AbstractBackground/AimsArgon plasma coagulation (APC) has some merits in the treatment of gastric neoplasms including a shorter operative time and fewer complications compared with endoscopic mucosal resection or endoscopic submucosal dissection. However, there are few reports on the outcomes of gastric neoplasms treated using APC. The aim of this study was to evaluate APC in the treatment of early gastric neoplasms in terms of clinical efficacy, safety, and local recurrence.
MethodsWe enrolled 28 patients who received APC therapy at the Kyungpook National University Hospital between May 2007 and April 2013. Clinical outcomes were analyzed.
ResultsThe median follow-up period was 24.8 months (range, 2 to 78). Among the 28 lesions treated using the APC procedure, tumor recurrence was encountered in seven lesions (25.0%). Recurrence was found in 50% (5/10) of single APC cases and 11% (2/18) of rescue APC cases. The mean time to recurrence was 16.1 months (range, 2 to 78). There were no serious APC-related complications such as perforation, bleeding, or infection.
INTRODUCTIONEndoscopic mucosal resection (EMR) and endoscopic submucosal dissection (ESD) are important treatments for early gastric cancer (EGC) or gastric adenoma.1,2,3 However, these endoscopic resections, particularly ESD, require specialist skills and a long operation time. In addition, endoscopic resection cannot be performed in cases with a highrisk of bleeding or perforation or in cases of non-lifting after submucosal injection.
Argon plasma coagulation (APC) is a noncontact tissue coagulation technique that transfers a high-frequency electric current through ionized argon gas to the target tissue.4,5 An APC applicator for endoscopic use was developed by Grund et al.4 in 1994. APC has been used mainly for hemorrhagic lesions and bleeding sites in the gastrointestinal tract.6,7 In EGC, APC has been used to treat patients who cannot undergo endoscopic resection or surgery. APC has several merits including a short operative time, ease of use, lack of serious complications, and operator independence. However, APC has been used in only a few patients because of the difficulty in predicting the depth of invasion and the inability to perform pathological evaluations, which are possible with complete resection.8,9,10
There have been few reports on the outcomes of patients who receive APC therapy for gastric neoplasms. Thus, the aim of this study was to determine the clinical outcomes of APC in patients with EGC or gastric adenoma.
MATERIALS AND METHODSPatientsThis study enrolled 32 patients (26 men and six women) who received singleor rescue APC (after ESD or EMR) for the treatment of EGC or gastric adenoma in our hospital between May 2007 and April 2013. Single APC treatment was selected instead of surgical or endoscopic resection for the following reasons: (1) the patient had a high surgical risk or refused surgery; (2) the lesion carried a high risk of complications or technical difficulties for endoscopic resection; or (3) the patient refused endoscopic resection owing to its high cost or the long duration of hospitalization. Rescue APC treatment was selected in cases with endoscopically uncertain or unclear margins. We observed well-differentiated EGCs in seven cases, moderately differentiated EGCs in two cases, poorly differentiated EGCs in five cases, and adenomas in 18 cases. Of these, three cases were excluded because of loss to follow-up after 3 months and one case was excluded for undergoing surgery for metachronous recurrence after 3 months. With the exception of one case, all excluded cases were adenomas. We obtained information on the patients who were lost to follow-up via telephone call with verbal consent. A total of 28 cases were retrospectively analyzed. This study was approved by the Institutional Review Board of the Kyungpook National University Medical Center.
APC procedureAll procedures were performed under conscious sedation. Cardiorespiratory functions were continually monitored th-roughout the procedure. The APC equipment included an argon gas source and a high-frequency generator (VIO300 D; Erbe Elektromedizin, TУМbingen, Germany). The argon gas flow rate was 1.5 L/min and the electrical current was set at 50 W.
DefinitionsMacroscopic EGC and adenoma types were classified according to Japanese Gastric Cancer Association criteria.11 The degree of differentiation was classified according to World Health Organization recommendations. Local recurrence was defined as recurrent cancer or adenoma at the resection site. Tumors detected at sites other than the primary resection site during follow-up endoscopy were regarded as metachronous recurrences. Significant bleeding was defined as bleeding with clinical symptoms or bleeding requiring transfusion.
