INTRODUCTION
Despite the low complication rates related to laparoscopic bariatric surgery, there is an increasing interest in endoluminal gastric instruments for weight loss procedures (Table 1). As we know, the intragastric balloon (IGB) made a breakthrough in endoluminal therapy. However, IGB is primarily used in short-term supplemental therapy. Recently, several companies and endoscopists have been developing various endoscopic bariatric devices for the treatment of obesity [1,2]. This summary highlights the various currently available non-balloon devices.
Table 1 summarizes the various endoscopic bariatric procedures according to the U.S. Food and Drug Administration (FDA) approval status [3]. An endoscopic bariatric procedure can be classified into four categories, namely, space occupying, gastric restriction, aspiration, and malabsorption devices. Our review focuses on the FDA approval status of the non-balloon devices.
ENDOSCOPIC SLEEVE GASTROPLASTY USING THE OVERSTITCHŌäó ENDOSCOPIC SUTURING SYSTEM
For gastric restriction, several techniques of endoscopic transoral gastroplasty are being developed. Endoscopic sleeve gastroplasty (ESG) is one of the methods that uses endoscopic suturing to decrease the intraluminal space [4,5]. Moreover, ESG can delay gastric motility function. This procedure makes a slim hole, and unlike laparoscopic sleeve gastrectomy, it does not require gastric resection.
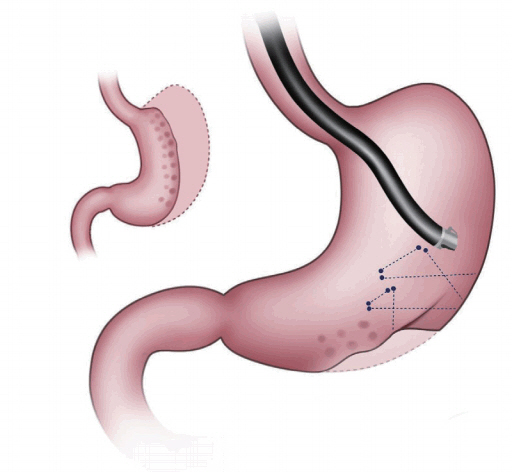
The OverStitchŌäó endoscopic suturing system is an advanced suturing device that allows the endoscopist to place sutures inside the stomach using an endoscope. It has a wide range of applications in minimally invasive surgery (Fig. 1). The OverStitchŌäó system can be used in conjunction with bariatric procedures, such as endoscopic sleeve gastrectomy, to greatly improve patient outcomes. The procedure is performed using an endoscope that is inserted through the mouth to reach the stomach. It involves placement of a row of stitches within the stomach along the greater (outer) curve, which when tightened creates folds or plications that reduce the size of the stomach. About 5ŌĆō6 running stitches are placed. The plications extend almost to the junction of the stomach and esophagus. A similar row of interrupted or running stitches is placed along the lesser (inner) curve creating a sleeve. This procedure considerably reduces the size of the stomach, promoting early satiety and reduced food intake, leading to weight loss. In 2012, the initial case study of ESG using OverStitchŌäó was performed as a multicenter trial [6]. A prospective study of 25 patients with a mean body mass index (BMI) of 35.5 kg/m2 showed a total weight loss (TWL) of 54%┬▒40% at 12 months and 45%┬▒41% at 20 months [7]. Total 10 patients showed excess weight loss (EWL) of 33 % and 30% EWL at 6 months [8]. Another study showed a TWL of 18.7%┬▒10.7% and a 7.3┬▒4.2 kg/m2 decrease in BMI at 1 year in 25 patients [9]. Some complications associated with this procedure were inflammatory fluid collection, pneumoperitoneum, and pulmonary thromboembolism. Postprandial and fasting ghrelin levels decreased by 29.4% at 3 months. Insulin sensitivity increased at 3 months.
PRIMARY OBESITY SURGERY ENDOLUMINAL
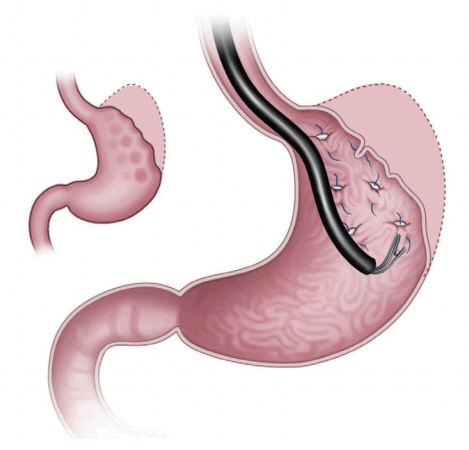
Primary obesity surgery endoluminal (POSE) is one of the gastric restriction methods under FDA review (Fig. 2). This method uses the incisionless system (IOP). This device makes tissue folding by opposing and then deploying and anchoring the tissue repeatedly. It contains four working channels that accommodate an ultra-slim endoscope for visualization, specialized instruments for grasping tissue (G-Lix), and snow-shoe shaped tissue anchors (G-Prox). To perform this procedure, the IOP is retroflexed and used to make two parallel rows with 4ŌĆō5 plications each. After the forward view is replaced, a ridge of 3ŌĆō4 plications is made at the intersection of the stomach.
