See commentary "Cell block created from pancreatic duct lavage is another jigsaw puzzle to diagnose early pancreatic ductal adenocarcinoma" in Volume 56 on page 313 AbstractBackground/AimsThis study aimed to clarify the efficacy and safety of pancreatic duct lavage cytology combined with a cell-block method (PLC-CB) for possible pancreatic ductal adenocarcinomas (PDACs).
MethodsThis study included 41 patients with suspected PDACs who underwent PLC-CB mainly because they were unfit for undergoing endoscopic ultrasonography-guided fine needle aspiration. A 6-Fr double lumen catheter was mainly used to perform PLC-CB. Final diagnoses were obtained from the findings of resected specimens or clinical outcomes during surveillance after PLC-CB.
ResultsHistocytological evaluations using PLC-CB were performed in 87.8% (36/41) of the patients. For 31 of the 36 patients, final diagnoses (invasive PDAC, 12; pancreatic carcinoma in situ, 5; benignancy, 14) were made, and the remaining five patients were excluded due to lack of surveillance periods after PLC-CB. For 31 patients, the sensitivity, specificity, and accuracy of PLC-CB for detecting malignancy were 94.1%, 100%, and 96.8%, respectively. In addition, they were 87.5%, 100%, and 94.1%, respectively, in 17 patients without pancreatic masses detectable using endoscopic ultrasonography. Four patients developed postprocedural pancreatitis, which improved with conservative therapy.
INTRODUCTIONThe high diagnostic accuracy of endoscopic ultrasonography (EUS)-guided fine needle aspiration (FNA) cytology makes it the most accepted procedure for the preoperative histocytological diagnosis of pancreatic ductal adenocarcinomas (PDACs) with mass lesions detected on imaging studies.1-3 In particular, EUS-guided fine needle biopsy (EUS-FNB) needles, including Franseen and fork-tip needles, have recently been reported to provide high diagnostic accuracy for pancreatic solid masses compared to conventional EUS-FNA needles.2,3 Therefore, the reliability of EUS-FNA, including EUS-FNB, in detecting pancreatic malignancies became higher than before EUS-FNB needles became available. However, for the diagnosis of PDACs for which EUS-FNA technically fails or early stage PDACs without visible mass lesions, including pancreatic carcinoma in situ (PCIS), pancreatic juice cytology (PJC) is an alternative procedure and has been reported to be an effective method for determining PDACs.4
In our hospital, the cell-block method has been used for cytological evaluation of pancreatobiliary diseases because this method allows many kinds of histological staining, including immunostaining, to be performed simultaneously, even by using cytological specimens, thereby enabling more accurate and objective diagnoses of malignancies, such as histological diagnoses.5 In fact, our prospective randomized study suggested that the accuracy of this method for detecting malignancy was shown to be higher than that of a smear method.6
Recently, we have adopted pancreatic duct lavage cytology combined with a cell-block method (PLC-CB), mainly by using a double lumen catheter for patients with possible malignant intraductal papillary mucinous neoplasms7 or possible PDACs. Although the efficacy of PLC-CB in detecting malignancy in patients with intraductal papillary mucinous neoplasms has been reported,8 its use in patients with possible PDACs has not yet been studied. Therefore, we conducted a retrospective study to investigate the usefulness of PLC-CB in the diagnosis of malignancy in patients with possible PDACs.
METHODSStudy populationThis was a retrospective, single-center, cross-sectional study conducted at Sendai City Medical Center. In our hospital, histocytological evaluations using pancreatic specimens, including pancreatic juice, are performed if patients have any one of the following: a pancreatic mass, focal main pancreatic duct (MPD) stenosis lacking secondary etiology of this finding, such as chronic pancreatitis, or focal pancreatic parenchymal atrophy replaced by fatty tissues. For patients with the above-mentioned indications, EUS-FNA (or transpapillary bile duct biopsy) is first performed if pancreatic masses are detected on imaging. On the other hand, PCL-CB is performed when the following situations occur: (1) patients do not have visible pancreatic masses; (2) EUS-FNA is technically difficult to perform; (3) patients refuse to undergo EUS-FNA because of the possibility of dissemination of tumor cells; and (4) PCL-CB is complementarily performed in addition to EUS-FNA for the purpose of improving diagnostic accuracy in detecting malignancy.