RESULTSA flowchart of the enrolled patients is shown in Fig. 1. The median follow-up period (from the date of initial treatment to the date of final endoscopic examination or recurrence) was 24.8 months (range, 2 to 78). Table 1 summarizes the baseline characteristics of the 28 patients. Eleven patients had underlying diseases, including seven patients with cardiovascular disease (five with poorly controlled hypertension and two with ischemic heart disease). In addition, six patients had diabetes mellitus and two patients had other malignancies (one with esophageal cancer and one with bladder cancer). Nine patients were older than 70 years.
Among the 28 lesions treated with an APC procedure, tumor recurrence was encountered in seven lesions (25.0%) during the follow-up period. Recurrence was found in 50% (5/10) of single APC cases and 11% (2/18) of rescue APC cases. Recurrence was located in the middle region in four patients, the lower region in one patient, and the upper region in two patients. The histological diagnosis of these lesions was EGC with poor differentiation in three cases and adenoma in four cases. The mean time to recurrence was 16.1 months (range, 2 to 78). The individual time to recurrence was 3 months in three patients and 2, 5, 19, and 78 months in one patient each. With the single APC procedure, three lesions did not recur after more than 64 months. One EGC lesion with poor differentiation recurred after 78 months. The patient had no metastasis and was followed up regularly before the diagnosis of recurrence. The recurring lesion was easily managed by APC retreatment with no complications.
DISCUSSIONAPC has been used to treat a range of gastrointestinal problems including bleeding ulcers,6 Dieulafoy's lesions,5 hemorrhagic vascular ectasia,7 and tumors.8 Few reports on APC as a first-line treatment for EGC have been published. The results of clinical trials8,9,10,12,13,14,15 of APC for the first-line treatment of EGC or gastric adenoma are summarized in Table 4.
The present study found that APC was useful for the treatment of EGC or gastric adenoma. With a single APC procedure, three lesions did not recur for more than 64 months and one EGC lesion with poor differentiation recurred after 78 months. With the rescue APC procedure, 11% lesions (2/18) recurred and one EGC lesion has not recurred after more than 57 months.
The recurring lesions were frequently observed to be large. Two local recurrences measured 50 mm. Almost all recurrent lesions were of elevated type IIa rather than the depressed type. This is potentially due to insufficient coagulation by APC therapy, since the tumor volume is greater in type IIa lesions than in depressed type lesions of the same size. APC is therefore a satisfactory and effective modality for flat and depressed lesions of a relatively small size. This finding has been notedin other studies.9,13,15 One study observed that all recurrent lesions were of elevated type IIa, regardless of the endoscopist's experience.9 Another study found that all recurrent lesions following APC were of the superficial elevated type.15 Yet another study found that no patient with intramucosal carcinoma had a residual tumor or recurrence after a single session or multiple sessions of APC.13 By contrast, three of the five patients (60%) with submucosal tumors had a residual tumor or recurrence. One of these three patients had large tumors that were treated with a single session of APC. The remaining two patients received two sessions of APC and had no evidence of local recurrence. This suggests that large protruding-type tumors or tumors with submucosal invasion have a high risk of residual tumor cells because of inadequate treatment after one session of APC.13
In a study in which 27 patients received APC for the treatment of EGC, 26 showed no recurrence during 30 months of follow-up.8 Most studies have shown that APC is an effective and safe treatment option for early gastric neoplasms (Table 4). APC is useful for the follow-up treatment of EGC after EMR.12 Taken together, these results suggest that the APC procedure canbe a therapeutic strategy for EGC, especially in elderly and high-risk patients. However, the risk factors for local recurrence have not been adequately studied. Non-lifting after submucosal saline injection and a lower power setting of 40 W are both risk factors for local recurrence after APC, with a lower reported rate of local recurrence in lifting and 60 or 80 W groups than in non-lifting and 40 W groups.10 In that study, the authors concluded that a higher power setting of 60 or 80 W may be safe and effective for the treatment of gastric neoplasms by APC.10
