AbstractBackground/AimsThis study aimed to examine the synergistic effect of independent risk factors on post-endoscopic retrograde cholangiopancreatography (ERCP) pancreatitis (PEP).
MethodsThis multicenter retrospective study included 1,273 patients with native papillae who underwent ERCP for bile dust stones in Japan. Independent PEP risk factors were identified using univariate and multivariate analyses. Significant risk factors for PEP in the multivariate analysis were included in the final analysis to examine the synergistic effect of independent risk factors for PEP.
ResultsPEP occurred in 45 of 1,273 patients (3.5%). Three factors including difficult cannulation Ōēź10 minutes, pancreatic injection, and normal serum bilirubin level were included in the final analysis. The incidences of PEP in patients with zero, one, two, and three factors were 0.5% (2/388), 1.9% (9/465), 6.0% (17/285), and 12.6% (17/135), respectively. With increasing risk factors for PEP, the incidence of PEP significantly increased (1 factor vs. 2 factors, p=0.006; 2 factors vs. 3 factors, p=0.033).
INTRODUCTIONEndoscopic retrograde cholangiopancreatography (ERCP) is a standard treatment for biliary and pancreatic diseases. However, post-ERCP pancreatitis (PEP) is the most common adverse event of ERCP, and guidelines document the independent risk factors for PEP.1-3
However, patients often present with a combination of risk factors for PEP in clinical practice. A prospective study revealed that the odds ratios in female sex alone, and the combination of a normal serum bilirubin level plus female sex, and normal serum bilirubin plus female sex plus difficult cannulation were 2.5, 4.8, and 16.2, respectively.4 Another randomized controlled study reported the PEP incidence in patients with Ōēź3 risk factors to be 32 of 183 (17.5%).5
Although endoscopists should consider the interactions between independent PEP risk factors, there is little evidence on this topic. Therefore, this study aimed to investigate the synergistic effect of independent PEP risk factors in patients with common bile duct (CBD) stones, the most common indication for ERCP.
METHODSPatientsThis multicenter retrospective study included 1,273 patients with native papillae who underwent ERCP for CBD stones at three institutions in Japan (Saiseikai Kumamoto Hospital, Kumamoto Chuo Hospital, and Kumamoto City Hospital) between April 2012 and March 2020. The exclusion criteria were as follows: (1) individuals with prior ERCP, (2) patients with a Billroth II or Roux-en-Y reconstruction, (3) patients with biliary pancreatitis, (4) patients who underwent unsuccessful cannulation, and (5) patients with no stones found on ERCP. The present study was approved by the institutional review boards of each participating institution (approval number: 601) and opt-out consent was obtained.
Endoscopic treatmentWe performed ERCP using a side-view duodenoscope. Pethidine hydrochloride and midazolam were administered for appropriate sedation. After selective biliary cannulation, endoscopic papillary balloon dilation using a small balloon of <12-mm diameter, endoscopic sphincterotomy, or endoscopic papillary large balloon dilation using a large balloon of diameter Ōēź12-mm diameter was conducted for stone extraction or biliary drainage. The decision of whether to use of preventive methods for PEP, such as rectal nonsteroidal anti-inflammatory drugs (NSAIDs) or prophylactic pancreatic stent placement, was based on the operatorŌĆÖs decision.
Study definitions1) Post-ERCP pancreatitisThe consensus criterion for endoscopic adverse events by Cotton et al.6 was used for the diagnosis and grading of PEP. To elaborate, PEP was defined as the occurrence of typical abdominal pain accompanied by amylase levels exceeding three times the normal range. We classified PEP into three grades: mild, moderate, and severe PEP based on the need for unplanned hospital admission or the length of hospital stay, which were Ōēż3, 4 to 10, and >10 nights, respectively.
2) Precut sphincterotomyAlthough various pre-cutting techniques have been reported based on the current guidelines,7 transpancreatic sphincterotomy using a short-tipped sphincterotome over the guidewire was selected as the pre-cutting method in patients with guidewire insertion into the main pancreatic duct. In patients without guidewire insertion into the main pancreatic duct, we selected a pre-cutting method that used a needle knife from the papillary orifice toward the oral side of the papillary bulge.
