Dietrich and Hocke: Elastography of the Pancreas, Current View
Abstract
Ultrasound elastography (USE) of the pancreas allows pancreatic tissue stiffness assessment by virtual palpation. Two main types of USE are used. For the pancreas strain elastography applying by endoscopic ultrasound has been established for the characterisation of small solid pancreatic lesions (SPL). In larger SPL >30 mm the results are less convincing mainly due to the heterogenicity of the lesions but also by concomitant changes of the surrounding pancreatic parenchyma. The current role of shear wave elastography has to be determined. This article reviews the current use of elastography of the pancreas.
Keywords: Endoscopic ultrasound; Neuroendocrine tumour; Shear wave elastography; Ultrasound
INTRODUCTION
Ultrasound elastography (USE) of the pancreas allows pancreatic tissue stiffness assessment by palpation. Prerequisite of all kinds of elastography is the entire visualization of the gland [ 1- 3]. Two main types of USE are used: Ultrasound strain elastography (SE) using the endoscopic and transcutaneous route and ultrasound shear wave elastography (SWE) only using the transcutaneous route [ 4- 6]. The types of USE vary on how the stress is applied and tissue displacement (strain) is measured. SE can be performed with qualitative and semiquantitative information and SWE with qualitative and quantitative data. The description of the basic principles and the terminology has been generally accepted [ 4, 5]. SE allows the semi-quantitative evaluation of the strain-ratio (SR) between two regions of interest and the strain-histograms (SH) of a certain pancreatic region of interest [ 7- 10]. Computer-aided diagnostic techniques using artificial neural networks might additionally improve the accuracy for the differential diagnosis of focal pancreatic masses [ 8, 11]. The advantages of SWE are used for assessment of liver fibrosis [ 4, 5, 12- 17], but only few studies used this technique for the pancreas. Recommendations have been published for the endoscopic and the transcutaneous approach of strain imaging on how to improve performance [ 18, 19]. SE applied by endoscopic ultrasound (EUS) has been established for the characterisation of small solid pancreatic lesions (SPL). In larger SPL >30 mm, the results are less convincing mainly due to desmoplastic reaction and the heterogenicity of larger lesions with regressive changes but also by concomitant changes of the surrounding pancreatic parenchyma. The current role of the transcutaneously applied SWE is less clear today compared to endoscopically applied strain imaging and has to be determined. This article reviews the current use of elastography of the pancreas.
HOW TO USE STRAIN IMAGING OF THE PANCREAS?
Ultrasound SE is a qualitative technique where a comparison is performed and relative stiffness differences of the pancreatic tissue are displayed by colours [ 8, 9, 12, 18- 23]. The transcutaneous examination technique has been described for the examination of lymph nodes [ 24- 29] with similiar elastographic features compared to the endoscopic USE approach to examine peripancreatic and other lymphadenopathy [ 25, 30- 33]. We prefer to denote blue as stiffer and red as softer but there are no convincing reasons for this except the historical use. The technical principles of real-time tissue elastography have been recently described in detail by the European Federation of Societies for Ultrasound in Medicine and Biology and the World Federation for Ultrasound in Medicine and Biology [ 4, 5, 12, 13, 16, 17, 34- 36]. Important parameters of SE to take into consideration are listed in the following check list.
appropriate transducer frequency selection frame rate line density palpation speed and amplitude noise filters persistence dynamic range of elasticity other qaulity parameters (e.g., strain graph display)
Since strain imaging displays the relative stiffness of tissue, the relation to enough sufficient normal or reference tissue surrounding the lesion is of major interest. The best image quality can be achieved when the lesion of interest covers up to 50% of the region of interest [ 18, 19, 37]. Too strong pre-compression should be avoided to achieve consistent, reproducible elastograms. The contact of the endoscopic transducer should be strictly applied to the center of the lesion to avoid falsely too stiff estimation. Care should be taken to avoid assessment of tissue adjacent to stiff areas, as soft tissue will strain more when it is above hard tissue [ 19]. EUS strain imaging allows the imaging of elasticity properties of SPL but is not suitable for examining larger pure cystic lesions. The blue/green/red sign is a useful artefact to determine the cystic nature of small pancreatic cystic lesion [ 38].
NORMAL STIFFNESS OF THE PANCREAS
The entire pancreas has an intermediate stiffness and the shear wave speed is about 1.4 m/sec [ 39]. Pancreatic stiffness increases during aging which is true for SE [ 40, 41] and SWE [ 42- 44]. Size, body weight, body mass index and gender do not significantly influence pancreatic stiffness [ 42- 46] but published data are sparse.