In endoscopic treatment, histological assessment is crucial for determining whether the lesions were treated successfully and whether additional surgery is needed. However, histology cannot be evaluated in APC treatment because the coagulation resulting from APC causes necrosis of the tumorous tissue. This is one reason that APC treatment alone has not been used as a first-line therapy for EGC until now. However, a strict histological assessment of the resected specimen may not always be necessary in patients without operable justification. The treatment options for EGC are nonuniform, even if tumors can be treated by endoscopic resection. Consequently, a different approach to the treatment of EGC may be selected in primary or geriatric hospitals.9
The treatment of low-grade dysplasia (LGD) can be different from that of high-grade dysplasia or EGC. APC has been shown to be a suitable treatment option for patients with a gastric LGD тЄ2.0 cm when compared with ESD.15 Because of reduced hospital admissions, lower medical costs, fewer complications, and more favorable outcomes, APC can be considered a first-line treatment option for patients with small gastric LGDs. Moreover, a recent study9 showed that the outcomes of APC did not differ according to endoscopic experience, with equal therapeutic outcomes achieved by experienced and non-experienced endoscopists. Therefore, non-experienced endoscopists may treat small gastric LGD lesions without a long training period such as that required for ESD.9,15
Our study had several limitations. First, this was a single-center study with a limited number of patients. It was a retrospective cohort study that involved the review of the records of patients with EGCs or adenomas. Second, there was no control group. The relatively short duration of follow-up may be another limitation. Further randomized prospective studies are necessary to confirm that APC is an effective and reasonable treatment for early gastric neoplasms.
In conclusion, APC can be a favorable therapeutic modality for the rescue treatment of early gastric neoplasms after EMR or ESD. In addition, APC can bea safe and effective first-line treatment of early gastric neoplasms, especially in elderly and high-risk patients.
References1. Gotoda T, Yamamoto H, Soetikno RM. Endoscopic submucosal dissection of early gastric cancer. J Gastroenterol 2006;41:929т942. 17096062.
2. Ono H, Kondo H, Gotoda T, et al. Endoscopic mucosal resection for treatment of early gastric cancer. Gut 2001;48:225т229. 11156645.
3. Ahn JY, Jung HY, Choi KD, et al. Endoscopic and oncologic outcomes after endoscopic resection for early gastric cancer: 1370 cases of absolute and extended indications. Gastrointest Endosc 2011;74:485т493. 21741645.
4. Grund KE, Storek D, Farin G. Endoscopic argon plasma coagulation (APC) first clinical experiences in flexible endoscopy. Endosc Surg Allied Technol 1994;2:42т46. 8081915.
5. Wahab PJ, Mulder CJ, den Hartog G, Thies JE. Argon plasma coagulation in flexible gastrointestinal endoscopy: pilot experiences. Endoscopy 1997;29:176т181. 9201466.
6. Chau CH, Siu WT, Law BK, et al. Randomized controlled trial comparing epinephrine injection plus heat probe coagulation versus epinephrine injection plus argon plasma coagulation for bleeding peptic ulcers. Gastrointest Endosc 2003;57:455т461. 12665753.
7. Sebastian S, McLoughlin R, Qasim A, O'Morain CA, Buckley MJ. Endoscopic argon plasma coagulation for the treatment of gastric antral vascular ectasia (watermelon stomach): long-term results. Dig Liver Dis 2004;36:212т217. 15046192.
8. Sagawa T, Takayama T, Oku T, et al. Argon plasma coagulation for successful treatment of early gastric cancer with intramucosal invasion. Gut 2003;52:334т339. 12584212.
9. Tomita T, Arai E, Kohno T, et al. Outcomes of treatment of argon plasma coagulation therapy in elderly or high-risk patients with early gastric cancer: a comparison of outcomes among experienced and nonexperienced endoscopists. J Clin Gastroenterol 2011;45:e54тe59. 20838235.
10. Ahn JY, Choi KD, Na HK, et al. Clinical outcomes of argon plasma coagulation for the treatment of gastric neoplasm. Surg Endosc 2013;27:3146т3152. 23443483.
