Reggiani, Pongeluppi, Ferreira, Felix, and de Oliveira Campoli: Endoscopic diagnosis of gastric metastases from malignant melanoma: systematic review
Abstract
Background/Aims
Metastases of malignant melanoma (MM) are rare and associated with poor prognosis. The objective of this study was to analyze the clinical and endoscopic characteristics of gastric metastases of MM by systematically reviewing cases and case series involving patients diagnosed using upper gastrointestinal endoscopy.
Methods
The PubMed and LILACS databases were searched. Reports containing individual patient data were included. Outcomes such as clinical data, endoscopic findings, treatments, and survival were analyzed.
Results
A total of 88 studies with individual data from 113 patients with gastric metastases of MM were included. The primary sites of MM were the skin (62%), eyes (10%), and mucous membranes (6%). Most patients (56%) had multiple metastases in the stomach, located predominantly in the gastric body (approximately 80%). The overall survival rate at 2 years was 4%. There was a significant reduction in the survival of patients with multiple gastric metastases compared to that of patients with single metastasis (hazard ratio, 0.459; 95% confidence interval, 0.235ŌłÆ0.895; p=0.022).
Conclusions
Gastric metastases of MM have a poor prognosis, especially in patients with multiple implants in the stomach. Additional studies are needed to verify whether ocular and mucosal melanomas are associated with a higher risk of gastric metastases than that of cutaneous melanomas.
Keywords: Gastrointestinal endoscopy; Melanoma; Neoplasm metastasis; Stomach neoplasms; Systematic review
INTRODUCTION
Malignant melanoma (MM) is an aggressive neoplasm that carries a significant risk of locoregional and distant metastases. The differential diagnosis between gastric metastasis of MM and primary melanoma of the stomach is performed based on the failure to identify the primary MM at another site. Primary melanoma of the stomach is rarer than gastric metastasis; a recent systematic review identified only 25 case reports describing primary melanoma of the stomach. 1 Gastrointestinal involvement in cases of disseminated disease is common, especially in the small intestine. Secondary involvement of the stomach is rare and poorly described. 2
The diagnosis of gastric metastasis of MM is often made through a postmortem. 3 Few publications have described cases diagnosed using upper gastrointestinal endoscopy. Therefore, knowledge regarding the clinical characteristics, endoscopic data, treatment, and survival of this condition is limited. The present study aimed to compile relevant data and analyze the clinical and endoscopic characteristics of MM gastric metastases by systematically reviewing cases and case series describing patients diagnosed using upper gastrointestinal endoscopy.
METHODS
Search strategy
We performed a systematic review in accordance with the guidelines of the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA). 4 Case reports and case series of gastric metastases of MM diagnosed using upper gastrointestinal endoscopy were analyzed. The searches were performed without restrictions on language or publication date. High-sensitivity searches were performed using the Medline/PubMed and Latin American and Caribbean Health Sciences Literature (LILACS) databases using the following MeSH terms: stomach neoplasms, melanoma, and gastrointestinal endoscopy. To identify additional eligible studies, the references of the included studies were manually searched.
After screening the titles and abstracts of the articles retrieved from the databases, the full texts of potentially eligible studies were examined by independent reviewers, and the inclusion criteria were applied. Disagreements between the reviewers were resolved by consensus.
Inclusion and exclusion criteria
Case reports and case series describing metastatic MM of the stomach, diagnosed using upper gastrointestinal endoscopy, were included. We excluded cases of primary stomach melanoma. Case series that did not present individual patient data were also excluded. If the same study was published more than once, the publications with more complete information were selected.
Data extraction
Individual patient data were extracted by independent reviewers using a predefined form. Clinical data such as those of age, sex, primary site of MM, metastases in other organs, and symptoms presented by the patients were collected. We also collected information on the number and location of lesions in the stomach. Finally, data on the treatments and their outcomes were obtained.
Data analysis
Medians or means and standard deviations were calculated for continuous variables, while frequencies and percentages were calculated for categorical variables. Student t-test was used to compare continuous variables, and chi-square test or Fisher exact test was used to compare categorical variables. For individual patient data that included survival time, survival curves were calculated by Kaplan-Meier analysis, and differences between the curves were analyzed using the log-rank test. Statistical analyses were performed using the MedCalc ver. 20.011 (MedCalc Software, Ostend, Belgium). Differences were considered statistically significant at p<0.05.