Statistical analysisThe chi-square test or FisherŌĆÖs exact test for categorical variables and WelchŌĆÖs t-test for continuous variables were used in univariate analyses. A multivariate logistics regression model was used to identify the independent risk factors for PEP. The factors that met both a p-value of <0.05, as determined by univariate analyses, and the definitive or likely risk factors in the European Society of Gastrointestinal Endoscopy guidelines,2 were included in the logistic regression multivariate analysis. We used factors that were significant in multivariate analyses to examine the synergistic effect of independent PEP risk factors. The synergistic effects of independent risk factors for PEP were examined based on the number of risk factors. A two-sided p-value of <0.05 was denoted as the threshold for statistical significance. The EZR statistical software ver. 1.61 (Saitama Medical Center, Jichi Medical University) was used for all statistical analyses.8
RESULTSERCP indicationsThe ERCP indications in this study were acute cholangitis in 840 patients (66.0%), mild cholangitis in 442 patients (34.7%), moderate cholangitis in 295 patients (23.2%), and severe cholangitis in 103 patients (8.1%); cholestasis without cholangitis in 268 patients (21.1%); and silent CBD stones in 165 patients (13.0%).
Incidence rates and severity of PEPPEP occurred in 45 of 1,273 patients (3.5%). PEP was classified as mild in 28 patients (62.2%), moderate in 13 patients (28.9%), and severe in four patients (8.9%).
Risk factors for PEPThe results of the univariate and multivariate analyses of PEP risk factors are shown in Tables 1 and 2, respectively. In the univariate analysis, there were eight significant risk factors for PEP, including difficult cannulation Ōēź10 minutes, pancreatic injection, prolonged procedure time, prophylactic pancreatic stent placement, contrast-assisted cannulation, precut sphincterotomy, pancreatic guidewire-assisted cannulation, and normal serum bilirubin level. Of the 166 patients who underwent pancreatic guide wire-assisted cannulation, 159 patients underwent pancreatic injection. Of the 65 patients who underwent pre-cutting, transpancreatic sphincterotomy using a short-tipped sphincterotome was performed in 63 patients. In two patients without insertion of a guidewire into the pancreatic duct, we selected a pre-cutting method that used a needle knife from the papillary orifice toward the oral side of the papillary bulge. None of the patients underwent needle-knife fistulotomy.
In the multivariate analysis, difficult cannulation Ōēź10 minutes, pancreatic injection, and normal serum bilirubin level were significant PEP risk factors.
Synergistic effect of the independent risk factors for PEP
Figure 1 shows the incidence rates of PEP according to the number of risk factors. The PEP rates in patients with zero, one, two, and three factors were 0.5% (2/388), 1.9% (9/465), 6.0% (17/285), and 12.6% (17/135), respectively. As the risk factors for PEP increased, the incidence of PEP significantly increased (1 factor vs. 2 factors, p=0.006; 2 factors vs. 3 factors, p=0.033).
Each PEP risk in patients with two risk factors for PEP were as follows; difficult cannulation for Ōēź10 minutes and normal serum bilirubin: 3/36 (8.3%), difficult cannulation for Ōēź10 minutes and pancreatic injection: 8/109 (7.3%), and pancreatic injection and normal serum bilirubin: 6/140 (4.3%).
DISCUSSIONThis study aimed to examine the synergistic effects of the independent risk factors for PEP. Our findings suggest that the risk of PEP occurrence multiplied, rather than increased by addition, as the number of risk factors increased.
PEP is the most common and severe adverse event associated with ERCP. Numerous studies have investigated its incidence,9 and the current guidelines have clearly presented the independent risk factors for PEP.1-3
According to a large-scale meta-analysis,9 the incidence of PEP is 9.7%. The incidence of PEP in high-risk patients, defined as those with one or more risk factors, was 14.7%. The study also revealed that PEP was classified as mild in approximately 60% of patients, while it was observed to be moderate to severe in the remaining 40%.9 Another randomized controlled study found that the incidence of PEP was notably higher in patients with more than three risk factors, with an incidence of 17.5% (32 out of 183 patients).5 Our findings are in line with these results.