11. Japanese Gastric Cancer Association. Japanese classification of gastric carcinoma: 2nd English edition. Gastric Cancer 1998;1:10т24. 11957040.
12. Murakami M, Nishino K, Inoue A, et al. Argon plasma coagulation for the treatment of early gastric cancer. Hepatogastroenterology 2004;51:1658т1661. 15532798.
13. Kitamura T, Tanabe S, Koizumi W, Mitomi H, Saigenji K. Argon plasma coagulation for early gastric cancer: technique and outcome. Gastrointest Endosc 2006;63:48т54. 16377315.
Fig.Т 1Flowchart of inclusion criteria used in this study. The clinical outcomes of 28 patients with early gastric neoplasms were analyzed after argon plasma coagulation (APC). ESD, endoscopic submucosal dissection; EMR, endoscopic mucosal resection.
TableТ 2Data on Patients Who Underwent Single Argon Plasma Coagulation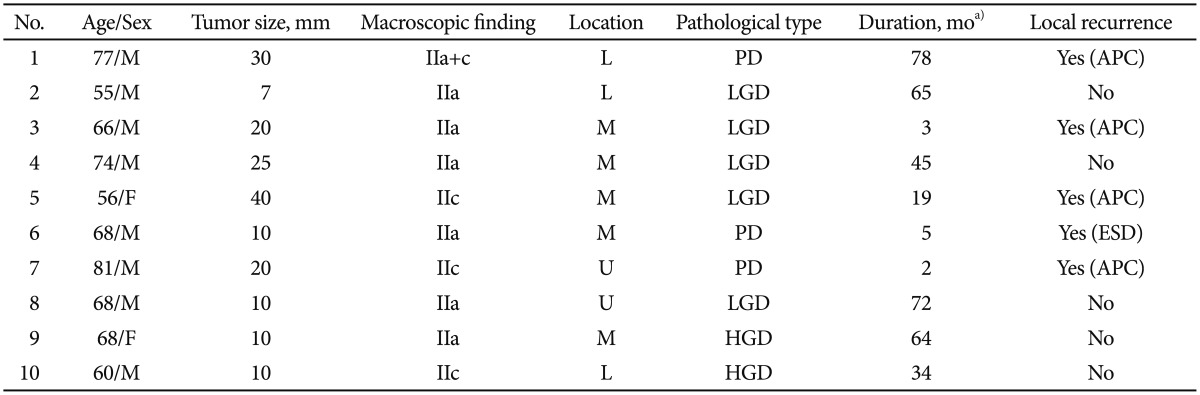 M, male; IIa, flat elevated; L, lower third; PD, poorly differentiated adenocarcinoma; APC, argon plasma coagulation; LGD, low-grade dysplasia; M, middle third; F, female; IIc, flat depressed; ESD, endoscopic submucosal dissection; U, upper third; HGD, high-grade