosenthal RJ; International Sleeve Gastrectomy Expert Panel, Diaz AA, et al. International Sleeve Gastrectomy Expert Panel Consensus Statement: best practice guidelines based on experience of >12,000 cases. Surg Obes Relat Dis 2012;8:8ŌĆō19.
19. Haffejee AA. Surgical management of high output enterocutaneous fistulae: a 24-year experience. Curr Opin Clin Nutr Metab Care 2004;7:309ŌĆō316.
20. Lloyd DA, Gabe SM, Windsor AC. Nutrition and management of enterocutaneous fistula. Br J Surg 2006;93:1045ŌĆō1055.
21. Schaheen L, Blackmon SH, Nason KS. Optimal approach to the management of intrathoracic esophageal leak following esophagectomy: a systematic review. Am J Surg 2014;208:536ŌĆō543.
22. Fernandez AZ Jr, DeMaria EJ, Tichansky DS, et al. Experience with over 3,000 open and laparoscopic bariatric procedures: multivariate analysis of factors related to leak and resultant mortality. Surg Endosc 2004;18:193ŌĆō197.
23. Dasari BV, Neely D, Kennedy A, et al. The role of esophageal stents in the management of esophageal anastomotic leaks and benign esophageal perforations. Ann Surg 2014;259:852ŌĆō860.
24. Kamarajah SK, Bundred J, Spence G, et al. Critical appraisal of the impact of oesophageal stents in the management of oesophageal anastomotic leaks and benign oesophageal perforations: an updated systematic review. World J Surg 2020;44:1173ŌĆō1189.
25. van Boeckel PG, Sijbring A, Vleggaar FP, et al. Systematic review: temporary stent placement for benign rupture or anastomotic leak of the oesophagus. Aliment Pharmacol Ther 2011;33:1292ŌĆō1301.
26. Rogalski P, Swidnicka-Siergiejko A, Wasielica-Berger J, et al. Endoscopic management of leaks and fistulas after bariatric surgery: a systematic review and meta-analysis. Surg Endosc 2021;35:1067ŌĆō1087.
27. Kobara H, Mori H, Nishiyama N, et al. Over-the-scope clip system: a review of 1517 cases over 9┬Āyears. J Gastroenterol Hepatol 2019;34:22ŌĆō30.
28. Vermeulen BD, Siersema PD. Diagnosis and endoscopic treatment of esophageal leakage: a systematic review. Tech Gastrointest Endosc 2019;21:58ŌĆō64.
29. Sharaiha RZ, Kumta NA, DeFilippis EM, et al. A large multicenter experience with endoscopic suturing for management of gastrointestinal defects and stent anchorage in 122 patients: a retrospective review. J Clin Gastroenterol 2016;50:388ŌĆō392.
30. Tavares G, Tustumi F, Trist├Żo LS, et al. Endoscopic vacuum therapy for anastomotic leak in esophagectomy and total gastrectomy: a systematic review and meta-analysis. Dis Esophagus 2021;34:doaa132.
31. Donatelli G, Spota A, Cereatti F, et al. Endoscopic internal drainage for the management of leak, fistula, and collection after sleeve gastrectomy: our experience in 617 consecutive patients. Surg Obes Relat Dis 2021;17:1432ŌĆō1439.
32. Hallit R, Calmels M, Chaput U, et al. Endoscopic management of anastomotic leak after esophageal or gastric resection for malignancy: a multicenter experience. Therap Adv Gastroenterol 2021;14:17562848211032823.
33. Lorenzo D, Guilbaud T, Gonzalez JM, et al. Endoscopic treatment of fistulas after sleeve gastrectomy: a┬Ācomparison of internal drainage versus closure. Gastrointest Endosc 2018;87:429ŌĆō437.
34. Farnik H, Driller M, Kratt T, et al. Indication for 'Over the scope' (OTS)-clip vs. covered self-expanding metal stent (cSEMS) is unequal in upper gastrointestinal leakage: results from a retrospective head-to-head comparison. PLoS One 2015;10:e0117483.
35. do Monte Junior ES, de Moura DT, Ribeiro IB, et al. Endoscopic vacuum therapy versus endoscopic stenting for upper gastrointestinal transmural defects: systematic review and meta-analysis. Dig Endosc 2021;33:892ŌĆō902.
36. Scognamiglio P, Reeh M, Karstens K, et al. Endoscopic vacuum therapy versus stenting for postoperative esophago-enteric anastomotic leakage: systematic review and meta-analysis. Endoscopy 2020;52:632ŌĆō642.