Based on the findings of this study, aggressive prophylaxis of PEP is particularly important in patients with several risk factors. The current guidelines provide recommendations for PEP prophylaxis.1-3 Prophylaxis by pancreatic stent is an effective strategy for the occurrence of PEP and some meta-analyses have reported a significant reduction in the overall occurrence of PEP (odds ratio, 0.22ŌĆō0.39) and a reduced occurrence of severe PEP.10-15
Rectal NSAIDs are a well-known pharmacological preventive measure for PEP.1-3 Rectal NSAIDs are reported to reduce the occurrence of PEP with an odds ratio between 0.24 and 0.63.2 Aggressive hydration is also an effective approach for PEP prevention.2,3 Recent meta-analyses have demonstrated that the administration of 35 to 45 mL/kg of RingerŌĆÖs lactate solution over 8 to 10 hours as part of aggressive hydration contributed to a reduction in PEP incidence, with odds ratios ranging from 0.29 to 0.47.16-18 Additionally, aggressive hydration decreased the occurrence of moderateŌĆōsevere PEP, with an odds ratio of 0.16.16 According to a network meta-analysis, the combination of aggressive hydration and rectal indomethacin is the most effective PEP prevention strategy. Its preventive efficacy was observed to be 70% to 99% higher than that of single prophylactic measures.19 Therefore, aggressive prophylaxis for PEP with these strategies should be considered in patients with several risk factors for PEP.
Recent research has suggested that silent CBD stones and choledocholithiasis without acute cholangitis are significant risk factors for PEP.20-24ŃĆĆSuch patients, including those with silent CBD stones, tend to have lower levels of cholestasis than in patients with cholangitis. Therefore, these patients often present multiple risk factors, including normal serum bilirubin levels and difficulty with selective cannulation. In addition, pancreatic injections are frequently administered to patients who experience challenging cannulation. Therefore, our study findings align with those of previous studies that found a higher risk of PEP in patients without acute cholangitis, including those with asymptomatic CBD stones.
A scoring system would be useful; however, because of the limited number of studies on this topic, no established system is available for the estimation of the synergistic effects of PEP risk factors.25-29
This study had several limitations. First, the major limitation of the present study is its retrospective design, which may lead to potential selection bias. Furthermore, although we identified the risk factors for PEP via a multivariate analysis, unmeasured confounding factors associated with PEP incidence may be residual. Second, the sample size of the patients with PEP was small. Further prospective studies are warranted to verify the synergistic effects of the independent risk factors for PEP.
In conclusion, the risk of PEP incidence may not be additive; rather, the risk multiplies as the risk factors for PEP increase. Aggressive prophylaxis is strongly recommended, particularly in patients with several risk factors for PEP.
NOTESAcknowledgments
We would like to thank the staff involved in endoscopic retrograde cholangiopancreatography at the participating institutions.
Author Contributions
Conceptualization: HS; Data curation: HS, YK, TK; Formal analysis: HS, TK; Investigation: HS, YK, TS, KK, AU, JN, MU, IM, SH, ST; Methodology: all authors; Project administration: HS, SH, ST; Resources: HS, YK, TS, KK, AU, JN, MU, IM, SH, ST; Software: HS, TK; Supervision: SH, ST; Validation: HS, TK, SH, ST; Visualization: HS, ST; WritingŌĆōoriginal draft: HS; WritingŌĆōreview & editing: YK, TS, KK, AU, JN, MU, IM, TK, SH, ST.