A flowchart of this study is presented in Figure 1. There were 384 patients with possible PDACs from whom pancreatic specimens were obtained endoscopically for histocytological evaluation between October 2015 and October 2018. Of these, 41 patients who underwent PLC-CB were included in the study. Of the 41 patients, eight were used for another retrospective study conducted in our hospital to clarify the diagnostic ability of MPD stenosis to detect PDACs without forming mass lesions, including PCIS.9
Methods1) Outcome measurementWe retrospectively evaluated the following: (1) the success rate of histocytological evaluations using PLC-CB, (2) the accuracy of PLC-CB for detecting malignancy, (3) the accuracy of PLC-CB in detecting malignancy in patients with possible PDACs in whom pancreatic masses were not detected using EUS, and (4) postprocedural adverse events.
2) Endoscopic proceduresPancreatography was performed using a duodenoscope (JF-260V or TJF-260V; Olympus) and a 1.7 mm diameter cannula (PR-104Q-1 or PR-109Q-1; Olympus). A 0.025-inch J-shaped tip guidewire (Revowave SJ; Piolax Medical Devices Inc.) was carefully advanced into the tail side of the MPD, and then the cannula was changed to the following sampling catheters over the guidewire: a single lumen catheter with side holes (PR-130Q; Olympus) or a double lumen catheter (Uneven Double Lumen Cannula; Piolax Medical Devices Inc.) (Fig. 2). The tip of the sampling catheter was placed in the MPD near the target, which is indicative of possible PDACs. After collecting as much pancreatic juice flowing into the MPD as possible through a sampling catheter with negative pressure using a syringe, saline lavage inside the MPD was performed to obtain specimens derived from the pancreatic duct epithelium. When a single catheter was used, saline injection into the MPD and suction of fluid in the MPD were alternated through one lumen. On the other hand, when a double lumen catheter was used, the two processes were simultaneously performed through two separate lumens, and saline injection was carefully performed while checking whether the amount of fluid specimen collected was almost the same as that of saline injected to avoid an increase in internal pressure of the MPD (Fig. 3). Saline lavage was continued until adequate floating of small tissue pieces was macroscopically confirmed.
3) Histocytological evaluationsAll collected fluid specimens were used for histocytological evaluations using the cell-block method. Cell block sections were processed using the sodium alginate method and subjected to hematoxylin and eosin (H&E) staining for all subjects and immunostaining, if necessary.6,10 The antibodies used for immunostaining were as follows: Ki-67 (MIB-1; Immunotech), p53 protein (DO-7; DAKO), and MUC1 glycoprotein (Ma695; Novocastra). In addition, antibodies used for immunostaining were also used for the resected specimens.
The findings obtained from H&E staining were first used for the evaluation of PLC-CB specimens, and malignancy was defined as Class IIIbŌĆōV on the basis of the Papanicolaou classification system.11 If possible, immunostaining was additionally used for the evaluation of malignancy. The presence of atypical cells positive for p53 and/or MUC1 staining with a high Ki-67 labeling index of Ōēź10% was defined as an indicator of malignancy.5,6,10 When both H&E staining and immunostaining could be used to evaluate malignancy, histocytological diagnoses using PLC-CB specimens were comprehensively determined from the results of these staining methods. All PLC-CB results were prospectively determined before pancreatic surgery or before surveillance without pancreatic surgery, and were retrospectively collected and analyzed.
4) Determination of definitive benignancy and malignancyFor patients who underwent pancreatic surgery, definitive malignancy was determined as having histologically confirmed PDACs, including pancreatic carcinoma in situ (PCIS). For patients who did not undergo pancreatic surgery after PLC-CB, definitive benignancy was diagnosed when the following two conditions were met: (1) there were no changes in imaging findings of the pancreatic lesions during a surveillance period of Ōēź12 months after PLC-CB, and (2) clinical courses and imaging findings after PLC-CB were consistent with those of a benign disease.