ACUTE AND CHRONIC PANCREATITIS, AUTOIMMUNE PANCREATITIS
Necroses in acute pancreatitis are softer as compared to the healthy pancreatic parenchyma. Acute pancreatitis induces complex changes of the pancreas with no clear cut stiffness values [ 46- 49]. The value of elastography for the diagnosis of chronic pancreatitis is controversially discussed [ 40, 44, 46, 48, 50- 68] with stiffer parenchyma during the course of the disease both using SE (SR and SH) [ 40, 69] and SWE [ 44, 46, 56, 57, 70]. Typically a heterogeneous (honeycombed) stiffness pattern can be displayed with predominantly stiff strands and calcifications. EUS elastography is especially helpful in identifying patients with autoimmune pancreatitis since the entire organ shows stiffer tissue (and hypervascularity) before B-mode changes are visible ( Fig. 1) [ 20, 71- 74]. Tuberculosis is also stiffer [ 75, 76]. Elastogaphic methods have also been used to estimate the risk of fistula formation after pancreatic surgery. Interestingly, the softer values are risk factors analysed by SWE [ 50, 51, 67, 77] and SE [ 54, 59, 68].
STRAIN IMAGING
Recently a study was performed with 218 patients with SPL Ōēż15 mm and a definite diagnosis [ 23]. It could be shown that in patients with small pancreatic lesions, EUS elastography can rule out malignancy with a high level of certainty if the lesion is displayed as soft ( Fig. 2). A stiff lesion can be either benign or malignant [ 78]. The most important differential diagnosis of pancreatic ductal adenocarcinoma (PDAC) ( Fig. 3) are pancreatic neuroendocrine tumors ( Fig. 2). Other SPL include metastases (e.g., renal cell, lung and colorectal carcinoma) ( Fig. 4), lymphoma, serous microcystic neoplasia with only microscopically detectable cysts mimicking a solid lesion, mesenchymal pancreatic tumors and and intrapancreatic accessory spleens. These may present as stiffer or softer lesions compared to the surrounding pancreatic parenchyma [ 38- 42]. In patients with SPL lesions <15 mm, it is more likely to diagnose lesions other than ductal adenocarcinoma compared to larger SPL. In multivariate analysis a lesion size of Ōēź15 mm was associated with PDAC with an Odds ratio of 20.2 [ 79]. In small pancreatic tumors (Ōēż25 mm), the risk of ductal adenocarcinoma was correlated to increasing size with a risk of ductal adenocarcinoma of 4.3% in lesions Ōēż15 mm, of 22.8% of lesions measuring 16ŌĆō20 mm, and of 42.1% of leasions measuring 21ŌĆō25 mm [ 80]. In a large cohort of solid pancreatic tumors (3.4ŌĆō130 mm; median 32 mm) diagnosed using EUS-guided fine needle aspiration (EUS-FNA), 40 lesions were Ōēż10 mm in diameter, 121 had a diameter of 10ŌĆō20 mm, and 835 >20 mm. In the group of lesions Ōēż10 mm, only 22.5% were diagnosed to be PDAC, but 40% proved to be pancreatic neuroendocrine tumours (P-NET). In the group with lesions of intermediate size (10ŌĆō20 mm) 58.7% were ductal adenocarcinoma and 14% were NET. In lesions larger than 20 mm PDAC was by far the most common diagnosis (81.8%), and only 2.8% of lesions were P-NET [ 81]. Dawwas et al. reported modest accuracy for differentiating malignant lesions [ 82]. They studied 104 patients with evidence of a solid pancreatic mass on cross-sectional imaging and/or endosonography, with 111 quantitative EUS elastography procedures. Multiple elastographic measurements of the mass lesion and soft-tissue reference areas were undertaken, and the corresponding SRs calculated. Malignant masses had a higher SR ( p=0.01) and lower mass elasticity ( p=0.003) than inflammatory lesions. The areas under the receiver-operating characteristic curve for the detection of pancreatic malignancy of both SR and mass elasticity were 0.69 and 0.72, respectively. At the cut-off points providing the highest accuracy in this cohort (4.65% for SR and 0.27% for