Introduction
Barrett’s esophagus (BE) is considered the principal risk factor for esophageal adenocarcinoma, a cancer whose incidence has increased dramatically over the past few decades. BE is characterized by the replacement of normal squamous epithelium of the esophagus by a columnar epithelium with intestinal metaplasia, which can progress to neoplasia through low-grade dysplasia (LGD), high-grade dysplasia (HGD), and adenocarcinoma [1-3]. The metaplastic mucosa of BE can be successfully eradicated with radiofrequency ablation (RFA) to prevent the progression to adenocarcinoma in patients with dysplasia [4]. A concern associated with this ablation technique is the potential persistence of residual areas of columnar epithelium underneath the newly formed squamous epithelium, known as “buried BEâ€, that may progress to adenocarcinoma by escaping surveillance [5-7].
Case Report
Here, we report the case of a 63-year-old Caucasian man with a past medical history of anti-reflux surgery and smoking habits as well as a BE diagnosis in 1998 (Prague Classification: C4M5). The patient started once-daily proton-pump inhibitor administration and underwent regular endoscopic surveillance every 3–5 years; biopsies (Seattle protocol) were negative for dysplasia. In 2010, surveillance endoscopy revealed no visible esophageal lesions but the biopsies revealed LGD. The biopsy samples were reviewed by an independent and expert gastrointestinal pathologist who confirmed the LGD diagnosis.
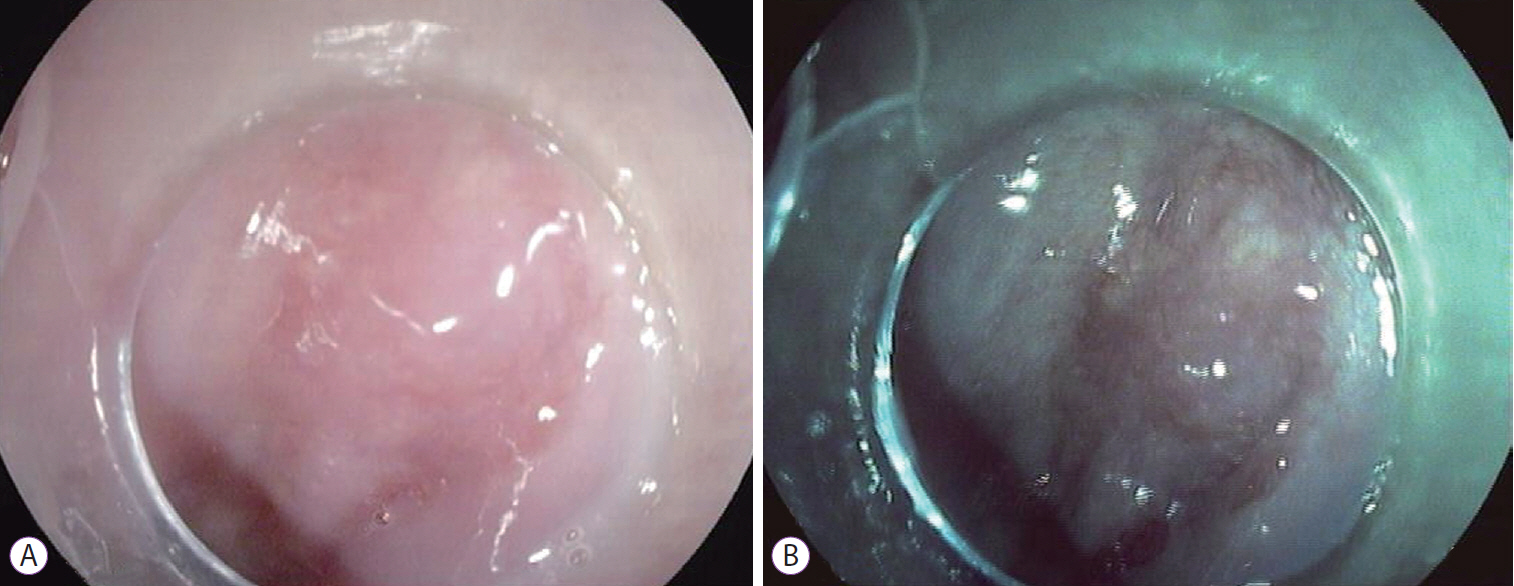
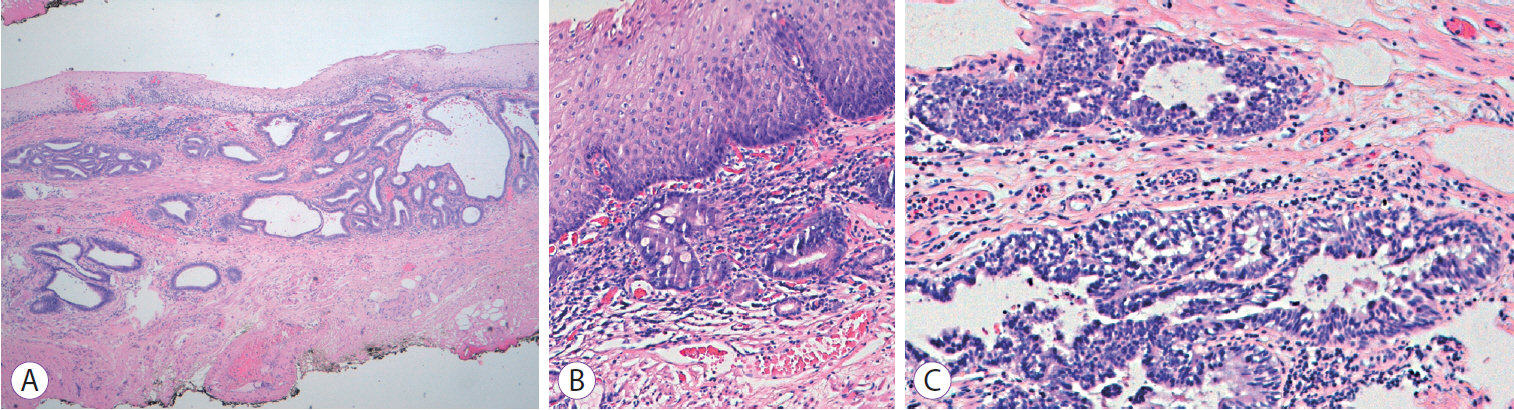
The patient was scheduled for RFA and underwent one session with HALO360 catheter (Barrx Medical Inc., Sunnyvale, CA, USA) followed by one session with HALO90. After each RFA procedure, the patient was prescribed a 2-week course of ranitidine at bedtime, sucralfate 4 times daily, and esomeprazole bid. An upper endoscopy performed 3 months after the RFA exhibited a