5) Procedure-related adverse eventsAdverse events associated with endoscopic retrograde cholangiopancreatography (ERCP), such as post-ERCP pancreatitis (PEP), perforation, bleeding, and adverse events related to the cardiovascular and pulmonary systems, were evaluated in all subjects. The diagnosis and severity of PEP were determined based on criteria developed by Cotton et al.12
RESULTSBaseline characteristics of 41 subjectsNineteen men and 22 women with a median age of 70 years (IQR, 64ŌĆō76 years) were included in this study. Imaging studies showed that 17 patients (41.5%) had pancreatic masses with a median size of 21 mm (IQR, 12ŌĆō40 mm), and nine of these patients underwent EUS-FNA for tissue acquisition (histological diagnoses of EUS-FNA specimens: malignancy, 3; indeterminate diagnosis, 6). Of the nine patients, five underwent EUS-FNA for initial tissue acquisition. The remaining four patients underwent EUS-FNA for additional tissue acquisition a few days after undergoing PLC-CB to improve the diagnostic accuracy for detecting malignancy by using specimens obtained endoscopically at the physicianŌĆÖs discretion. On the other hand, 8 of the 17 patients with pancreatic masses initially underwent PLC-CB for initial tissue acquisition for the following reasons: (1) difficulty in undergoing EUS-FNA due to poor visualization of their pancreatic masses using EUS (n=3) and (2) a refusal to undergo EUS-FNA due to the possibility of tumor cell dissemination (n=5). All 24 patients without pancreatic masses initially underwent PLC-CB to obtain pancreatic tissues (Tables 1, 2).
Definitive diagnoses of PDACs were made in 18 patients (invasive, n=13; PCIS, n=5). Based on the Union for International Cancer Control 8th edition, the stages of the PDACs were determined as follows: stage 0 (PCIS) in five patients (Figs. 4, 5), stage IA in four patients (T1b, n=2; T1c, n=2), stage IB in four patients (T2, n=4), stage IIA in two patients (T3, n=2), stage IIB in two patients (T1bN1, n=1; T2N1, n=1), and stage III in one patient (T3N2, n=1). Imaging studies or resected specimens confirmed that these PDACs were not derived from concomitant pancreatic cysts.
Results of PLC-CB and clinical courses after PLC-CBA double lumen catheter was used in 88% (n=36) of the patients. The median amounts of injected saline and fluid specimens obtained were 39 mL (IQR, 16ŌĆō40 mL) and 40 mL (IQR, 15ŌĆō45 mL), respectively. Fluid specimens obtained through ERCP were histocytologically evaluated in 88% (n=36) of the subjects. For the remaining five patients, the obtained fluid specimens could not be histocytologically evaluated because of insufficient tissue specimens. Immunostaining was performed for all 36 patients, excluding five patients with inadequate PLC-CB specimens.
Of the 36 patients whose fluid specimens could be evaluated histocytologically, 16 were diagnosed with malignancy using the initial PLC-CB. Of these 16 patients, 15 underwent surgery and the remaining patients underwent preoperative chemotherapy. All 15 patients who underwent surgery were definitively diagnosed with malignancy by using resected pancreatic specimens (invasive PDAC, n=10; PCIS, n=5). The remaining patient, who did not undergo surgery because of progression to inoperable PDAC after preoperative chemotherapy, was determined to have a definitive malignancy based on his clinical course after PLC-CB.
Of the 20 patients diagnosed with benignancy using initial PLC-CB, two underwent pancreatic surgery after initial PLC-CB in consideration of the possibility of false-negative results due to MPD stenosis with marked dilation of the upstream MPD. Both patients were diagnosed with low-grade pancreatic intraepithelial neoplasia (PanIN) by using resected specimens. Of the 18 patients who underwent surveillance without surgery depending on the PLC-CB results of benignancy, 12 were determined to be benign based on imaging findings and clinical courses during a surveillance period of Ōēź12 months after PLC-CB (median surveillance period after initial PLC-CB, 641 days (IQR, 494ŌĆō1,084 days); benign MPD stenosis, n=8; mass-forming pancreatitis, n=3; autoimmune pancreatitis, n=1). Of the other six patients, five were determined to have indeterminate final diagnoses due to the lack of surveillance periods after PLC-CB, and the remaining patient was shown to have a low echoic mass 7 mm in size adjacent to the MPD stenosis using EUS three months after the initial PLC-CB. The mass lesion was determined to be malignant based on EUS-FNA results.