mass elasticity), quantitative EUS elastography had a sensitivity of 100.0% and 95.7%, specificity of 16.7% and 22.2%, positive predictive value of 86.1% and 86.4%, negative predictive value of 100.0% and 50.0%, and overall accuracy of 86.5% and 83.8%, respectively [ 82]. The authors suggested that EUS-SE may only supplement rather than supplant the role of pancreatic tissue sampling in the future. EUS elastography was reported to be useful for the differentiation of focal pancreatic masses, particularly between pseudotumoral chronic pancreatitis and pancreatic cancer, and in the presence of a strong suspicion of pancreatic cancer and false-negative EUS-FNA results [ 83]. In a retrospective study design, 109 patients with SPL were assessed by EUS elastography. Tissue elasticity distribution and elasticity semiquantification, using the SR of tissue elasticity, were used. Elastography for all PDAC patients showed intense blue coloration, indicating hard tissue. In contrast, mass-forming pancreatitis presented with a mixed coloration pattern of green, yellow, and low-intensity blue. Normal controls showed an even distribution of green to red. The mean SR was 23.66┬▒12.65 for mass-forming pancreatitis and 39.08┬▒20.54 for PDAC. Semiquantitative analysis of elasticity using the SR may allow the differentiation of mass-forming pancreatitis from PDAC [ 84]. Mass forming pancreatitis seems to be a computed tomography phenomenon since it relies mainly on swelling whereas EUS shows most often focal lesions. One prospective study from 2008, of 70 patients with undifferentiated pancreatic masses, reported a much lower overall sensitivity of elastography for malignancy of 41%, specificity of 53%, and accuracy of only 45% in larger lesions. The subanalysis revealed much better results for smaller lesions [ 21]. Recent efforts to improve the reproducibility, accuracy, and clinical utility of elastography in pancreatic imaging have moved toward developing quantitative scoring systems for elastography to better delineate the relative differences in the elasticity of solid pancreatic masses [ 9]. SR is a tool used for quantifying relative tissue stiffness, normally used to measure the stiffness of a discrete mass lesion [ 18]. Histogram analysis has been applied in diffuse chronic pancreatic diseases, where the colour pattern displayed in the elastogram is related to the fibrous structure caused by the chronic inflammatory disease [ 18]. Both topics have been extensivle discussed elsewhere [ 18, 19, 23, 85].
SHEAR WAVE ELASTOGAPHY
Shear wave velocities are significantly higher in PDAC compared to the surrounding pancreatic parenchyma [ 46, 86, 87] with shear wave velocities >3 m/s [ 46, 86- 88].
CONCLUSIONS
Ultrasound based SE allows improved visualisation and relative quantification of pancreatic tissue stiffness, an area not accessible to direct palpation. SE [ 5, 8- 11, 19- 21, 31, 32, 40, 41, 44, 52- 54, 57- 63, 69, 78, 84, 89- 112] and SWE [ 5, 39, 42- 47, 50, 51, 53, 55- 57, 86- 90, 113- 115] have been widely used to examine the pancreatic parenchyma and to differentiate SPL. The EUS approach has been established for the differential diagnosis of small SPL. A hypoechoic SPL <30 mm on B-mode with low strain signal (hard) in otherwise healthy pancreatic parenchyma can be malignant or benign whereas a soft SPL is almost always benign. The transcutaneous and intraoperative approaches are promising as well but data are less extensive and less convincing. Elastographic methods are not able to decisively differentiate focal pancreatitis from PDAC. Transabdominal and endoscopic USE may be also helpful tools for diagnosing and staging of chronic pancreatitis. Strain imaging is also of use in diagnosing autoimmune pancreatitis. Finally, the combination of imaging methods should be used.
Fig.┬Ā1.
