Application of artificial intelligence for diagnosis of early gastric cancer based on magnifying endoscopy with narrow-band imaging
Article information
Abstract
Although magnifying endoscopy with narrow-band imaging is the standard diagnostic test for gastric cancer, diagnosing gastric cancer using this technology requires considerable skill. Artificial intelligence has superior image recognition, and its usefulness in endoscopic image diagnosis has been reported in many cases. The diagnostic performance (accuracy, sensitivity, and specificity) of artificial intelligence using magnifying endoscopy with narrow band still images and videos for gastric cancer was higher than that of expert endoscopists, suggesting the usefulness of artificial intelligence in diagnosing gastric cancer. Histological diagnosis of gastric cancer using artificial intelligence is also promising. However, previous studies on the use of artificial intelligence to diagnose gastric cancer were small-scale; thus, large-scale studies are necessary to examine whether a high diagnostic performance can be achieved. In addition, the diagnosis of gastric cancer using artificial intelligence has not yet become widespread in clinical practice, and further research is necessary. Therefore, in the future, artificial intelligence must be further developed as an instrument, and its diagnostic performance is expected to improve with the accumulation of numerous cases nationwide.
INTRODUCTION
Gastric cancer has one of the highest cancer mortality rates in the world, although the number of cases detected at an early stage is increasing and the mortality rate is decreasing due to advances in endoscopic technology,1-3 including magnifying endoscopy with narrow-band imaging (ME-NBI).4,5 ME-NBI with vessel plus surface classification is a standard diagnostic test for gastric cancer as the mucosa can be observed at 80 to 100× magnification and evaluation of surface structures and vascular patterns allows for the diagnosis of cancer.5,6 However, because the diagnosis of gastric cancer with ME-NBI requires considerable skill,7 its use has been limited.
Artificial intelligence (AI) has superior image recognition, and its usefulness in endoscopic image diagnosis has been reported in many cases.8-13 The usefulness of AI for the detection and diagnosis of gastric cancer using endoscopic images was first reported in 201810 and has been reported in several other subsequent studies.11-16 Additionally, we have reported the diagnostic performance of AI for gastric cancer using ME-NBI still images17 and videos.18 If more reports emerge that AI can support endoscopists using ME-NBI, its use may become widespread and beneficial in daily practice. However, although other reports on the subject are expected, there is currently no comprehensive review of this topic.
Therefore, in this review, based on a literature search, we aim to clarify the current status of the application of AI in the diagnosis of early gastric cancer based on ME-NBI.
DIAGNOSTIC PERFORMANCE OF AI USING ME-NBI STILL IMAGES FOR GASTRIC CANCER
The diagnostic performance of AI using ME-NBI still images for gastric cancer was reported in a previous study of 1,492 cancerous and 1,078 noncancerous images, which were used to educate the AI (Fig. 1),17 and 151 cancerous and 107 noncancerous images (continuous cases of images as external validation) were used to evaluate its diagnostic performance (accuracy, sensitivity, and specificity as primary endpoints).17 Cancerous images included both differentiated and undifferentiated types. The AI used a convolutional neural network (CNN) system that enables the classification of input images.

Endoscopic images used to educate the convolutional neural network. (A) Differentiated-type cancer: irregular vessels (yellow arrows indicate demarcation line). (B) Differentiated-type cancer: irregular vessels and structures (yellow arrows indicate demarcation lines). (C) Undifferentiated-type cancer: irregular vessels and structure. (D) Undifferentiated-type cancer: irregular vessels. (E) Gastritis: atrophy of fundic gland. (F) Gastritis: intestinal metaplasia. Reproduced from Horiuchi et al. Dig Dis Sci 2020;65:1355–1363, with permission.17
Diagnostic performance was defined in terms of accuracy (the sum of the number of images accurately diagnosed as cancer and noncancerous divided by the total number of images), sensitivity (the number of images accurately diagnosed as cancer divided by the total number of cancerous images), and specificity (the number of images accurately diagnosed as noncancerous divided by the total number of noncancerous images). The area under the receiver operating characteristic curve (AUROC curve) was calculated to evaluate the performance of the CNN system.
The CNN system diagnosed the images at a rate of 51.8 images/s (0.02 seconds per image). The accuracy was 85.3% (220/258), with a sensitivity of 95.4% and a specificity of 71.0%. Endoscopy is a screening test, and its sensitivity (the ability to detect cancer) is of utmost importance. The sensitivity was particularly high in this study. Therefore, this study demonstrated the usefulness of AI for the diagnosis of gastric cancer.