RESULTS
Study selection and population characteristics
The databases were searched from their inception to November 20, 2021, and 2,812 publications were identified. After screening the titles and abstracts, 2,652 articles were excluded. Twenty-six additional articles were identified by a manual search, resulting in 186 potentially eligible studies. After analyzing the full texts using the eligibility criteria, 88 articles remained for the analysis ( Fig. 1). Among the 88 included studies, eight were case series, 2,3,5-10 and the other 80 11-90 reported only one case each. Thus, the individual data of 113 patients with gastric metastases of MM were analyzed ( Fig. 2). The patientsŌĆÖ ages ranged from 25 to 89 years (median, 63 years), and 64% of the patients were male. The most common symptoms were digestive bleeding (34.5%) and abdominal pain (34.5%).
The primary site of MM was reported in 78% of the patients and the skin was the most reported site (62%). Primary melanoma with an ocular location was described in 10% of the cases, and melanoma originated from the mucosal surfaces in 6% of the cases ( Table 1). Among the 113 patients with gastric metastases, 24 had metastases in the stomach only (21%) while 89 had metastases in other organs (79%) as well. The number of distant metastases in each of the 113 patients ranged from one to seven different sites ( Table 2). In addition to the stomach, the other most affected organs were the lungs, liver, lymph nodes, duodenum, and central nervous system ( Table 2). A high percentage of the patients received chemotherapy (46%) or only palliative care (24%). Only 20 patients (18%) underwent surgery for gastric metastases.
Endoscopic findings
Single metastasis in the stomach was reported in 48 patients (42%) and multiple metastases were observed in 63 patients (56%). Data regarding the number of stomach metastases were not reported for two patients. Metastases was predominantly located in the gastric body (in approximately 80% of the cases). In only 5% of the patients, metastases were exclusively located in the gastric antrum ( Table 3). The reports on the endoscopic characteristics of the lesions could not be validated as they varied widely, thus precluding the synthesis of these data.
Survival
In the present study, the overall 1-year survival rate was 16% and the 2-year survival rate was only 4%. The median survival time was 3 months. There was a significant reduction in the survival of patients with multiple gastric metastases compared to that of patients with single metastasis (hazard ratio for death, 0.459; 95% confidence interval, 0.235ŌĆō0.895; p=0.022) ( Fig. 3).
DISCUSSION
The present systematic review confirmed that patients with gastric metastases of MM have very low overall survival rate (2-year survival rate of 4%) and median survival of only three months. In addition, this study demonstrated that patients with single metastasis in the stomach had significantly higher overall survival than patients with multiple gastric metastases. A study by Kim et al. 3 reported a similar result; the authors analyzed 37 patients with gastric metastases from several primary sites. Surgical treatment of MM metastases in the gastrointestinal tract is associated with increased survival. 7 Thus, early detection followed by gastrectomy is key to obtaining better results. According to a study involving more than 133,000 cases of MM, the prevalence of cutaneous melanoma, ocular melanoma, and mucosal melanoma was 94.9%, 3.7%, and 1.4%, respectively. 91 However, in the present study, the prevalence of cutaneous melanoma, ocular melanoma, and mucosal melanoma was 61.9%, 9.7%, and 6.2%, respectively. Despite the small number of cases in the present review and the high percentage of unreported primary sites (22.1%), the hypothesis is that primary ocular and mucosal melanomas present a greater risk of dissemination to the stomach. Primary mucosal melanomas confer a high risk of distant metastases, ranging from 16.7% to 27.7%. 92,93