37. Jung CF, Hallit R, M├╝ller-Dornieden A, et al. Endoscopic internal drainage and low negative-pressure endoscopic vacuum therapy for anastomotic leaks after oncologic upper gastrointestinal surgery. Endoscopy 2022;54:71ŌĆō74.
38. Alazmi W, Al-Sabah S, Ali DA, et al. Treating sleeve gastrectomy leak with endoscopic stenting: the Kuwaiti experience and review of recent literature. Surg Endosc 2014;28:3425ŌĆō3428.
39. El Mourad H, Himpens J, Verhofstadt J. Stent treatment for fistula after obesity surgery: results in 47 consecutive patients. Surg Endosc 2013;27:808ŌĆō816.
40. Shehab HM, Hakky SM, Gawdat KA. An endoscopic strategy combining mega stents and over-the-scope clips for the management of post-bariatric surgery leaks and fistulas (with video). Obes Surg 2016;26:941ŌĆō948.
41. Southwell T, Lim TH, Ogra R. Endoscopic therapy for treatment of staple line leaks post-laparoscopic sleeve gastrectomy (LSG): experience from a large bariatric surgery centre in New Zealand. Obes Surg 2016;26:1155ŌĆō1162.
42. van Wezenbeek MR, de Milliano MM, Nienhuijs SW, et al. A specifically designed stent for anastomotic leaks after bariatric surgery: experiences in a tertiary referral hospital. Obes Surg 2016;26:1875ŌĆō1880.
43. Sakran N, Goitein D, Raziel A, et al. Gastric leaks after sleeve gastrectomy: a multicenter experience with 2,834 patients. Surg Endosc 2013;27:240ŌĆō245.
44. Simon F, Siciliano I, Gillet A, et al. Gastric leak after laparoscopic sleeve gastrectomy: early covered self-expandable stent reduces healing time. Obes Surg 2013;23:687ŌĆō692.
45. van Halsema EE, van Hooft JE. Clinical outcomes of self-expandable stent placement for benign esophageal diseases: a pooled analysis of the literature. World J Gastrointest Endosc 2015;7:135ŌĆō153.
46. Boerlage TC, Houben GP, Groenen MJ, et al. A novel fully covered double-bump stent for staple line leaks after bariatric surgery: a retrospective analysis. Surg Endosc 2018;32:3174ŌĆō3180.
47. Hamid HK, Emile SH, Saber AA, et al. Customized bariatric stents for sleeve gastrectomy leak: are they superior to conventional esophageal stents? A systematic review and proportion meta-analysis. Surg Endosc 2021;35:1025ŌĆō1038.
48. de Moura DT, de Moura EG, Neto MG, et al. Outcomes of a novel bariatric stent in the management of sleeve gastrectomy leaks: a multicenter study. Surg Obes Relat Dis 2019;15:1241ŌĆō1251.
49. El Hajj II, Imperiale TF, Rex DK, et al. Treatment of esophageal leaks, fistulae, and perforations with temporary stents: evaluation of efficacy, adverse events, and factors associated with successful outcomes. Gastrointest Endosc 2014;79:589ŌĆō598.
50. Persson S, Rouvelas I, Kumagai K, et al. Treatment of esophageal anastomotic leakage with self-expanding metal stents: analysis of risk factors for treatment failure. Endosc Int Open 2016;4:E420ŌĆōE426.
51. Freeman RK, Ascioti AJ, Giannini T, et al. Analysis of unsuccessful esophageal stent placements for esophageal perforation, fistula, or anastomotic leak. Ann Thorac Surg 2012;94:959ŌĆō964.
52. Nedelcu M, Manos T, Cotirlet A, et al. Outcome of leaks after sleeve gastrectomy based on a new algorithm adressing leak size and gastric stenosis. Obes Surg 2015;25:559ŌĆō563.
53. Murino A, Arvanitakis M, Le Moine O, et al. Effectiveness of endoscopic management using self-expandable metal stents in a large cohort of patients with post-bariatric leaks. Obes Surg 2015;25:1569ŌĆō1576.
54. Puig CA, Waked TM, Baron TH Sr, et al. The role of endoscopic stents in the management of chronic anastomotic and staple line leaks and chronic strictures after bariatric surgery. Surg Obes Relat Dis 2014;10:613ŌĆō617.
55. Rodrigues-Pinto E, Pereira P, Sousa-Pinto B, et al. Retrospective multicenter study on endoscopic treatment of upper GI postsurgical leaks. Gastrointest Endosc 2021;93:1283ŌĆō1299.
56. Park KH, Lew D, Samaan J, et al. Comparison of no stent fixation, endoscopic suturing, and a novel over-the-scope clip for stent fixation in preventing migration of fully covered self-expanding metal stents: a retrospective comparative study (with video). Gastrointest Endosc 2022;96:771ŌĆō779.