Fig.┬Ā1.The incidence rates of post-endoscopic retrograde cholangiopancreatography pancreatitis based on the number of independent risk factors. PEP, post-endoscopic retrograde cholangiopancreatography pancreatitis. 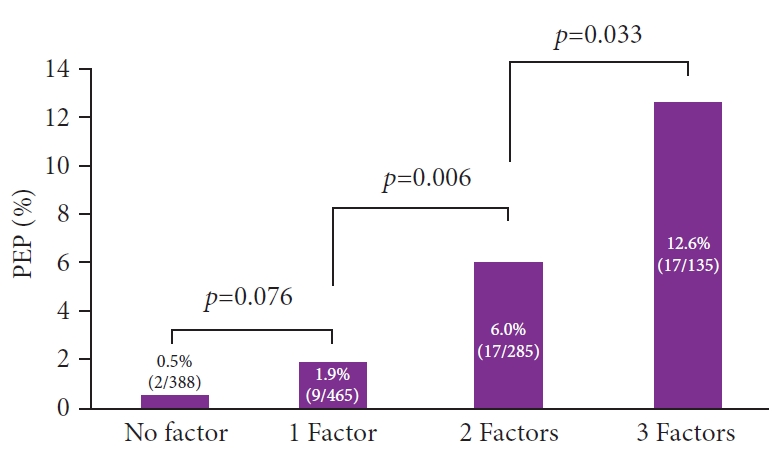
Table┬Ā1.Risk factors for PEP (univariate analyses) Table┬Ā2.Risk factors for PEP (multivariate analyses) REFERENCES1. Mine T, Morizane T, Kawaguchi Y, et al. Clinical practice guideline for post-ERCP pancreatitis. J Gastroenterol 2017;52:1013ŌĆō1022.
2. Dumonceau JM, Kapral C, Aabakken L, et al. ERCP-related adverse events: European Society of Gastrointestinal Endoscopy (ESGE) Guideline. Endoscopy 2020;52:127ŌĆō149.
3. ASGE Standards of Practice Committee, Chandrasekhara V, Khashab MA, et al. Adverse events associated with ERCP. Gastrointest Endosc 2017;85:32ŌĆō47.
4. Freeman ML, DiSario JA, Nelson DB, et al. Risk factors for post-ERCP pancreatitis: a prospective, multicenter study. Gastrointest Endosc 2001;54:425ŌĆō434.
5. Sofuni A, Maguchi H, Mukai T, et al. Endoscopic pancreatic duct stents reduce the incidence of post-endoscopic retrograde cholangiopancreatography pancreatitis in high-risk patients. Clin Gastroenterol Hepatol 2011;9:851ŌĆō858.
6. Cotton PB, Eisen GM, Aabakken L, et al. A lexicon for endoscopic adverse events: report of an ASGE workshop. Gastrointest Endosc 2010;71:446ŌĆō454.
7. Ryozawa S, Itoi T, Katanuma A, et al. Japan Gastroenterological Endoscopy Society guidelines for endoscopic sphincterotomy. Dig Endosc 2018;30:149ŌĆō173.
8. Kanda Y. Investigation of the freely available easy-to-use software ŌĆśEZRŌĆÖ for medical statistics. Bone Marrow Transplant 2013;48:452ŌĆō458.
9. Kochar B, Akshintala VS, Afghani E, et al. Incidence, severity, and mortality of post-ERCP pancreatitis: a systematic review by using randomized, controlled trials. Gastrointest Endosc 2015;81:143ŌĆō149.
10. Vadal├Ā di Prampero SF, Faleschini G, Panic N, et al. Endoscopic and pharmacological treatment for prophylaxis against postendoscopic retrograde cholangiopancreatography pancreatitis: a meta-analysis and systematic review. Eur J Gastroenterol Hepatol 2016;28:1415ŌĆō1424.
11. Fan JH, Qian JB, Wang YM, et al. Updated meta-analysis of pancreatic stent placement in preventing post-endoscopic retrograde cholangiopancreatography pancreatitis. World J Gastroenterol 2015;21:7577ŌĆō7583.
12. Mazaki T, Mado K, Masuda H, et al. Prophylactic pancreatic stent placement and post-ERCP pancreatitis: an updated meta-analysis. J Gastroenterol 2014;49:343ŌĆō355.