Additionally, several studies have reported the diagnostic performance of AI for gastric cancer using ME-NBI still images (Table 1).17,19-21 While the aforementioned study was conducted on differentiated- and undifferentiated-type cancers, another study regarding differentiated-type gastric cancer reported an accuracy of 98.7%, a sensitivity of 98%, and a specificity of 100% using a CNN system.19 In another study, the CNN system outperformed expert endoscopists with regard to sensitivity (CNN, 91.2%; expert 1, 78.2%; expert 2, 81.2%) and non-expert endoscopists with regard to accuracy (CNN, 90.9%; non-expert 1, 69.8%; non-expert 2, 73.6%), sensitivity (CNN, 91.2%; non-expert 1, 77.7%; non-expert 2, 74.1%), and specificity (CNN, 90.6%; non-expert 1, 62.0%; non-expert 2, 73.1%).20 A comparison of the diagnostic performances of the CNN system and expert endoscopists, which was constructed as a diagnostic model for gastric cancer, reported that expert endoscopists improved their accuracy and sensitivity by referring to the CNN system, although the difference was not significant.21
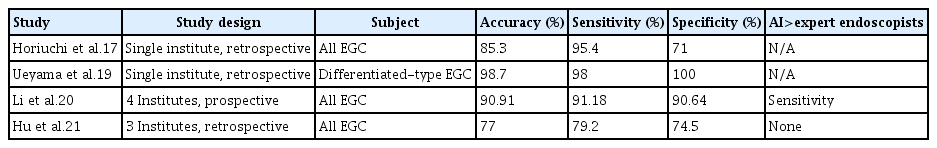
Studies regarding the diagnostic performance of AI using magnifying endoscopy with narrow band still images for gastric cancer
In conclusion, the diagnostic performance of AI using ME-NBI still images for gastric cancer was acceptable, and the diagnostic speed was high.
DIAGNOSTIC PERFORMANCE OF AI USING ME-NBI VIDEOS FOR GASTRIC CANCER
AI using ME-NBI still images improves the diagnostic accuracy of the secondary judgment of endoscopic images at health checkups and is useful for determining treatment strategies. However, to demonstrate whether AI can improve the accuracy of the real-time detection of gastric cancer during endoscopy, the results must be validated using videos. This is because actual endoscopy does not use still images to detect lesions, but rather uses moving images obtained by manipulating the endoscope to detect lesions. Therefore, we previously conducted a study to clarify the diagnostic performance of AI using ME-NBI videos for gastric cancer.18 The CNN system used in the still image study17 was also used to evaluate 174 videos of continuous cases as external validation (87 cancerous and 87 noncancerous areas).18 Using the same videos, the diagnostic performance of 11 expert endoscopists was calculated and compared with that of the CNN system. The diagnostic performance was evaluated using the accuracy, sensitivity, and specificity of the CNN system. The AUROC curves were determined.
When endoscopic videos were used, the CNN system had an accuracy of 85.1%, a sensitivity of 87.4%, a specificity of 82.8%, and an AUROC curve of 0.868. The accuracy of the CNN system was superior to that of two endoscopists, inferior to that of one endoscopist, and not significantly different from that of the other eight endoscopists. The CNN system had superior sensitivity to that of three endoscopists and was not significantly different from that of eight endoscopists; its specificity was superior to that of two endoscopists, inferior to that of three endoscopists, and not significantly different from that of six endoscopists. These results demonstrate the usefulness of the CNN system for gastric cancer diagnosis using ME-NBI videos.
The diagnostic performance of AI for gastric cancer using ME-NBI videos has been previously reported (Table 2).18,22 A multicenter, retrospective study reported an accuracy of 87.2%, a sensitivity of 96.9%, and a specificity of 82.3%, with significantly better sensitivity than expert endoscopists.22 In addition, the study reported that the CNN system improved the diagnostic performance of endoscopists.

Studies regarding the diagnostic performance of AI using magnifying endoscopy with narrow-band imaging videos for gastric cancer
Therefore, the diagnostic performance of AI using ME-NBI still images and videos for gastric cancer was high, suggesting its usefulness in clinical practice.