57. Schiemer M, Bettinger D, Mueller J, et al. Reduction of esophageal stent migration rate with a novel over-the-scope fixation device (with video). Gastrointest Endosc 2022;96:1ŌĆō8.
58. Ngamruengphong S, Sharaiha R, Sethi A, et al. Fully-covered metal stents with endoscopic suturing vs. partially-covered metal stents for benign upper gastrointestinal diseases: a comparative study. Endosc Int Open 2018;6:E217ŌĆōE223.
59. Choi CW, Kang DH, Kim HW, et al. Full covered self-expandable metal stents for the treatment of anastomotic leak using a silk thread. Medicine (Baltimore) 2017;96:e7439.
60. Sdralis EIK, Petousis S, Rashid F, et al. Epidemiology, diagnosis, and management of esophageal perforations: systematic review. Dis Esophagus 2017;30:1ŌĆō6.
61. Haito-Chavez Y, Law JK, Kratt T, et al. International multicenter experience with an over-the-scope clipping device for endoscopic management of GI defects (with video). Gastrointest Endosc 2014;80:610ŌĆō622.
62. Baron TH, Song LM, Ross A, et al. Use of an over-the-scope clipping device: multicenter retrospective results of the first U.S. experience (with videos). Gastrointest Endosc 2012;76:202ŌĆō208.
63. Honegger C, Valli PV, Wiegand N, et al. Establishment of Over-The-Scope-Clips (OTSC┬«) in daily endoscopic routine. United European Gastroenterol J 2017;5:247ŌĆō254.
64. Keren D, Eyal O, Sroka G, et al. Over-the-Scope Clip (OTSC) system for sleeve gastrectomy leaks. Obes Surg 2015;25:1358ŌĆō1363.
65. Hagel AF, Naegel A, Lindner AS, et al. Over-the-scope clip application yields a high rate of closure in gastrointestinal perforations and may reduce emergency surgery. J Gastrointest Surg 2012;16:2132ŌĆō2138.
66. Mercky P, Gonzalez JM, Aimore Bonin E, et al. Usefulness of over-the-scope clipping system for closing digestive fistulas. Dig Endosc 2015;27:18ŌĆō24.
67. Cai JX, Khashab MA, Okolo PI 3rd, et al. Full-thickness endoscopic suturing of staple-line leaks following laparoscopic sleeve gastrectomy. Endoscopy 2014;46 Suppl 1 UCTN:E623ŌĆōE624.
68. B├Čhm G, Mossdorf A, Klink C, et al. Treatment algorithm for postoperative upper gastrointestinal fistulas and leaks using combined vicryl plug and fibrin glue. Endoscopy 2010;42:599ŌĆō602.
69. Kotzampassi K, Eleftheriadis E. Tissue sealants in endoscopic applications for anastomotic leakage during a 25-year period. Surgery 2015;157:79ŌĆō86.
70. Plat VD, Bootsma BT, van der Wielen N, et al. The role of tissue adhesives in esophageal surgery, a systematic review of literature. Int J Surg 2017;40:163ŌĆō168.
71. Surace M, Mercky P, Demarquay JF, et al. Endoscopic management of GI fistulae with the over-the-scope clip system (with video). Gastrointest Endosc 2011;74:1416ŌĆō1419.
72. Vilallonga R, Himpens J, Bosch B, et al. Role of percutaneous glue treatment after persisting leak after laparoscopic sleeve gastrectomy. Obes Surg 2016;26:1378ŌĆō1383.
73. Chan SM, Auyeung KK, Lam SF, et al. Current status in endoscopic management of upper gastrointestinal perforations, leaks and fistulas. Dig Endosc 2022;34:43ŌĆō62.
74. Gutschow CA, Schlag C, Vetter D. Endoscopic vacuum therapy in the upper gastrointestinal tract: when and how to use it. Langenbecks Arch Surg 2022;407:957ŌĆō964.
75. Schorsch T, M├╝ller C, Loske G. Endoscopic vacuum therapy of perforations and anastomotic insufficiency of the esophagus. Chirurg 2014;85:1081ŌĆō1093.
76. Laukoetter MG, Mennigen R, Neumann PA, et al. Successful closure of defects in the upper gastrointestinal tract by endoscopic vacuum therapy (EVT): a prospective cohort study. Surg Endosc 2017;31:2687ŌĆō2696.
77. Bludau M, Fuchs HF, Herbold T, et al. Results of endoscopic vacuum-assisted closure device for treatment of upper GI leaks. Surg Endosc 2018;32:1906ŌĆō1914.