Upper gastrointestinal endoscopy plays an important role in the diagnosis of gastric metastases. In 1962, Reed et al. 90 published the first case of gastric metastasis of MM diagnosed using endoscopic biopsy. Before the emergence of modern upper gastrointestinal endoscopy, gastric metastases of MM were described as ŌĆ£bullŌĆÖs-eyeŌĆØ or ŌĆ£target-signŌĆØ lesions on radiological examinations. 94,95 However, this radiological imaging pattern may be found in several other diseases, such as lymphoma, carcinoma, KaposiŌĆÖs sarcoma, and other uncommon benign diseases. 88
In 1978, Nelson and Lanza 96 presented a proposal for an endoscopic classification system that consisted of three types of gastric lesions: (1) nodules varying in size, usually appearing to arise on the crest of the normal rugae, often ulcerated at the tip, and invariably melanotic; (2) raised, submucosal tumor-like masses with ulcerated centers which usually produced the so-called bullŌĆÖs-eye configuration on radiologic examination, and did or did not exhibit melanin grossly; and (3) mass lesions with varying degrees of necrosis and melanosis. This classification system has been used by several authors. 86,88 In 2001, Oda et al. 97 presented a proposal for an endoscopic classification system of metastatic tumors of the stomach that included several primary sites. The lesions were classified into two main patterns: submucosal tumors (with or without central depression) or primary gastric cancer. Lesions similar to primary gastric cancer were subdivided into those similar to early gastric cancer and those similar to advanced gastric cancer. Finally, lesions such as advanced gastric cancer were classified as follows: type 1, polypoid; type 2, ulcerated with well-defined margins; type 3, ulcers with undefined margins; type 4, tumors with diffuse infiltration. Several authors have adopted this classification. 3,6,30,98 In addition, elevated lesions without infiltration of the edges and with central ulceration are commonly referred to as volcano-like or donut-shaped. These various proposals for endoscopic characterization generated high variability among the included studies, which precluded the compilation of lesion characteristics in the present review. An important endoscopic finding in this review was that of the high percentage of metastases located in the gastric body. This characteristic has been described earlier, 99 and recently by other authors. 3,97,100 The pathophysiological mechanism underlying the greater preference for involvement of the gastric body remains unknown. A strength of the present study was that relevant data pertaining to an infrequent but highly severe condition were obtained. Using individual patient data, it was possible to construct and compare survival curves. The limitations of this review include the limited scope of the search, which was conducted in only two databases, and the retrospective nature of the cases reported in the included studies.
In conclusion, the spread of MM to the stomach is associated with poor prognosis, particularly in cases with multiple gastric metastases. Further studies are needed to assess whether ocular melanomas and mucosal surface melanomas are associated with a higher risk of gastric metastases than that of cutaneous melanomas. Finally, standardization of the macroscopic description of gastric metastases is necessary to facilitate better standardization of reports.
Fig.┬Ā1.
The Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) flow diagram of study selection. LILACS, Latin American and Caribbean Health Science Literature. 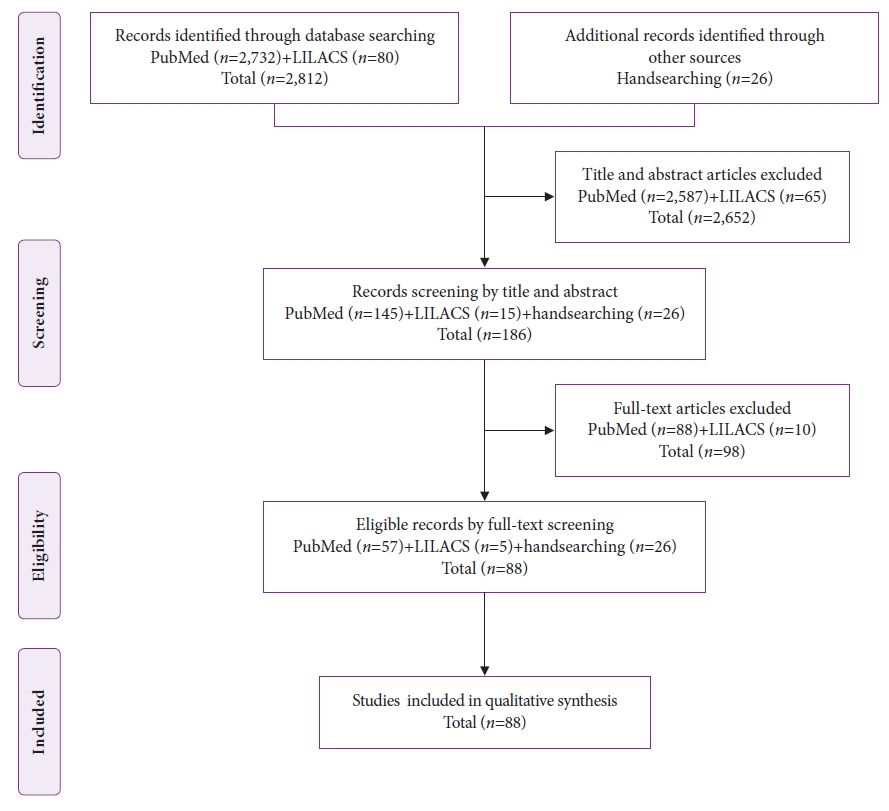
Fig.┬Ā2.