HISTOLOGICAL DIAGNOSIS OF GASTRIC CANCER BY AI USING ME-NBI STILL IMAGES
Indications for endoscopic submucosal dissection (ESD) differ depending on the histological type of gastric cancer (differentiated or undifferentiated). For differentiated-type gastric cancer, ESD is indicated for intramucosal carcinoma without ulceration and with ulceration that is 3 cm or less in diameter. However, for undifferentiated-type gastric cancer, ESD is indicated only for intramucosal carcinomas measuring 2 cm or less in diameter without ulceration. In contrast, although biopsy is the gold standard for histological diagnosis,23-25 biopsy results deviate from the histological type after treatment in approximately 18.4% of cases, resulting in a correct diagnosis rate of 81.6%.26-29 As histological diagnosis can affect the treatment strategy, a discrepancy between pre- and post-treatment diagnoses can affect clinical outcomes. For example, if an intramucosal carcinoma with a tumor diameter of 3 cm is diagnosed as undifferentiated-type gastric cancer before treatment, the patient will likely undergo surgical gastrectomy. However, if the tumor is diagnosed postoperatively as a differentiated-type intramucosal carcinoma with a diameter of 3 cm, the patient may have been cured with ESD instead of surgical gastrectomy, indicating the possibility of overtreatment.30
In contrast, typical ME-NBI findings have been reported for both differentiated and undifferentiated types of gastric cancer (Fig. 2).31-37 The overall accuracy of AI in differentiating the histological types of gastric cancer using still images has been reported as 86.2%. For differentiated types, the sensitivity was 88.6% and the specificity was 78.6%; for undifferentiated types, the sensitivity was 78.6% and the specificity was 88.6%.38 In addition, the CNN system was superior to the expert endoscopist group in overall accuracy and sensitivity of differentiated types. The agreement between the CNN system and post-treatment pathology results was 0.641, which was higher than that of each expert endoscopist. These results suggest that AI is a promising tool for histological diagnosis of gastric cancer.
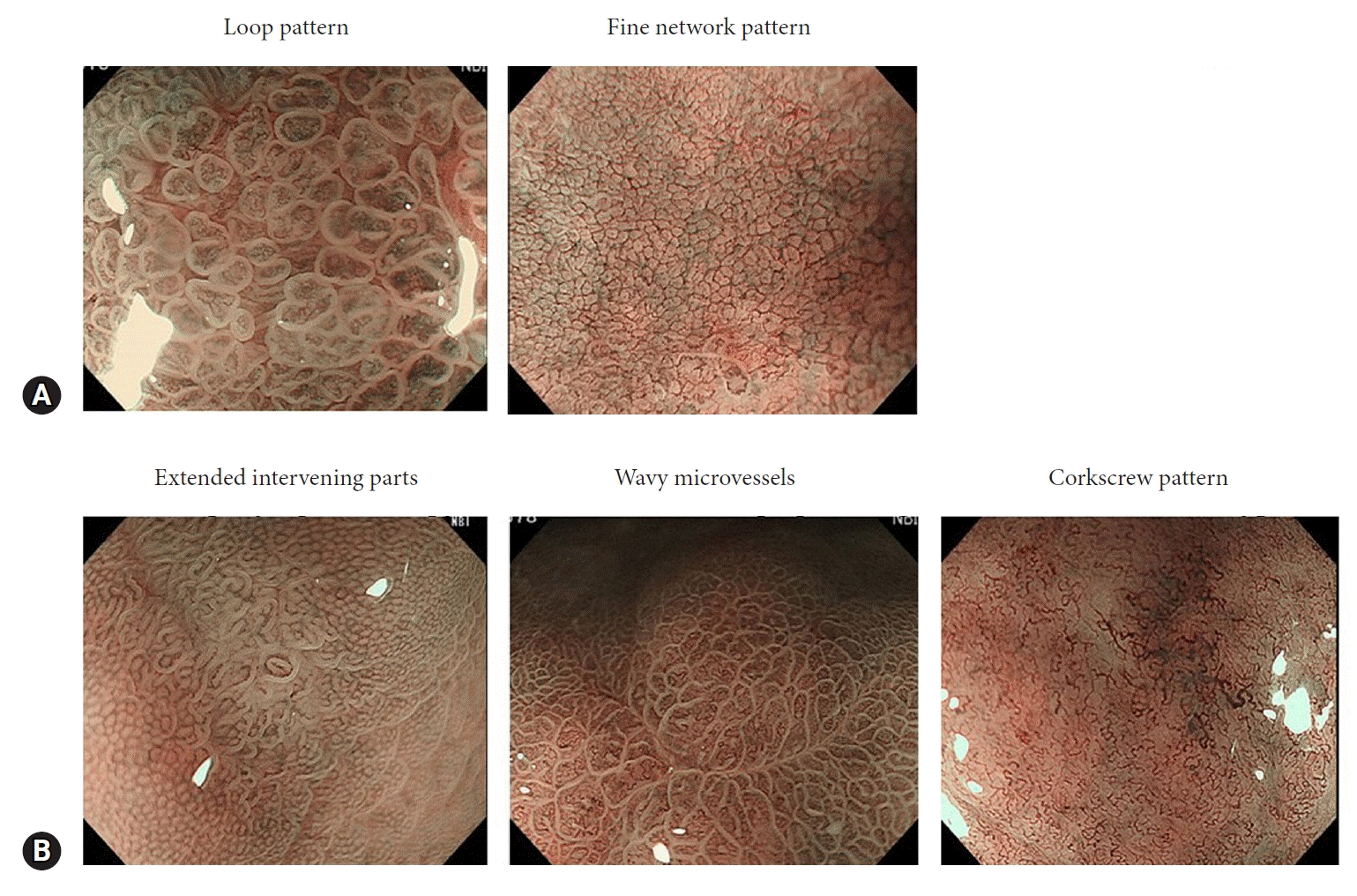
Magnifying endoscopy with narrow-band imaging findings. (A) Differentiated-type findings defined by the fine network or loop pattern. (B) Undifferentiated-type findings defined by extended intervening parts, wavy microvessels, or a corkscrew pattern. Reproduced Horiuchi et al. Dig Dis Sci 2020;65:591–599, with permission.37
CONCLUSIONS AND FUTURE PROSPECTS