Accuracy of PLC-CB to detect malignancy for patients with possible PDACsOf the 41 patients who underwent PLC-CB, five were excluded from the evaluation of the diagnostic ability to detect malignancy due to lack of surveillance periods after PLC-CB, and the accuracy of PLC-CB for detecting malignancy was evaluated using 36 patients whose final diagnoses could be determined (Table 3).
First, from 36 patients with definitive diagnoses, we selected 31 patients with adequate PLC-CB specimens to investigate the diagnostic ability of PLC-CB to detect malignancy. The sensitivity, specificity, positive predictive value (PPV), negative predictive value (NPV), and accuracy in detecting malignancy were 94.1% (16/17), 100% (14/14), 100% (16/16), 93.3% (14/15), and 96.8% (30/31), respectively. For one patient whose diagnosis of initial PLC-CB was determined to be a false negative (Class II), a pancreatic mass lesion was detected three months after initial PLC-CB, followed by a diagnosis of malignancy using secondary PLC-CB (Class V) and EUS-FNA (adenocarcinoma).
Of the 31 patients, 17 had no visible pancreatic masses detected using EUS (final diagnosis: invasive PDAC, 3; PCIS, 5; low-grade PanIN, 1; benign MPD stenosis, 8). For the 17 patients, the sensitivity, specificity, PPV, NPV, and accuracy of PLC-CB for detecting malignancy were 87.5% (7/8), 100% (9/9), 100% (7/7), 90.0% (9/10), and 94.1% (16/17), respectively. Eight of the 17 patients were diagnosed with PDACs using resected specimens, and the final stages on the basis of the 8th edition of the Union for International Cancer Control were stage 0 in five patients and IA in three patients (T1b, n=2; T1c, n=1).
In addition, we investigated the diagnostic ability of PLC-CB to detect malignancy in all 36 patients with definitive diagnoses, including five patients with inadequate PLC-CB specimens. In this situation, regardless of which final diagnoses were made for five patients with inadequate PLC-CB specimens, the diagnoses of PLC-CB for those five patients were regarded as incorrect. Thus, when all 36 patients were included in this analysis, the sensitivity, specificity, PPV, NPV, and accuracy of PLC-CB for detecting malignancy were calculated as 88.9% (16/18), 77.8% (14/18), 100% (16/16), 93.3% (14/15), and 83.3% (30/36), respectively. When 21 patients without visible imaging mass lesions were selected from the 36 patients for the evaluation of the sensitivity, specificity, PPV, NPV, and accuracy of PLC-CB in detecting malignancy, they were calculated to be 87.5% (7/8), 69.2% (9/13), 100% (7/7), 90.0% (9/10), and 76.2% (16/21), respectively.
Post-ERCP adverse eventsFor 35 subjects (85.4%), prophylactic pancreatic stenting using a 5 Fr, one-sided pigtail-type pancreatic stent (Pit-stent; Gaderius Medical Co., Tokyo, Japan) was performed. In addition, nonsteroidal anti-inflammatory drugs (NSAIDs; diclofenac sodium suppository, 25 or 50 mg/body) were administered before ERCP as a preventative measure in 23 of the subjects (56.1%) (Table 4).
PEP developed in four patients (9.8%; moderate severity, 3; mild severity, 1). Of the four patients with PEP, one patient with moderate PEP did not undergo pancreatic stent replacement and administration of NSAIDs, whereas the remaining three underwent both prophylactic treatments. The PEP in these patients improved with conservative treatment. No other adverse events associated with ERCP were observed in any of the patients.