Among the 88 studies, 80 included one case. The other eight studies were case series. Four studies had two patients each, two studies had three patients, one study had nine patients, and one study had ten patients. 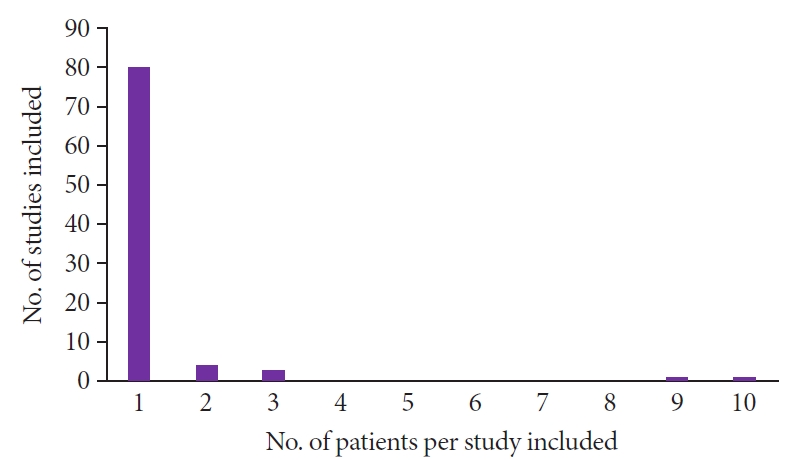
Fig.┬Ā3.
Estimated overall survival by Kaplan-Meier analysis. Patients with multiple gastric metastases were compared to patients with single gastric metastasis. CI, confidence interval. a)Number of patients at risk of survival. 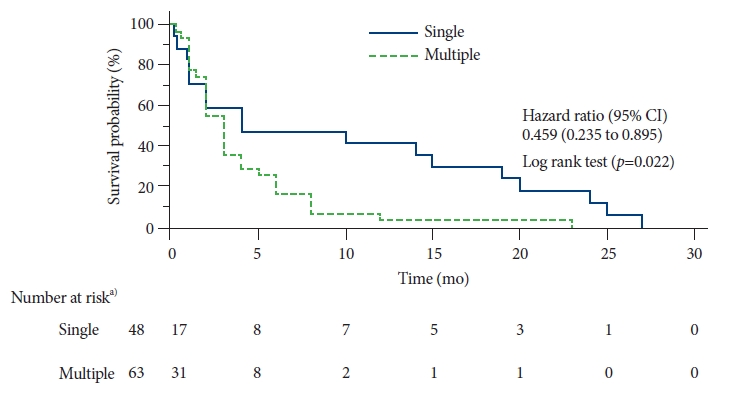
Table┬Ā1.