Autoimmune pancreatitis. The entire organ shows diffuse stiffness. In comparision, pancreatic cancer shows circumscriptive stiffness only. 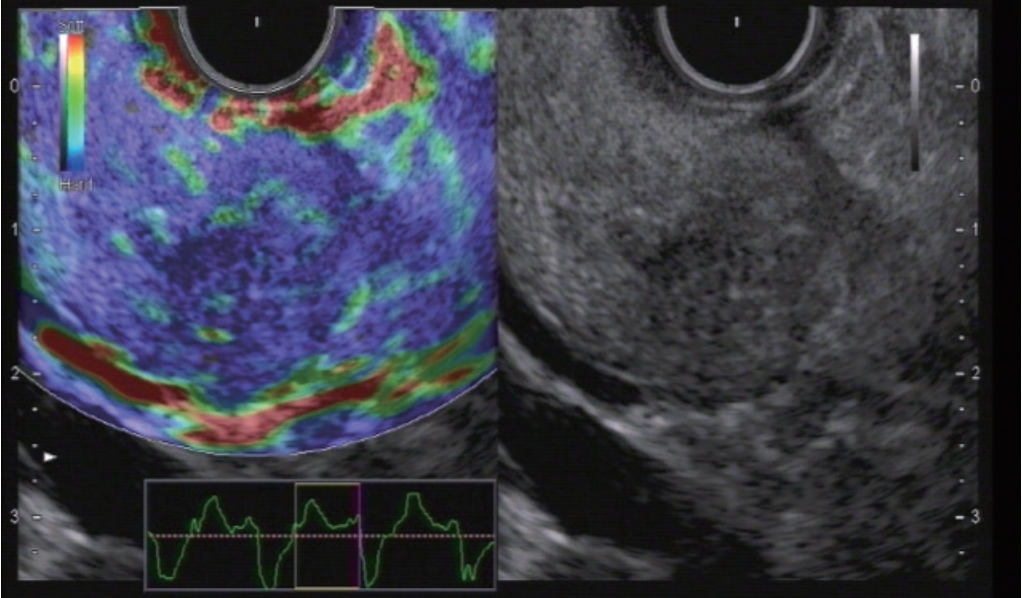
Fig.┬Ā2.
Neuroendocrine tumour, benign. Endoscopic ultrasound elastography can rule out malignancy with a high level of certainty if the lesion is displayed as soft. 
Fig.┬Ā3.
Pancreatic ductal adenocarcinoma. Elastography show a typical heterogenous stiff pattern. 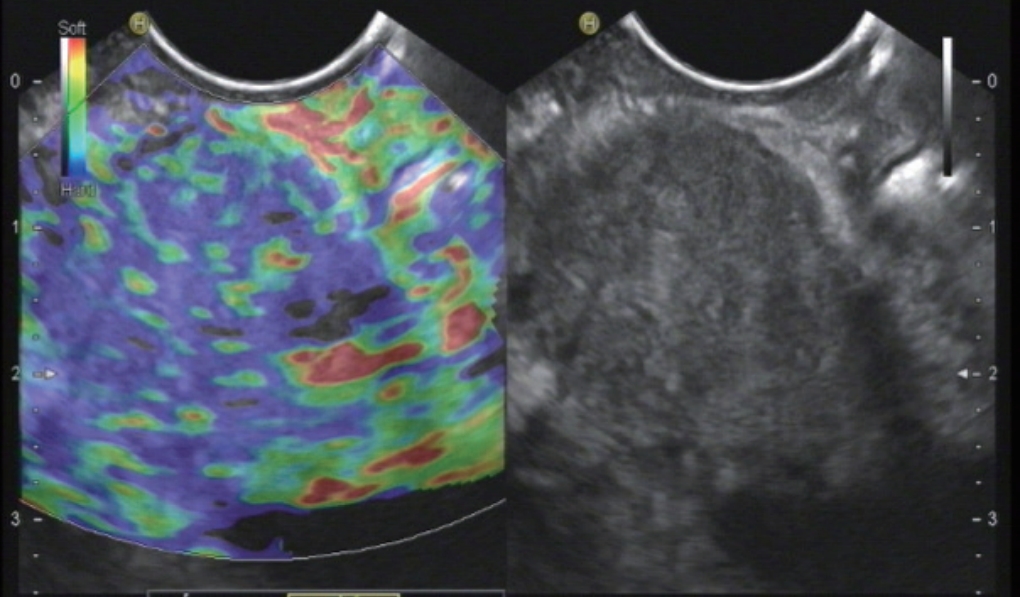
Fig.┬Ā4.