To clarify the risk factors for PEP related to PLC-CB procedures, we investigated the relationship between PEP and the following factors: sex, body mass index, NSAID administration, pancreatic duct stent placement, sphincterotomy, intraductal ultrasound, and total amount of injected saline, as shown in Table 4. However, no significant risk factors for PEP were identified by univariate analysis (Fisher's exact test).
DISCUSSIONThis study indicates that PLC-CB can be used to detect malignancy in patients with possible PDACs for which EUS-FNA is inadequate, with a high sensitivity for detecting malignancy and a relatively acceptable rate of PEP. Since EUS-FNA is sometimes ineffective for patients with early stage PDACs, including PCIS, because of the lack of target pancreatic masses, PLC-CB may be a promising method for these patients in consideration of the high diagnostic accuracy of this method.
The methodology in this study was characterized by the use of a combination of pancreatic duct lavage and cell-block methods. There have been no reports on the diagnostic ability of the pancreatic duct lavage method for detecting malignancy in patients with PDAC. In this study, a commercially available double lumen catheter was mainly used for the pancreatic duct lavage method. This catheter allowed us to perform injection and suction simultaneously, and larger volumes of fluid specimens were obtained (a median of 40 mL). However, this method is not suitable for cytological evaluation using the smear method because the fluid specimens obtained using this method are too large to be evaluated. We believe that the cell-block method is an optimal choice for evaluating larger volumes of fluid specimens histocytologically, and that the pancreatic duct lavage and cell-block methods make a good combination. In addition, the cell-block method enables us to evaluate structural atypia and many types of immunostaining, even for pancreatic juice specimens, contributing to a more objective and accurate diagnosis of malignancy in patients with possible PDACs.5,6,10
With regard to recent studies for the diagnosis of PCIS, the utility of serial pancreatic juice aspiration for cytologic examination using an endoscopic nasopancreatic drainage (ENPD) catheter has been reported.13,14 This method makes it possible to perform PJC several times using fluid specimens continuously obtained through an ENPD tube, which is the main reason why this method is effective for the early diagnosis of PDACs. However, this method has some disadvantages: (1) the necessity of competent cytologists who can perform cytological evaluations, including rapid on-site evaluation, (2) a burden on the staff in the pathology department who must perform cytology several times, (3) the burden of collecting PJC specimens several times through an ENPD tube on the attending doctors, and (4) a burden on the patient with an ENPD tube present for a long period. Although it is not clear which of the two methods is useful to detect PDACs at an early stage, like PCIS, PLC-CB may become an alternative method to serial pancreatic juice aspiration for cytologic examination for some of these PDACs, and a combination of the two methods may improve the accuracy of detecting PDACs.
This study had several limitations. First, it had a small sample size and was a retrospective study conducted in a single medical center. Therefore, the results of this study need to be verified in a multicenter, large-sample validation cohort. Second, the surveillance period after PLC-CB is relatively short. As it can take several years for PCISs to develop invasive lesions,15 patients determined to be benign by using benign clinical courses of Ōēź12 months may not necessarily be benign. Third, transpapillary approaches to obtaining pancreatic specimens, including the procedure in this study, include the risk of developing PEP. In particular, saline lavage inside the MPD may increase its internal pressure, which may further increase the risk of developing PEP. However, the incidence of PEP in this study was similar to previous results involving ENPD (4.1%ŌĆō12.4%),16,17 which may be due to the insertion of a prophylactic pancreatic stent and/or the administration of NSAIDs.18,19 At present, these two methods should be considered to avoid PEP as much as possible when PLC-CB is performed. In addition, it is important to pay attention to whether the infusion rate of saline is almost the same as the suction rate of the fluid specimens to avoid an increase in the internal pressure of the MPD. Fourth, PLC-CB sometimes provides indeterminate histocytological diagnoses owing to an inadequate volume of PLC-CB specimens (12.2%, 5/41). Of the five patients with inadequate PLC-CB specimens, one patient with definitive PDAC (no. 13 in Table 2) could not be determined to have malignancy using PLC-CB, which may be due to the small amount of saline used for lavage (only 5 mL). In contrast, the remaining four patients with inadequate PLC-CB specimens were determined to have benignancy based on the definitions of malignancy in this study. Although neoplastic epithelial cells may be relatively easy to detach from the neoplastic ductal epithelia in the pancreas due to their fragility, non-neoplastic epithelial cells may sometimes be difficult to detach from the pancreatic ducts even when using saline lavage. Fifth, despite the excellent sensitivity of PLC-CB to detect malignancy (almost 90%), not all patients with definitive PDACs can be diagnosed with malignancy using PLC-CB. Considering a case of PDAC (no. 12 in Table 2) whose mass lesion was obvious 3 months after the result of benignancy determined by using the first PLC-CB, if the first PLC-CB does not indicate malignancy in patients with possible PDACs, we should consider performing imaging studies, such as computed tomography and magnetic resonance imaging, and/or the second PLC-CB within 3 months after the first PLC-CB. Despite several limitations, this study indicates that it is possible to diagnose early stage PDACs, including PCIS, using PLC-CB. Moreover, PLC-CB may be an alternative to EUS-FNA for diagnosing PDACs for which EUS-FNA is technically difficult.