78. Jung DH, Huh CW, Min YW, et al. Endoscopic vacuum therapy for the management of upper GI leaks and perforations: a multicenter retrospective study of factors associated with treatment failure (with video). Gastrointest Endosc 2022;95:281ŌĆō290.
79. Watkins JR, Farivar AS. Endoluminal therapies for esophageal perforations and leaks. Thorac Surg Clin 2018;28:541ŌĆō554.
80. Ahrens M, Schulte T, Egberts J, et al. Drainage of esophageal leakage using endoscopic vacuum therapy: a prospective pilot study. Endoscopy 2010;42:693ŌĆō698.
81. Pournaras DJ, Hardwick RH, Safranek PM, et al. Endoluminal vacuum therapy (E-Vac): a treatment option in oesophagogastric surgery. World J Surg 2018;42:2507ŌĆō2511.
82. Chon SH, T├Čx U, Lorenz F, et al. A novel hybrid stent with endoscopic vacuum therapy for treating leaks of the upper gastrointestinal tract. Visc Med 2021;37:403ŌĆō409.
83. Lange J, Dormann A, Bulian DR, et al. VACStent: combining the benefits of endoscopic vacuum therapy and covered stents for upper gastrointestinal tract leakage. Endosc Int Open 2021;9:E971ŌĆōE976.
84. Chon SH, Scherdel J, Rieck I, et al. A new hybrid stent using endoscopic vacuum therapy in treating esophageal leaks: a prospective single-center experience of its safety and feasibility with mid-term follow-up. Dis Esophagus 2022;35:doab067.
85. Donatelli G, Dumont JL, Cereatti F, et al. Treatment of leaks following sleeve gastrectomy by endoscopic internal drainage (EID). Obes Surg 2015;25:1293ŌĆō1301.
86. Bouchard S, Eisendrath P, Toussaint E, et al. Trans-fistulary endoscopic drainage for post-bariatric abdominal collections communicating with the upper gastrointestinal tract. Endoscopy 2016;48:809ŌĆō816.
87. Gonzalez JM, Lorenzo D, Guilbaud T, et al. Internal endoscopic drainage as first line or second line treatment in case of postsleeve gastrectomy fistulas. Endosc Int Open 2018;6:E745ŌĆōE750.
88. Donatelli G, Dumont JL, Cereatti F, et al. Endoscopic internal drainage as first-line treatment for fistula following gastrointestinal surgery: a case series. Endosc Int Open 2016;4:E647ŌĆōE651.
89. Donatelli G, Catheline JM, Dumont JL, et al. Outcome of leaks after sleeve gastrectomy based on a new algorithm addressing leak size and gastric stenosis. Obes Surg 2015;25:1258ŌĆō1260.
90. Gjeorgjievski M, Imam Z, Cappell MS, et al. A comprehensive review of endoscopic management of sleeve gastrectomy leaks. J Clin Gastroenterol 2021;55:551ŌĆō576.
91. Hughes D, Hughes I, Khanna A. Management of staple line leaks following sleeve gastrectomy: a systematic review. Obes Surg 2019;29:2759ŌĆō2772.
92. Baretta G, Campos J, Correia S, et al. Bariatric postoperative fistula: a life-saving endoscopic procedure. Surg Endosc 2015;29:1714ŌĆō1720.
93. Campos JM, Ferreira FC, Teixeira AF, et al. Septotomy and balloon dilation to treat chronic leak after sleeve gastrectomy: technical principles. Obes Surg 2016;26:1992ŌĆō1993.
94. Manta R, Caruso A, Cellini C, et al. Endoscopic management of patients with post-surgical leaks involving the gastrointestinal tract: a large case series. United European Gastroenterol J 2016;4:770ŌĆō777.
95. Berlth F, Bludau M, Plum PS, et al. Self-expanding metal stents versus endoscopic vacuum therapy in anastomotic leak treatment after oncologic gastroesophageal surgery. J Gastrointest Surg 2019;23:67ŌĆō75.
96. B├©ge T, Emungania O, Vitton V, et al. An endoscopic strategy for management of anastomotic complications from bariatric surgery: a prospective study. Gastrointest Endosc 2011;73:238ŌĆō244.
97. Christophorou D, Valats JC, Funakoshi N, et al. Endoscopic treatment of fistula after sleeve gastrectomy: results of a multicenter retrospective study. Endoscopy 2015;47:988ŌĆō996.
98. Rodrigues-Pinto E, Repici A, Donatelli G, et al. International multicenter expert survey on endoscopic treatment of upper gastrointestinal anastomotic leaks. Endosc Int Open 2019;7:E1671ŌĆōE1682.
|
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