Characteristics of the patients
|
Characteristic |
Value |
|
Age (yr) |
|
|
ŌĆāMean┬▒SD |
62.2┬▒12.6 |
|
ŌĆāMedian (IQR) |
63 (18.5) |
|
ŌĆāRange |
25ŌĆō89 |
|
Sex |
|
|
ŌĆāMale |
72 (64.0) |
|
ŌĆāFemale |
41 (36.0) |
|
Symptom |
|
|
ŌĆāBleeding |
39 (34.5) |
|
ŌĆāAbdominal pain |
39 (34.5) |
|
ŌĆāAnorexia and weight loss |
26 (23.0) |
|
ŌĆāNausea and vomiting |
20 (17.7) |
|
ŌĆāWeakness |
7 (6.2) |
|
ŌĆāDyspepsia |
6 (5.3) |
|
ŌĆāDyspnea, cough, and chest pain |
5 (4.4) |
|
ŌĆāJaundice |
3 (2.7) |
|
ŌĆāDysphagia |
2 (1.8) |
|
ŌĆāOther symptoms |
4 (3.5) |
|
ŌĆāAsymptomatic |
11 (9.7) |
|
Primary location |
|
|
ŌĆāCutaneous |
70 (62.0) |
|
ŌĆāEye |
11 (9.7) |
|
ŌĆāMucosa |
7 (6.2) |
|
ŌĆāNot reported |
25 (22.1) |
Table┬Ā2.
Characteristics of metastases
|
Characteristic |
No. (%) |
|
Distant metastases |
|
|
ŌĆāStomach (only) |
24 (21.2) |
|
ŌĆāStomach plus a single organ |
31 (27.5) |
|
ŌĆāStomach plus two organs |
20 (17.7) |
|
ŌĆāStomach plus three organs |
20 (17.7) |
|
ŌĆāStomach plus four organs |
12 (10.6) |
|
ŌĆāStomach plus five organs |
5 (4.4) |
|
ŌĆāStomach plus six organs |
1 (0.9) |
|
Organs affected by metastases |
|
|
ŌĆāStomach |
113 (100) |
|
ŌĆāLung |
39 (34.5) |
|
ŌĆāLiver |
36 (31.9) |
|
ŌĆāLymph nodes |
25 (22.1) |
|
ŌĆāDuodenum |
22 (19.5) |
|
ŌĆāCentral nervous system |
20 (17.7) |
|
ŌĆāBone |
16 (14.2) |
|
ŌĆāAdrenal |
9 (8.0) |
|
ŌĆāSubcutaneous |
7 (6.2) |
|
ŌĆāJejunum/ileum |
7 (6.2) |
|
ŌĆāSpleen |
5 (4.4) |
|
ŌĆāEsophagus |
4 (3.5) |
|
ŌĆāPancreas |
4 (3.5) |
|
ŌĆāSkin |
3 (2.7) |
|
ŌĆāColon |
3 (2.7) |
|
ŌĆāRectum |
2 (1.8) |
|
ŌĆāPeritoneum |
2 (1.8) |
|
ŌĆāBreast |
1 (0.9) |
|
ŌĆāThyroid |
1 (0.9) |
|
ŌĆāAmygdala |
1 (0.9) |
|
ŌĆāBladder |
1 (0.9) |
|
ŌĆāPericardium |
1 (0.9) |
|
ŌĆāKidney |
1 (0.9) |
Table┬Ā3.
|
Variable |
No. (%) |
|
Gastric metastases |
|
|
ŌĆāSingle |
48 (42.5) |
|
ŌĆāMultiple |
63 (55.7) |
|
ŌĆāNot reported |
2 (1.8) |
|
Gastric location |
|
|
ŌĆāBody (only) |
68 (60.2) |
|
ŌĆāBody and antrum |
22 (19.5) |
|
ŌĆāAntrum (only) |
6 (5.3) |
|
ŌĆāNot reported |
17 (15.0) |
REFERENCES
2. Strippoli S, Ruggeri E, Fucci L, et al. The evolving landscape in the management of gastric metastases from melanoma: a case series. Clin Pract 2018;16:1079ŌĆō1084.  4. Moher D, Liberati A, Tetzlaff J, et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Ann Intern Med 2009;151:264ŌĆō269.   5. Trouillet N, Robert B, Charfi S, et al. Gastric metastases: an endoscopic series of ten cases. Gastroenterol Clin Biol 2010;34:305ŌĆō309.   