In conclusion, PLC-CB has a good ability to detect malignancy for possible PDACs without visible pancreatic masses or those for which EUS-FNA is technically difficult. A transpapillary approach using PLC-CB may contribute to the detection of early stage PDACs, including PCIS, for which good prognosis can be expected.
NOTESAcknowledgments
We would like to thank Fumiyoshi Fujishima, MD, PhD, Department of Pathology, Tohoku University School of Medicine, Miwa Uzuki, MD, PhD, Department of Nursing, Faculty of Medical Science and Welfare, Tohoku Bunka Gakuen University, and Toru Furukawa, MD, PhD, Department of Investigative Pathology, Tohoku University Graduate School of Medicine for histocylogical evaluations using cytological specimens and resected specimens from all subjects, and all staff in the Department of Pathology at Sendai City Medical Center for performing immunohistochemical staining in this study. In addition, we are grateful to Dr. Brian Breedlove, Associate Professor, Tohoku University School of Science, for English proofreading.
Author Contributions
Conceptualization: HK, SK; Data curation: HK, SK; Formal analysis: HK, SK, TT, TSaw, YN, KI; Investigation: HK, SK, YK, TO, TSak, KY, KM, FK, HA, KE, HO, MO; Methodology: HK, SK; Project administration: HK, SK; Supervision: SK, KI; Validation: SK, KI; WritingŌĆōoriginal draft: HK, SK; WritingŌĆōreview & editing: all authors.
Fig.┬Ā1.Flowchart for patient selection in this study. PDACs, pancreatic ductal adenocarcinomas; EUS-FNA, endoscopic ultrasound-guided fine needle aspiration; PLC-CB, pancreatic duct lavage cytology combined with a cell-block method. 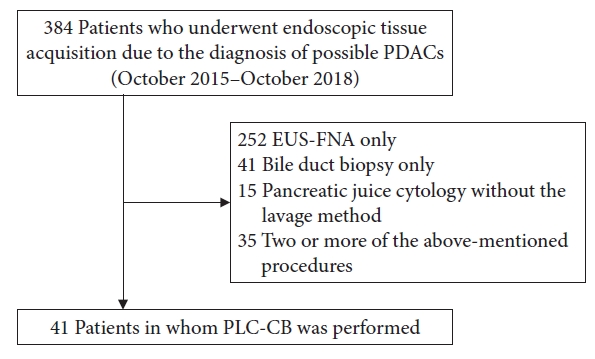
Fig.┬Ā2.Tip of a double lumen sampling catheter used in this study (Uneven Double Lumen Cannula; Piolax Medical Devices Inc.). 
Fig.┬Ā3.Endoscopic procedure for pancreatic duct lavage cytology by using a double lumen catheter (endoscopy assistant). The injection of saline into the main pancreatic duct (A) and the suction of fluid in the main pancreatic duct with negative pressure by using a syringe (B) were simultaneously performed through two separate lumens (0.025- and 0.035-inch lumen, respectively). 