Pancreatic metastasis of colorectal carcinoma. Elastography show a typical homogenous stiff pattern. 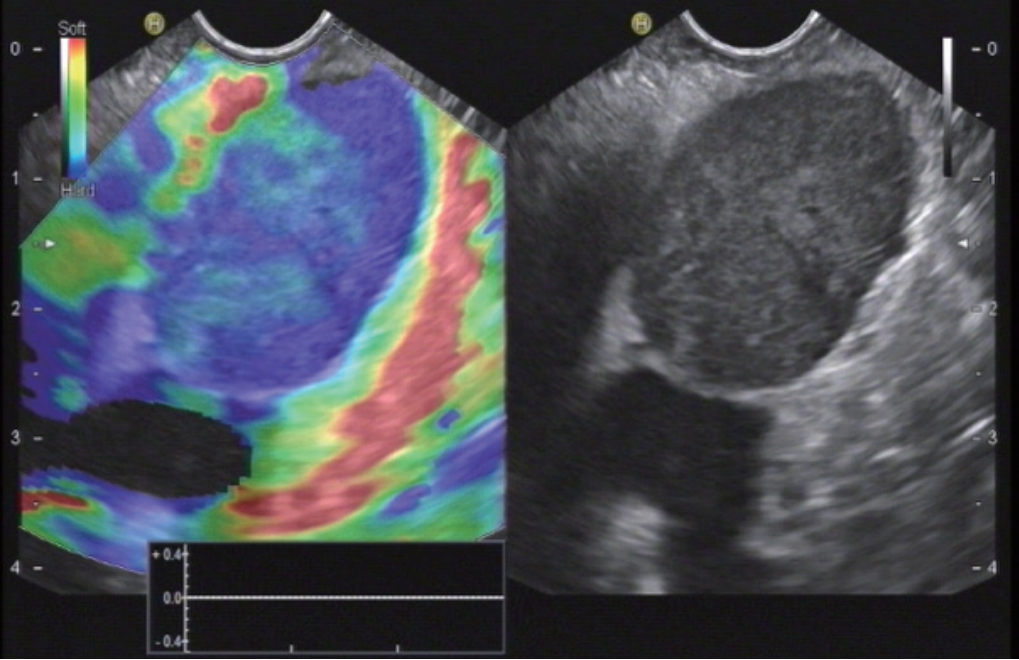
REFERENCES
2. Pirri C, Cui XW, De Molo C, Ignee A, Schreiber-Dietrich DG, Dietrich CF. The pancreatic head is larger than often assumed. Z Gastroenterol 2013;51:390ŌĆō394.   5. Shiina T, Nightingale KR, Palmeri ML, et al. WFUMB guidelines and recommendations for clinical use of ultrasound elastography: part 1: basic principles and terminology. Ultrasound Med Biol 2015;41:1126ŌĆō1147.   7. Saftoiu A, Vilman P. Endoscopic ultrasound elastography-- a new imaging technique for the visualization of tissue elasticity distribution. J Gastrointestin Liver Dis 2006;15:161ŌĆō165.  8. S─āftoiu A, Vilmann P, Gorunescu F, et al. Efficacy of an artificial neural network-based approach to endoscopic ultrasound elastography in diagnosis of focal pancreatic masses. Clin Gastroenterol Hepatol 2012;10:84ŌĆō90; e1.   10. S─āftoiu A, Vilmann P. Differential diagnosis of focal pancreatic masses by semiquantitative EUS elastography: between strain ratios and strain histograms. Gastrointest Endosc 2013;78:188ŌĆō189.   11. S─āftoiu A, Vilmann P, Gorunescu F, et al. Neural network analysis of dynamic sequences of EUS elastography used for the differential diagnosis of chronic pancreatitis and pancreatic cancer. Gastrointest Endosc 2008;68:1086ŌĆō1094.   13. Ferraioli G, Filice C, Castera L, et al. WFUMB guidelines and recommendations for clinical use of ultrasound elastography: part 3: liver. Ultrasound Med Biol 2015;41:1161ŌĆō1179.   15. Berzigotti A, Ferraioli G, Bota S, Gilja OH, Dietrich CF. Novel ultrasound-based methods to assess liver disease: the game has just begun. Dig Liver Dis 2018;50:107ŌĆō112.   17. Dietrich CF, Bamber J, Berzigotti A, et al. EFSUMB guidelines and recommendations on the clinical use of liver ultrasound elastography, update 2017 (long version). Ultraschall Med 2017;38:e16ŌĆōe47.  18. Ferraioli G, Wong VW, Castera L. Liver ultrasound elastography: an update to the world federation for ultrasound in medicine and biology guidelines and recommendations. Ultrasound Med Biol 2018;44:2419ŌĆō2440.   20. Dietrich CF, Hirche TO, Ott M, Ignee A. Real-time tissue elastography in the diagnosis of autoimmune pancreatitis. Endoscopy 2009;41:718ŌĆō720.   21. Hirche TO, Ignee A, Barreiros AP, et al. Indications and limitations of endoscopic ultrasound elastography for evaluation of focal pancreatic lesions. Endoscopy 2008;40:910ŌĆō917.   22. Havre RF, ├śdegaard S, Gilja OH, Nesje LB. Characterization of solid focal pancreatic lesions using endoscopic ultrasonography with real-time elastography. Scand J Gastroenterol 2014;49:742ŌĆō751.   24. Chiorean L, Barr RG, Braden B, et al. Transcutaneous ultrasound: elastographic lymph node evaluation. Current clinical applications and literature review. Ultrasound Med Biol 2016;42:16ŌĆō30.   32. Dietrich CF, S─āftoiu A, Jenssen C. Real time elastography endoscopic ultrasound (RTE-EUS), a comprehensive review. Eur J Radiol 2014;83:405ŌĆō414.   33. Janssen J, Dietrich CF, Will U, Greiner L. Endosonographic elastography in the diagnosis of mediastinal lymph nodes. Endoscopy 2007;39:952ŌĆō957.   