13. Shi QQ, Ning XY, Zhan LL, et al. Placement of prophylactic pancreatic stents to prevent post-endoscopic retrograde cholangiopancreatography pancreatitis in high-risk patients: a meta-analysis. World J Gastroenterol 2014;20:7040ŌĆō7048.
14. Akshintala VS, Sperna Weiland CJ, Bhullar FA, et al. Non-steroidal anti-inflammatory drugs, intravenous fluids, pancreatic stents, or their combinations for the prevention of post-endoscopic retrograde cholangiopancreatography pancreatitis: a systematic review and network meta-analysis. Lancet Gastroenterol Hepatol 2021;6:733ŌĆō742.
15. Choudhary A, Bechtold ML, Arif M, et al. Pancreatic stents for prophylaxis against post-ERCP pancreatitis: a meta-analysis and systematic review. Gastrointest Endosc 2011;73:275ŌĆō282.
16. Wu D, Wan J, Xia L, Chen J, Zhu Y, Lu N. The efficiency of aggressive hydration with lactated ringer solution for the prevention of post-ercp pancreatitis: a systematic review and meta-analysis. J Clin Gastroenterol 2017;51:e68ŌĆōe76.
17. Zhang ZF, Duan ZJ, Wang LX, et al. Aggressive hydration with lactated ringer solution in prevention of postendoscopic retrograde cholangiopancreatography pancreatitis: a meta-analysis of randomized controlled trials. J Clin Gastroenterol 2017;51:e17ŌĆōe26.
18. Radadiya D, Devani K, Arora S, et al. Peri-Procedural Aggressive Hydration for Post Endoscopic Retrograde Cholangiopancreatography (ERCP) Pancreatitis Prophylaxsis: Meta-analysis of Randomized Controlled Trials. Pancreatology 2019;19:819ŌĆō827.
19. M├Īrta K, Gede N, Szak├Īcs Z, et al. Combined use of indomethacin and hydration is the best conservative approach for post-ERCP pancreatitis prevention: a network meta-analysis. Pancreatology 2021;21:1247ŌĆō1255.
20. Saito H, Koga T, Sakaguchi M, et al. Post-endoscopic retrograde cholangiopancreatography pancreatitis in patients with asymptomatic common bile duct stones. J Gastroenterol Hepatol 2019;34:1153ŌĆō1159.
21. Kim SB, Kim KH, Kim TN. Comparison of outcomes and complications of endoscopic common bile duct stone removal between asymptomatic and symptomatic patients. Dig Dis Sci 2016;61:1172ŌĆō1177.
22. Xu XD, Qian JQ, Dai JJ, et al. Endoscopic treatment for choledocholithiasis in asymptomatic patients. J Gastroenterol Hepatol 2020;35:165ŌĆō169.
23. Hakuta R, Hamada T, Nakai Y, et al. Natural history of asymptomatic bile duct stones and association of endoscopic treatment with clinical outcomes. J Gastroenterol 2020;55:78ŌĆō85.
24. Saito H, Kadono Y, Shono T, et al. Increased post-endoscopic retrograde cholangiopancreatography pancreatitis for choledocholithiasis without acute cholangitis. J Gastroenterol Hepatol 2022;37:327ŌĆō334.
25. Friedland S, Soetikno RM, Vandervoort J, et al. Bedside scoring system to predict the risk of developing pancreatitis following ERCP. Endoscopy 2002;34:483ŌĆō488.
26. Jeurnink SM, Siersema PD, Steyerberg EW, et al. Predictors of complications after endoscopic retrograde cholangiopancreatography: a prognostic model for early discharge. Surg Endosc 2011;25:2892ŌĆō2900.
27. DiMagno MJ, Spaete JP, Ballard DD, et al. Risk models for post-endoscopic retrograde cholangiopancreatography pancreatitis (PEP): smoking and chronic liver disease are predictors of protection against PEP. Pancreas 2013;42:996ŌĆō1003.
|
|
|||||||||||||||||||||||||||||||||||||