Fig.┬Ā4.A 64-year-old patient with an initial diagnosis of acute pancreatitis (patient no. 14 in Table 2). The enlargement of the pancreatic tail with mild pancreatitis and localized atrophy of the pancreatic parenchyma at the proximal site of the enlarged pancreatic tail were detected (A, contrast-enhanced computed tomography, arrowhead). Endoscopic ultrasonography did not detect mass lesions, and magnetic resonance cholangiopancreatography did not show the main pancreatic duct stenosis at the pancreatic tail. We suspected pancreatic carcinoma in situ and performed pancreatic duct lavage cytology combined with a cell-block method using a double lumen catheter. Endoscopic retrograde pancreatography did not show clear stenosis of the main pancreatic duct at the pancreatic tail (B, pancreatography). Cell-block specimens obtained were firstly evaluated by using hematoxylin and eosin staining (C, ├Ś10; D, ├Ś40). Histocytological diagnosis was shown to be adenocarcinoma (Class V), and distal pancreatectomy was performed. For the histological findings of resected specimens, the atypical ductal epithelium was continuously observed from the main pancreatic duct (E, ├Ś20; arrowhead) to branch pancreatic duct, and invasive components derived from this atypical epithelium were not found. Thus, this patient was diagnosed with pancreatic carcinoma in situ (high-grade pancreatic intraepithelial neoplasm). 
Fig.┬Ā5.An 82-year-old patient (patient no. 17 in Table 2) with recurrent acute pancreatitis had a localized parenchymal atrophy of the pancreatic body (A, contrast-enhanced computed tomography; arrowhead, localized atrophic parenchyma in the pancreatic body). Although magnetic resonance cholangiopancreatography (B) showed a stenosis of the main pancreatic duct (MPD) in the pancreatic body (arrowhead), endoscopic ultrasonography did not detect mass lesions in this MPD stenosis. Pancreatic duct lavage cytology combined with a cell-block method using a double lumen catheter was conducted due to the diagnosis of suspected pancreatic carcinoma in situ. For histocytology of the specimens obtained through pancreatic duct lavage cytology combined with a cell-block method, some clusters of atypical cells were observed by using hematoxylin and eosin staining (C, ├Ś20). MUC1 staining (D, ├Ś40) was focally positive, a Ki-67 labeling index (E, ├Ś40) was approximately 20% and p53 staining was negative, indicating adenocarcinoma (Class IV). She underwent distal pancreatectomy, and histology (hematoxylin and eosin staining) of resected specimens showed an atypical epithelium mainly located in the MPD and branch ducts (F, ├Ś4; G, ├Ś20; black arrowhead: MPD, yellow arrowhead: branch duct), resulting in the final diagnosis of pancreatic carcinoma in situ (high-grade pancreatic intraepithelial neoplasm). 
Table┬Ā1.Baseline characteristics of 41 patients included in this study Values are presented as median (interquartile range) or number (%). MPD, main pancreatic duct; Ph, pancreatic head; Pb, pancreatic body; Pt, pancreatic tail; PLC-CB, pancreatic duct lavage cytology combined with a cell block method; EUS-FNA, endoscopic ultrasound-guided fine needle aspiration; PDAC, pancreatic ductal adenocarcinomas; PCIS, pancreatic carcinoma in situ; PanIN, pancreatic intraepithelial neoplasia; TNM, tu┬Łmor-node-metastasis; UICC, Union for International Cancer Control. Table┬Ā2.Demographic, procedural, and histocytological data for the respective 41 patients who underwent pancreatic duct lavage cytology combined with the cell-block method MPD, main pancreatic duct; EUS-FNA, endoscopic ultrasound-guided fine needle aspiration; PLC-CB, pancreatic duct lavage cytology combined with a cell-block method; PDAC, pancreatic ductal adenocarcinoma; UICC, Union for International Cancer Control; PEP, post-endoscopic retrograde cholangiopancreatography pancreatitis; NSAIDs, non-steroidal anti-inflammatory drugs; F, female; M, male; EUS, endoscopic ultrasonography; CT, computed tomography; MRI, magnetic resonance imaging; US, ultrasonography; Ph, pancreatic head; Pt, pancreatic tail; Pb, pancreatic body; N/A, not available; PCIS, pancreatic carcinoma in situ; PanIN, pancreatic intraepithelial neoplasia; MFP, mass forming pancreatitis; AIP, autoimmune pancreatitis. a)Indeternimate final diagnosis due to lack of surveillance periods (<12 months) after undergoing PLC-CB. Table┬Ā3.Diagnostic ability of PLCŌĆÉCB to detect malignancy Table┬Ā4.Possible risk factors for developing PEP PEP, post-endoscopic retrograde cholangiopancreatography pancreatitis; BMI, body mass index; NSAIDs, non-steroidal anti-inflammatory drugs, EST, endoscopic sphincterotomy; EPST, endoscopic pancreatic sphincterotomy; IDUS, intraductal ultrasonography; F, female; M, male; TGRY, total gastrectomy with Roux-en-Y reconstruction; DGBI, distal gastrectomy with Billroth I reconstruction. REFERENCES1. ASGE Standards of Practice Committee, Eloubeidi MA, Decker GA, et al. The role of endoscopy in the evaluation and management of patients with solid pancreatic neoplasia. Gastrointest Endosc 2016;83:17ŌĆō28.