34. Barr RG, Nakashima K, Amy D, et al. WFUMB guidelines and recommendations for clinical use of ultrasound elastography: part 2: breast. Ultrasound Med Biol 2015;41:1148ŌĆō1160.   35. Cosgrove D, Barr R, Bojunga J, et al. WFUMB guidelines and recommendations on the clinical use of ultrasound elastography: part 4. Thyroid. Ultrasound Med Biol 2017;43:4ŌĆō26.   36. Barr RG, Cosgrove D, Brock M, et al. WFUMB guidelines and recommendations on the clinical use of ultrasound elastography: part 5. Prostate. Ultrasound Med Biol 2017;43:27ŌĆō48.   37. Havre RF, Elde E, Gilja OH, et al. Freehand real-time elastography: impact of scanning parameters on image quality and in vitro intra- and interobserver validations. Ultrasound Med Biol 2008;34:1638ŌĆō1650.   38. Cho N, Moon WK, Chang JM, Kim SJ, Lyou CY, Choi HY. Aliasing artifact depicted on ultrasound (US)-elastography for breast cystic lesions mimicking solid masses. Acta Radiol 2011;52:3ŌĆō7.   41. Chantarojanasiri T, Hirooka Y, Kawashima H, et al. Age-related changes in pancreatic elasticity: when should we be concerned about their effect on strain elastography? Ultrasonics 2016;69:90ŌĆō96.   42. Kawada N, Tanaka S, Uehara H, et al. Potential use of point shear wave elastography for the pancreas: a single center prospective study. Eur J Radiol 2014;83:620ŌĆō624.   45. Arda K, Ciledag N, Aktas E, Aribas BK, K├Čse K. Quantitative assessment of normal soft-tissue elasticity using shear-wave ultrasound elastography. AJR Am J Roentgenol 2011;197:532ŌĆō536.   46. Goertz RS, Schuderer J, Strobel D, Pfeifer L, Neurath MF, Wildner D. Acoustic radiation force impulse shear wave elastography (ARFI) of acute and chronic pancreatitis and pancreatic tumor. Eur J Radiol 2016;85:2211ŌĆō2216.   48. Mateen MA, Muheet KA, Mohan RJ, et al. Evaluation of ultrasound based acoustic radiation force impulse (ARFI) and eSie touch sonoelastography for diagnosis of inflammatory pancreatic diseases. JOP 2012;13:36ŌĆō44.  49. G├Čya C, Hamidi C, Hattapo─¤lu S, et al. Use of acoustic radiation force impulse elastography to diagnose acute pancreatitis at hospital admission: comparison with sonography and computed tomography. J Ultrasound Med 2014;33:1453ŌĆō1460.   50. DŌĆÖOnofrio M, Tremolada G, De Robertis R, et al. Prevent pancreatic fistula after pancreatoduodenectomy: possible role of ultrasound elastography. Dig Surg 2018;35:164ŌĆō170.   51. Harada N, Ishizawa T, Inoue Y, et al. Acoustic radiation force impulse imaging of the pancreas for estimation of pathologic fibrosis and risk of postoperative pancreatic fistula. J Am Coll Surg 2014;219:887ŌĆō894; e5.   53. Kuwahara T, Hirooka Y, Kawashima H, et al. Quantitative evaluation of pancreatic tumor fibrosis using shear wave elastography. Pancreatology 2016;16:1063ŌĆō1068.   54. Kuwahara T, Hirooka Y, Kawashima H, et al. Usefulness of endoscopic ultrasonography-elastography as a predictive tool for the occurrence of pancreatic fistula after pancreatoduodenectomy. J Hepatobiliary Pancreat Sci 2017;24:649ŌĆō656.   55. Kuwahara T, Hirooka Y, Kawashima H, et al. Usefulness of shear wave elastography as a quantitative diagnosis of chronic pancreatitis. J Gastroenterol Hepatol 2018;33:756ŌĆō761.   56. Pozzi R, Parzanese I, Baccarin A, et al. Point shear-wave elastography in chronic pancreatitis: a promising tool for staging disease severity. Pancreatology 2017;17:905ŌĆō910.   