dysplasia. a)Duration, interval between APC procedure and local recurrence. TableТ 3Data on Patients Who Underwent Rescue Argon Plasma Coagulation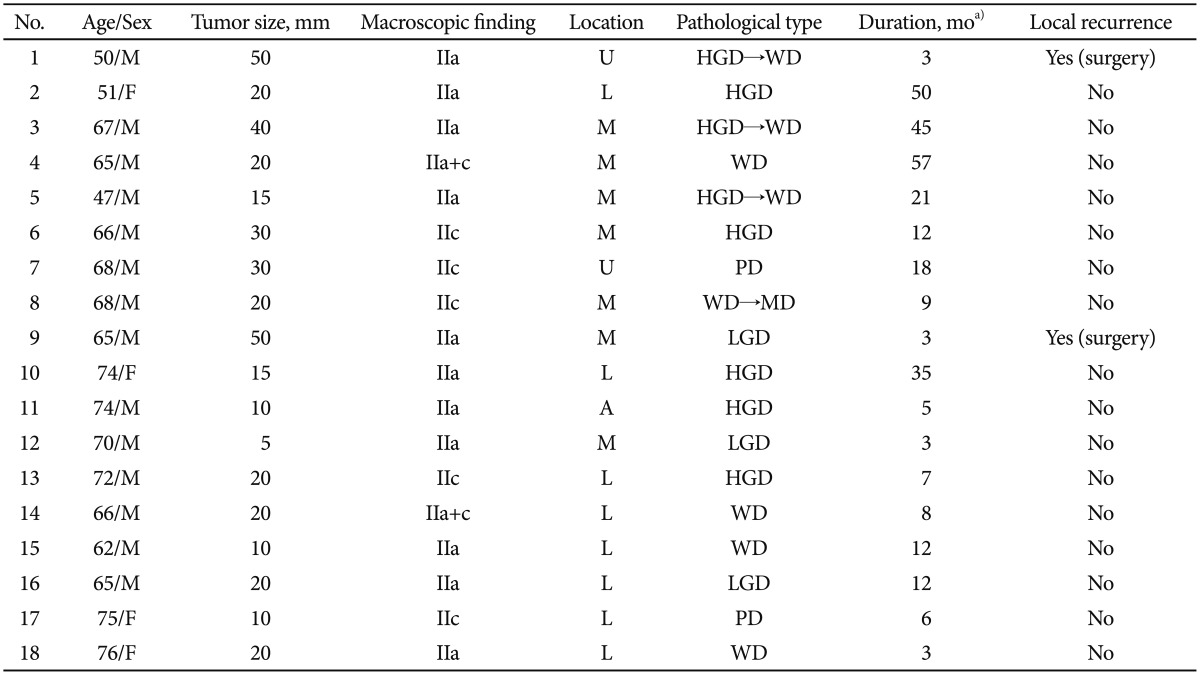 M, male; IIa, flat elevated; U, upper third; HGD, high-grade dysplasia; WD, well-differentiated adenocarcinoma; F, female; L, lower third; M, middle third; IIc, flat depressed; PD, poorly differentiated adenocarcinoma; MD, moderately differentiated adenocarcinoma; LGD, low-grade dysplasia; A, anastomosis site. a)Duration, interval between APC procedure and local recurrence. TableТ 4Clinical Trials of Argon Plasma Coagulation for the First-Line Treatment of Early Gastric Cancer or Gastric Adenoma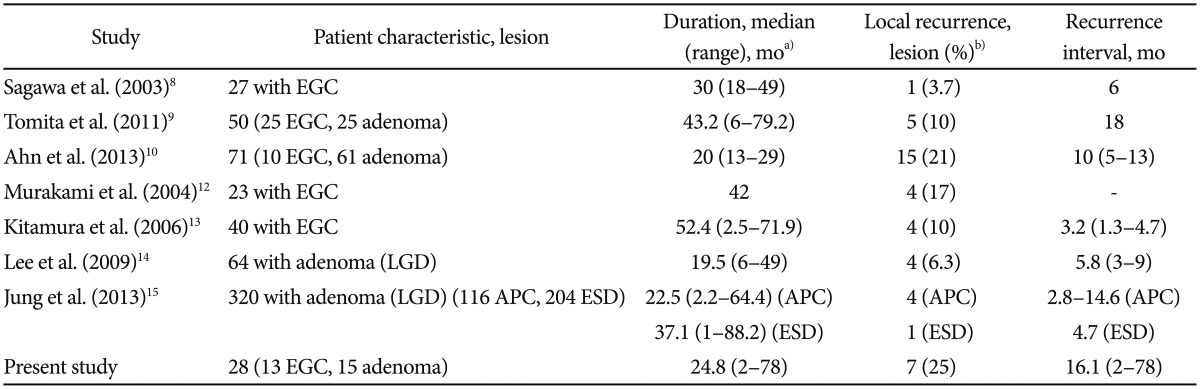 EGC, early gastric cancer; LGD, low-grade dysplasia; APC, argon plasma coagulation; ESD, endoscopic submucosal dissection. a)Duration, follow-up period from the date of initial APC treatment to the date of final endoscopic examination or recurrence; b)Recurrence interval, period from the date of initial APC treatment to the date of local recurrence. |
|
|||||||||||||||||||||||||||||||||||||||||||||||