7. Liang KV, Sanderson SO, Nowakowski GS, et al. Metastatic malignant melanoma of the gastrointestinal tract. Mayo Clin Proc 2006;81:511ŌĆō516.   8. Panagiotou I, Brountzos EN, Bafaloukos D, et al. Malignant melanoma metastatic to the gastrointestinal tract. Melanoma Res 2002;12:169ŌĆō173.   9. Kovcin V, Josifovski J, Opric M. Gastric metastases of melanoma: two case reports. Melanoma Res 1998;8:90ŌĆō91.   10. Geboes K, De Jaeger E, Rutgeerts P, et al. Symptomatic gastrointestinal metastases from malignant melanoma. A clinical study. J Clin Gastroenterol 1988;10:64ŌĆō70.   12. Chowdhury AAM, Khan MU, Hussain MM. Secondary metastatic gastric melanoma. J Bangladesh Coll Phys Surg 2021;39:68ŌĆō75.   15. Lee MW, Lee HJ. Numerous black elevations in the stomach and duodenum. Am J Med Sci 2020;360:78.   16. Lim AH, Argyrides J. Gastrointestinal bleeding from metastatic melanoma. N Engl J Med 2020;382:e7.  19. Jain A, Jaju A, Iyer M, et al. Metastases of malignant melanoma to stomach: an unusual presentation. Indian J Pathol Oncol 2020;7:480ŌĆō482.  20. Sachdeva S, Dalal A, Kumar A, et al. Multifocal gastric metastasis of malignant melanoma: an ominous endoscopic appearance: gastric metastasis of malignant melanoma. Dig Liver Dis 2020;52:1512.   21. Dinh B, Mossad D, Krishnamurthy P. S2953 Rare metastatic gastric melanoma causing iron deficiency anemia. Am J Gastroenterol 2020;115:S1552ŌĆōS1553.  22. Umeda Y, Tanaka K, Tsuboi J, et al. Small gastric metastases of malignant melanoma mimicking gastric erosion (with video). Gastrointest Endosc 2020;92:423ŌĆō424.   24. Januszewicz W, Corrie P, Liu H, et al. A sinister black finding in the stomach. Lancet 2019;393:1149.   25. Santos-Seoane SM, Perez-Casado L, Helguera-Amezua C, et al. Metastatic melanoma of the stomach. Cir Esp (Engl Ed) 2019;97:51.   26. Hussain S. Gastric and umbilical metastasis of cutaneous malignant melanoma. Int J Innov Sci Res Technol 2019;4:819ŌĆō821.
27. Morita S, Suda T, Terai S. Gastric metastasis from uveal melanoma. Clin Gastroenterol Hepatol 2019;17:A20.  28. Masuda TS, Kuiava VA, dos Santos PH, et al. Gastric metastases of a malignant melanoma. Clin Biomed Res 2019;39:179ŌĆō180.  29. Rausei S, Pappalardo V, Boni L, et al. Laparoscopic intragastric resection of melanoma cardial lesion. Surg Oncol 2018;27:642.   32. Borahma M, Essamri W, Afifi R, et al. Metastatic melanoma in the gastric body. Austin J Gastroenterol 2018;5:1093.
33. Queiroz CA, Soares RH, de Andrade LP, et al. Gastric metastasis of malignant melanoma. Rev Med Minas Gerais 2017;27:eŌĆō1898.