A study of 147 obese patients demonstrated a TWL of 15.1%┬▒7.8% and EWL of 44.9%┬▒24.4% at one year [10]. A study of 45 patients (mean BMI= 36.7┬▒3.8 kg/m2) reported BMI decrease of 5.8 kg/m2 at 6 months [11]. EWL was 49.4%┬▒21.5% and TWL was 15.5%┬▒6.1% at 6 months. In cases of younger patients with a higher initial BMI, this method demonstrated a higher success rate. No complications were noted.
ASPIREASSIST
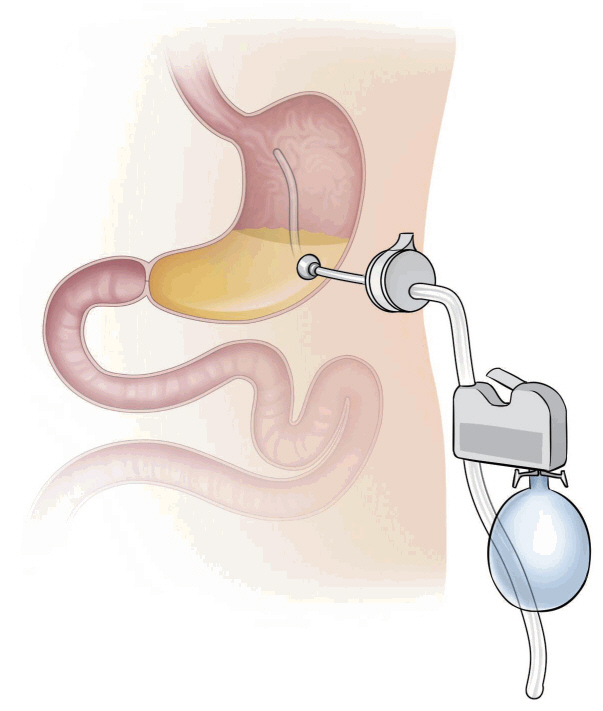
Aspiration therapy using the AspireAssist® system removes gastric foods after a meal through a percutaneous endoscopic gastrostomy (PEG) tube (Fig. 3). Therefore, this therapy reduces the amount of chyme reaching the small intestine for absorption. This aspiration instrument is located as in the conventional PEG tube, and the skin port is supplied after about 14 days. After feeding, the food migrates to the gastric space, where it is located temporarily. During first 60 minutes after eating, the food begins to break down and passes into the small intestine. The AspireAssist® helps patients aspirate about 30% of the stomach food, causing weight loss. The gastric food aspiration procedure is carried out about 30 minutes after each meal, and it takes about 5 to 10 minutes to complete. This aspiration procedure is performed in the restroom, and the food is discarded into the toilet [12].
In 2013, a study about the aspiration therapy was reported; 18 patients were registered and 10 AspireAssist® and 4 control group patients completed this study [13]. At 12 months, TWL was 18.6%±2.3% with AspireAssist® and 5.9%±5.0% in the control group. Seven of the 10 patients in the AspireAssist® group continued treatment for one year and accomplished a TWL of 20.1%±3.5%. Furthermore, during the study, there was no evidence of adverse effects on eating patterns or eating disorder psychopathology. Complications, such as infection and fistula were mainly associated with the PEG tube. AspireAssist® was approved in the USA for patients with BMI between 35 kg/m2 and 55 kg/m2 in 2016. FDA approval was based on a randomized controlled trial [14], which enrolled 171 patients in ten centers across the USA. TWL was 14.2%±9.8% and 4.9% in the active and control patients, respectively. This result is in accordance with that of previous studies [13,15].
CONCLUSIONS
The OverStitchŌäó Endoscopic Suturing System and POSE procedure are the currently available gastric restriction methods for the treatment of obesity. The ESG method might be more effective than the currently available IGB. Moreover, we suggest that the ESG is less invasive and reversible modality for bariatric treatment compared to the laparoscopic sleeve gastrectomy. The AspireAssist┬« method is a noninvasive, cost-effective, and safe option for bariatric treatment. However, there are many debates regarding the prolonged efficacy, economic feasibility, and durability of these procedures.
In conclusion, the role of an endoscopist is crucial in obesity management and concerns managing complications occurring after bariatric surgery as well as during endoscopic endoluminal treatment of obesity. In the future, several endoscopic treatment modalities may be introduced, which may play a vital role in the treatment of obesity and obesity-related diseases.