normal neosquamous epithelium with biopsies showing no intestinal metaplasia or dysplasia. He continued undergoing regular endoscopic surveillance with biopsies of the neosquamous mucosa according to the Seattle protocol. On follow-up endoscopy performed 4 years after the RFA, a slightly elevated 6-mm nodular area (Paris classification: 0-IIa) covered by squamous mucosa was detected immediately above the gastroesophageal junction (Fig. 1). Targeted biopsies of the nodular lesion revealed squamous epithelium with subjacent columnar epithelium and areas of intestinal metaplasia and cytoarchitectural features indefinite for dysplasia. The lesion was managed with ligation-assisted endoscopic mucosal resection (Duette Multiband Mucosectomy Kitâ„¢; Cook Medical, Limerick, Ireland) with en bloc resection. Morphologically, it was a polypoid fragment lined by squamous epithelium with subjacent columnar epithelium exhibiting focal HGD with lateral and deep margins free of dysplasia, findings consistent with buried BE and HGD (Fig. 2). After the procedure, the patient continued undergoing sessions of endoscopic surveillance with high-resolution endoscopy, and biopsies of the scar and random biopsies of the neo-squamous mucosa showed normal neosquamous epithelium with no buried glands or dysplasia. Currently, 24 months after the buried BE diagnosis, no sign of recurrence has been seen.
Discussion
The presence of buried BE, also known as buried or subsquamous metaplasia, after the endoscopic ablation of BE has been described in several studies [8-10]. However, far higher rates of buried metaplasia have been noted after photodynamic therapy (PDT) and other endoscopic ablative therapies than after RFA [9]. Actually, according to a systematic review, buried metaplasia was detected in only 9 of 1,004 patients (0.9%) who underwent endoscopic biopsy of neosquamous epithelium after RFA versus 135 of 953 patients (14.2%) after PDT [10].