34. Iadevaia MD, Sgambato D, Miranda A, et al. Amelanotic metastatic melanoma of the stomach presenting with iron deficiency anemia. Acta Gastroenterol Belg 2017;80:327ŌĆō328.  36. Hachiya M, Satoh K, Takami S, et al. A case of metastatic uveal melanoma of the liver and digestive tract. Nihon Shokakibyo Gakkai Zasshi 2017;114:1978ŌĆō1986.  38. Shustef E, Torres-Cabala CA, Curry JL, et al. Intraepithelial melanoma in the stomach after treatment with immune checkpoint blockade therapy. Am J Dermatopathol 2017;39:e116ŌĆōe118.   40. Hiramoto S, Kyogoku K. A case report of gastroduodenal metastasis from skin melanoma. Nihon Shokakibyo Gakkai Zasshi 2016;113:1001ŌĆō1004.  41. Cooper CJ, Mardini H. Gastrointestinal bleeding as the initial presentation of gastric melanoma metastasis. Am J Gastroenterol 2016;111:S1139ŌĆōS1140.  46. Eivazi-Ziaei J, Esmaili H. Metastatic malignant melanoma affecting stomach. J Cancer Res Ther 2014;10:733ŌĆō736.   47. Laskaratos FM, Gillmore R, Clark I, et al. Dark macules in the upper gastrointestinal tract: an ominous sign. Dig Liver Dis 2014;46:1133.   48. Ruiz-Cuesta P, Herv├Īs-Molina AJ, Villar-Pastor CM, et al. Met├Īstasis gastrica tardia de melanoma cutaneo Late gastric metastasis from cutaneous melanoma]. Gastroenterol Hepatol 2014;37:564ŌĆō565.   49. Rozsa A, Mellar E, Poczik S, et al. Gastrointestinal locations of primary melanoma. Magy Seb 2014;67:9ŌĆō14.  50. Mostafa MG, Hussein MR, El-Ghorory RM, et al. Gastric metastases from invasive primary mucosal epithelioid malignant melanoma of the hard palate: report of the first case in the English literature. Expert Rev Gastroenterol Hepatol 2014;8:15ŌĆō19.   55. Kraft Rovere R, Pires de Souza ME, Fernanda Hilgert S, et al. Melanoma metastasis to the gastric mucosa preceded by guillain-barr├® as a paraneoplastic syndrome. Gastrointest Cancer Res 2013;6:150ŌĆō151.   56. Crin├▓ SF, Scalisi G, Pallio S, et al. Malignant melanoma rather than malignant cutaneous melanoma? Eur J Gastroenterol Hepatol 2013;25:503ŌĆō506.   57. Canhotoa M, Barbeiroa S, Arroja B, et al. Multiple gastric metastases of malignant melanoma. GE Port J Gastroenterol 2013;20:279ŌĆō281.  58. Rana SS, Chaudhary V, Bhasin DK. Narrow band imaging appearance of gastric metastasis from malignant melanoma. Ann Gastroenterol 2013;26:353.   59. Li Z, Linghu E. One case report: endoscopic manifestation of gastric metastatic melanoma. Am J Gastroenterol. 2013;108: S603.  60. Das HS, Panda C, Padhi S, et al. Endoscopic diagnosis of a case of malignant melanoma. Trop Gastroenterol 2012;33:296ŌĆō297.   61. Wu PR, Yen HH, Chen CJ. Gastrointestinal: primary esophageal melanoma with gastric metastasis. J Gastroenterol Hepatol 2011;26:1338.   62. Kawaguti FS, Maluf-Filho F, Medeiros RS, et al. Ocular melanoma with multiple gastrointestinal metastases. Dig Endosc 2011;23:208.   65. Kasza J, Espinel F, Khambaty F, et al. Laparoscopy for stage IV melanoma in two organs. Surg Laparosc Endosc Percutan Tech 2010;20:295ŌĆō297.   66. Matsubayashi H, Takizawa K, Nishide N, et al. Metastatic malignant melanoma of the gastric mucosa. Intern Med 2010;49:1243ŌĆō1244.   67. Carneiro JQ, Landim MR, Mendes JV, et al. Metastatic gastric melanoma: case report. Rev Soc Bras Clin Med 2010;8:461ŌĆō463.
69. Padhi S, Kar A, Behera PK, et al. Occult malignant melanoma metastasizing to the stomach in an elderly patient. Indian J Pathol Microbiol 2008;51:461ŌĆō462.  70. Koklu S, Gultuna S, Yuksel I, et al. Diffuse gastroduodenal metastasis of conjunctival malignant melanoma. Am J Gastroenterol 2008;103:1321ŌĆō1323.   72. Erzin Y, Akyuz U, Pata C. What is your diagnosis? Small black spots in the stomach and duodenum. Neth J Med 2008;66:129ŌĆō131.  73. Bargiggia S, Parente F, Ucci G, et al. Bleeding gastric metastatic melanoma. Dig Liver Dis 2008;40:699.   74. Pommer B, Probst A, Messmann H. Gastric metastases from malignant melanoma. Endoscopy 2008;40 Suppl 2:E30ŌĆōE31.   75. Parisian KR, Mcfarland JE, Shah AN. Metastatic malignant melanoma of the gastrointestinal tract. Clin Gastroenterol Hepatol 2008;6:A24ŌĆōA24.e1.  76. Rocha ME, Rodrigues GP, Borges SA, et al. Metastatic melanoma of the stomach. ABCD Arq Bras Cir Dig 2008;21:205ŌĆō207.  77. Cohen VM, Ahmadi-lari S, Hungerford JL. Gastric metastases from conjunctival melanoma. Melanoma Res 2007;17:255ŌĆō256.   78. Prado AB, Vargas SF, Clemente LJ. Gastric metastasis of cutaneous melanoma. Gastroenterol Latinoam 2007;18:35ŌĆō38.