57. Llamoza-Torres CJ, Fuentes-Pardo M, ├ülvarez-Higueras FJ, Alberca-de-Las-Parras F, Carballo-├ülvarez F. Usefulness of percutaneous elastography by acoustic radiation force impulse for the non-invasive diagnosis of chronic pancreatitis. Rev Esp Enferm Dig 2016;108:450ŌĆō456.   58. Dominguez-Mu├▒oz JE, Iglesias-Garcia J, Casti├▒eira Alvari├▒o M, Luaces Regueira M, Lari├▒o-Noia J. EUS elastography to predict pancreatic exocrine insufficiency in patients with chronic pancreatitis. Gastrointest Endosc 2015;81:136ŌĆō142.   60. Iglesias-Garcia J, Larino-Noia J, Abdulkader I, Forteza J, Dominguez-Munoz JE. Quantitative endoscopic ultrasound elastography: an accurate method for the differentiation of solid pancreatic masses. Gastroenterology 2010;139:1172ŌĆō1180.   62. Janssen J, Schl├Črer E, Greiner L. EUS elastography of the pancreas: feasibility and pattern description of the normal pancreas, chronic pancreatitis, and focal pancreatic lesions. Gastrointest Endosc 2007;65:971ŌĆō978.   64. Dom├Łnguez-Mu├▒oz JE. Predicting pancreatic exocrine insufficiency with EUS elastography. Gastroenterol Hepatol (N Y) 2016;12:511ŌĆō512.   65. Uchida H, Hirooka Y, Itoh A, et al. Feasibility of tissue elastography using transcutaneous ultrasonography for the diagnosis of pancreatic diseases. Pancreas 2009;38:17ŌĆō22.   66. Friedrich-Rust M, Schlueter N, Smaczny C, et al. Non-invasive measurement of liver and pancreas fibrosis in patients with cystic fibrosis. J Cyst Fibros 2013;12:431ŌĆō439.   67. Sugimoto M, Takahashi S, Kojima M, et al. What is the nature of pancreatic consistency? Assessment of the elastic modulus of the pancreas and comparison with tactile sensation, histology, and occurrence of postoperative pancreatic fistula after pancreaticoduodenectomy. Surgery 2014;156:1204ŌĆō1211.   69. Kim SY, Cho JH, Kim YJ, et al. Diagnostic efficacy of quantitative endoscopic ultrasound elastography for differentiating pancreatic disease. J Gastroenterol Hepatol 2017;32:1115ŌĆō1122.   70. DŌĆÖOnofrio M, Crosara S, De Robertis R, Canestrini S, Demozzi E, Pozzi Mucelli R. Elastography of the pancreas. Eur J Radiol 2014;83:415ŌĆō419.   75. Dong Y, J├╝rgensen C, Puri R, et al. Ultrasound imaging features of isolated pancreatic tuberculosis. Endosc Ultrasound 2018;7:119ŌĆō127.   76. Barreiros AP, Braden B, Schieferstein-Knauer C, Ignee A, Dietrich CF. Characteristics of intestinal tuberculosis in ultrasonographic techniques. Scand J Gastroenterol 2008;43:1224ŌĆō1231.   77. Lee TK, Kang CM, Park MS, et al. Prediction of postoperative pancreatic fistulas after pancreatectomy: assessment with acoustic radiation force impulse elastography. J Ultrasound Med 2014;33:781ŌĆō786.   79. Wang W, Shpaner A, Krishna SG, et al. Use of EUS-FNA in diagnosing pancreatic neoplasm without a definitive mass on CT. Gastrointest Endosc 2013;78:73ŌĆō80.   82. Dawwas MF, Taha H, Leeds JS, Nayar MK, Oppong KW. Diagnostic accuracy of quantitative EUS elastography for discriminating malignant from benign solid pancreatic masses: a prospective, single-center study. Gastrointest Endosc 2012;76:953ŌĆō961.   86. Onoyama T, Koda M, Fujise Y, et al. Utility of virtual touch quantification in the diagnosis of pancreatic ductal adenocarcinoma. Clin Imaging 2017;42:64ŌĆō67.   