The biology and clinical relevance of subsquamous metaplasia remains unclear. To date, few cases of subsquamous neoplasia have been reported after the successful eradication of BE, the majority of which were associated with ablative therapies other than RFA [11,12]. Titi et al. were the first to describe neosquamous neoplasia after the successful RFA of dysplastic BE [6]. Table 1 summarizes the cases of buried neoplasia after successful RFA of BE published to the date [6,12-15]. Fewer than 20 cases of subsquamous neoplasia after the successful RFA of BE have been reported. The present report describes a case of the development of subsquamous neoplasia that occurred later after RFA treatment.
The real risk of cancer development of the buried glands is unknown, with different competing hypotheses [9]. Genetic abnormalities acquired during the carcinogenic process might confer survival advantages rendering neoplastic Barrett’s cells more resistant than non-neoplastic cells to ablative therapies. This might promote the selective destruction of normal rather than neoplastic cells, predisposing patients to buried neoplastic glands with a higher risk of malignancy [9,14,16]. A tortuous distal esophagus, a large hiatal hernia, or surgical anastomosis may contribute to inadequate energy delivery via RFA, increasing the risk of buried metaplasia [6]. On the other hand, some authors believe that the potential for neoplastic progression of buried BE is reduced since the overlying layers of the neosquamous epithelium protect the buried glands from the trophic influence of acid reflux and bile. Actually, fewer DNA content abnormalities and lower crypt proliferation rates have been observed in buried metaplasia than in the surface metaplastic epithelium [17]. However, according to Orlando, the neosquamous epithelium has a defective barrier function that is more permeable and leaky than normal squamous esophageal epithelium [18]. Besides the question of “risk†is the question of “originâ€. In the present case, the subsquamous HGD area was detected 4 years after the last RFA session. It is unclear whether it progressed from buried glands with dysplasia ab initio and was inadequately eradicated and remained quiescent for this long period of time or if it was derived from non-dysplastic buried glands with the development of a de novo lesion [15]. Considering the last hypothesis, the most acceptable pathways of cellular reprogramming in Barrett’s metaplasia are transdifferentiation (direct or via squamous cell de-differentiation) and transcommitment (from the esophageal submucosal glands, gastric cardia/gastroesophageal junction epithelium, or circulating bone marrow–derived progenitor cells) [19]. In this case, we believe that a squamous cell origin can be ruled out since the squamous cell epithelium was destroyed by the RFA procedure.
The subsquamous neoplasia of this patient presented as a visible lesion, enabling early endoscopic recognition and adequate endoscopic management. The limitation of standard white light endoscopy for detecting subsquamous neoplasia is concerning. Optical coherence tomography and volumetric laser endomicroscopy have been developed as valuable imaging techniques that can image the superficial layers of the esophagus over a large surface area, allowing the depiction of subepithelial structures such as subsquamous neoplasia. Studies have shown divergent results of the overall prevalence of buried glands with these imaging methods; however, all emphasize the need for endoscopic surveillance of the neosquamous epithelium, eventually with deeper imaging-guided sampling [5,9].
In conclusion, RFA is not a perfect technique, with a known risk of buried glands even after apparently successful RFA treatment [9]. Although the real risk of subsquamous neoplasia (adenocarcinoma or HGD) after successful RFA is unknown, cases reported to date, including the present one, highlight the need for continued surveillance. In patients with LGD, after the complete eradication of BE, surveillance every year for 2 years and every 3 years thereafter is recommended [20]. We believe that high-resolution endoscopy, ideally performed using advanced imaging modalities like narrow-band imaging, should be used during follow-up. A detailed endoscopic inspection of the neosquamous epithelium to detect any residual columnar mucosa or mucosal irregularities that should be subsequently treated with complementary surveillance with biopsies according to the Seattle protocol are advised. Likewise, we consider a detailed endoscopic inspection of BE before RFA necessary to exclude any visible lesions and ensure its application only in flat mucosa to promote adequate energy delivery and cellular destruction [7].