Our findings suggest that the diagnostic performance of AI using ME-NBI still images and videos for gastric cancer is better than that of expert endoscopists. However, studies on the use of AI to diagnose gastric cancer are limited, and large-scale studies are necessary to examine whether a high diagnostic performance can be achieved. Additionally, the diagnosis of gastric cancer using AI has not yet become widespread in clinical practice, and further research is necessary. The diagnostic performance of AI varies between studies; therefore, in the future, AI must be further developed as an instrument, and its diagnostic performance is expected to improve with the accumulation of many cases nationwide.
The histological diagnosis of gastric cancer by AI is promising because histological diagnosis has a significant impact on the selection of treatment for gastric cancer. However, the overall accuracy of AI is 86.2%,38 and the possibility of incorrect pretreatment histological diagnosis influencing the treatment strategy remains a concern with the use of AI and ME-NBI. Pretreatment misdiagnoses occur because of mixed-type gastric cancers that include both differentiated and undifferentiated components. The diagnostic performance of pretreatment biopsy alone versus a combination of pretreatment biopsy and ME-NBI findings was compared in 192 lesions of mixed-type gastric cancer.37 The accuracy (77.6% vs. 92.2%, p<0.0001), sensitivity (87.8% vs. 96.8%, p=0.0002), and specificity (33.3% vs. 72.2%, p=0.0002) were significantly higher when pretreatment biopsy was combined with ME-NBI findings. The combination of pretreatment biopsy and ME-NBI findings is expected to further improve the diagnostic performance of histological typing of gastric cancer using AI.
In conclusion, it is challenging for endoscopists to master ME-NBI diagnostic techniques, which require training at specialized facilities. The findings of this review suggest that endoscopists and patients will greatly benefit from the use of AI to assist endoscopists in diagnosing gastric cancer. This review can serve as a catalyst for further understanding and development of AI for gastric cancer diagnosis.
Notes
Conflicts of Interest
Yusuke Horiuchi received a Grant-in-Aid for Early-Career Scientists (21K15962), a Japanese Foundation for Research and Promotion of Endoscopy Grant, an academic grant from the Japanese Gastric Cancer Association Research Committee, and personal fees for specific speaking and teaching commitments involving honoraria from Olympus Corp. and Kaken Pharmaceutical Co. Ltd. The other authors have no potential conflicts of interest.
Funding
None.
Author Contributions
Conceptualization: all authors; Data curation: YH; Formal analysis: YH; Investigation: YH; Methodology: YH; Project administration: YH, JF; Resources: YH; Software: YH; Supervision: all authors; Validation: YH; Visualization: YH; Writing–original draft: YH; Writing–review & editing: all authors.