87. DŌĆÖOnofrio M, De Robertis R, Crosara S, et al. Acoustic radiation force impulse with shear wave speed quantification of pancreatic masses: a prospective study. Pancreatology 2016;16:106ŌĆō109.   93. Iglesias Garc├Ła JJ, Lari├▒o Noia J, Alvarez Castro A, Cigarr├Īn B, Dom├Łnguez Mu├▒oz JE. Second-generation endoscopic ultrasound elastography in the differential diagnosis of solid pancreatic masses. Pancreatic cancer vs. inflammatory mass in chronic pancreatitis. Rev Esp Enferm Dig 2009;101:723ŌĆō730.   96. Iordache S, Costache MI, Popescu CF, Streba CT, Cazacu S, S─āftoiu A. Clinical impact of EUS elastography followed by contrast-enhanced EUS in patients with focal pancreatic masses and negative EUS-guided FNA. Med Ultrason 2016;18:18ŌĆō24.   97. Kawada N, Tanaka S, Uehara H, et al. Alteration of strain ratio evaluated by transabdominal ultrasound elastography may predict the efficacy of preoperative chemoradiation performed for pancreatic ductal carcinoma: preliminary results. Hepatogastroenterology 2014;61:480ŌĆō483.  98. Kongkam P, Lakananurak N, Navicharern P, et al. Combination of EUS-FNA and elastography (strain ratio) to exclude malignant solid pancreatic lesions: a prospective single-blinded study. J Gastroenterol Hepatol 2015;30:1683ŌĆō1689.   100. Rustemovi─ć N, Kalauz M, Grubeli─ć Ravi─ć K, et al. Differentiation of pancreatic masses via endoscopic ultrasound strain ratio elastography using adjacent pancreatic tissue as the reference. Pancreas 2017;46:347ŌĆō351.   101. Dietrich CF. [Elastography, the new dimension in ultrasonography]. Praxis (Bern 1994) 2011;100:1533ŌĆō1542.   102. Dietrich CF, Cantisani V. Current status and perspectives of elastography. Eur J Radiol 2014;83:403ŌĆō404.   105. Jafri M, Sachdev AH, Khanna L, Gress FG. The role of real time endoscopic ultrasound guided elastography for targeting EUS-FNA of suspicious pancreatic masses: a review of the literature and a single center experience. JOP 2016;17:516ŌĆō524.   107. Pei Q, Zou X, Zhang X, Chen M, Guo Y, Luo H. Diagnostic value of EUS elastography in differentiation of benign and malignant solid pancreatic masses: a meta-analysis. Pancreatology 2012;12:402ŌĆō408.   108. Popescu A, Ciocalteu AM, Gheonea DI, et al. Utility of endoscopic ultrasound multimodal examination with fine needle aspiration for the diagnosis of pancreatic insulinoma - a case report. Curr Health Sci J 2012;38:36ŌĆō40.   112. Rustemovic N, Opacic D, Ostojic Z, et al. Comparison of elastography methods in patients with pancreatic masses. Endosc Ultrasound 2014;3(Suppl 1):S4.  113. He Y, Wang H, Li XP, Zheng JJ, Jin CX. Pancreatic elastography from acoustic radiation force impulse imaging for evaluation of diabetic microangiopathy. AJR Am J Roentgenol 2017;209:775ŌĆō780.   114. Sa─¤lam D, Bilgici MC, Kara C, Yilmaz GC, ├ćaml─▒da─¤ I. Acoustic radiation force impulse elastography in determining the effects of type 1 diabetes on pancreas and kidney elasticity in children. AJR Am J Roentgenol 2017;209:1143ŌĆō1149.   115. Zaro R, Lupsor-Platon M, Cheviet A, Badea R. The pursuit of normal reference values of pancreas stiffness by using acoustic radiation force impulse (ARFI) elastography. Med Ultrason 2016;18:425ŌĆō430.  
|
|