79. Lufrano M, Storch I, Feldstein R, et al. Metastatic melanoma in a dialysis patient with vomiting diagnosed by gastric polyp biopsy. Am J Gastroenterol 2007;102:S343.  80. Ueno N, Yoneda M, Inamori M, et al. Metastatic malignant melanoma in the upper alimentary tract. Gastrointest Endosc 2006;64:1002; discussion 1003.   81. Benedeto-Stojanov DA, Nagorni AV, Zivkovic VV, et al. Metastatic melanoma of the stomach and the duodenum. Arch Oncol 2006;14:60ŌĆō61.  83. Mimica M, Tomic I. Endoscopic diagnosis of malignant melanoma metastatic to the stomach. Am J Gastroenterol 2002;97:1572ŌĆō1573.  84. Hokama A, Sugama R, Kinjo F, et al. At the focal point...metastatic malignant melanoma. Gastrointest Endosc 1999;50:241.   85. Woollons A, Derrick EK, Price ML, et al. Gastrointestinal malignant melanoma. Int J Dermatol 1997;36:129ŌĆō131.   86. Hsu CC, Chen JJ, Changchien CS. Endoscopic features of metastatic tumors in the upper gastrointestinal tract. Endoscopy 1996;28:249ŌĆō253.   88. Morini S, Bassi O, Colavolpe V. Malignant melanoma metastatic to the stomach: endoscopic diagnosis and findings. Endoscopy 1980;12:86ŌĆō89.   89. B├żckman H, Davidsson L. Metastases of malignant melanoma in the stomach and small intestine. Acta Med Scand 1965;178:329ŌĆō335.   90. Reed PI, Raskin HF, Graff PW. Malignant melanoma of the stomach. JAMA 1962;182:298ŌĆō299.   91. McLaughlin CC, Wu XC, Jemal A, et al. Incidence of noncutaneous melanomas in the U.S. Cancer 2005;103:1000ŌĆō1007.   92. Bishop KD, Olszewski AJ. Epidemiology and survival outcomes of ocular and mucosal melanomas: a population-based analysis. Int J Cancer 2014;134:2961ŌĆō2971.   93. Tas F, Keskin S, Karadeniz A, et al. Noncutaneous melanoma have distinct features from each other and cutaneous melanoma. Oncology 2011;81:353ŌĆō358.   94. Pomerantz H, Margolin HN. Metastases to the gastrointestinal tract from malignant melanoma. Am J Roentgenol Radium Ther Nucl Med 1962;88:712ŌĆō717.  95. Potchen EJ, Khung CL, Yatsuhashi M. X-ray diagnosis of gastric melanoma. N Engl J Med 1964;271:133ŌĆō136.   96. Nelson RS, Lanza F. Malignant melanoma metastatic to the upper gastrointestinal tract: endoscopic and radiologic correlations, form and evolution of lesions, and value of directed biopsy in diagnosis. Gastrointest Endosc 1978;24:156ŌĆō158.   97. Oda I, Kondo H, Yamao T, et al. Metastatic tumors to the stomach: analysis of 54 patients diagnosed at endoscopy and 347 autopsy cases. Endoscopy 2001;33:507ŌĆō510.   98. Gilg MM, Grochenig HP, Schlemmer A, et al. Secondary tumors of the GI tract: origin, histology, and endoscopic findings. Gastrointest Endosc 2018;88:151ŌĆō158.e1.   100. Haendchen Bento L, Kazuyoshi Minata M, Pires Batista C, et al. Clinical and endoscopic aspects of metastases to the gastrointestinal tract. Endoscopy 2019;51:646ŌĆō652.  
|
|