2. Renelus BD, Jamorabo DS, Boston I, et al. Endoscopic ultrasound-guided fine needle biopsy needles provide higher diagnostic yield compared to endoscopic ultrasound-guided fine needle aspiration needles when sampling solid pancreatic lesions: a meta-analysis. Clin Endosc 2021;54:261ŌĆō268.
3. Chung MJ, Park SW, Kim SH, et al. Clinical and technical guideline for endoscopic ultrasound (EUS)-guided tissue acquisition of pancreatic solid tumor: Korean Society of Gastrointestinal Endoscopy (KSGE). Clin Endosc 2021;54:161ŌĆō181.
4. Nakaizumi A, Tatsuta M, Uehara H, et al. Usefulness of simple endoscopic aspiration cytology of pancreatic juice for diagnosis of early pancreatic neoplasm: a prospective study. Dig Dis Sci 1997;42:1796ŌĆō1803.
5. Koshita S, Noda Y, Ito K, et al. Pancreatic juice cytology with immunohistochemistry to detect malignancy and histologic subtypes in patients with branch duct type intraductal papillary mucinous neoplasms of the pancreas. Gastrointest Endosc 2017;85:1036ŌĆō1046.
6. Noda Y, Fujita N, Kobayashi G, et al. Prospective randomized controlled study comparing cell block method and conventional smear method for pancreatic juice cytology. Dig Endosc 2012;24:168ŌĆō174.
7. Koshita S, Noda Y, Kanno Y, et al. Value of repeated cytology for intraductal papillary mucinous neoplasms of the pancreas with high risk potential of malignancy: is it a promising method for monitoring a malignant transformation? Pancreatology 2020;20:1164ŌĆō1174.
8. Sai JK, Nobukawa B, Matsumura Y, et al. Pancreatic duct lavage cytology with the cell block method for discriminating benign and malignant branch-duct type intraductal papillary mucinous neoplasms. Gastrointest Endosc 2013;77:726ŌĆō735.
9. Kanno Y, Koshita S, Ogawa T, et al. Predictive value of localized stenosis of the main pancreatic duct for early detection of pancreatic cancer. Clin Endosc 2019;52:588ŌĆō597.
10. Noda Y, Fujita N, Kobayashi G, et al. Diagnostic efficacy of the cell block method in comparison with smear cytology of tissue samples obtained by endoscopic ultrasound-guided fine-needle aspiration. J Gastroenterol 2010;45:868ŌĆō875.
11. Guidelines of the Papanicolaou Society of Cytopathology for fine-needle aspiration procedure and reporting. Diagn Cytopathol 1997;17:239ŌĆō247.
12. Cotton PB, Lehman G, Vennes J, et al. Endoscopic sphincterotomy complications and their management: an attempt at consensus. Gastrointest Endosc 1991;37:383ŌĆō393.
13. Iiboshi T, Hanada K, Fukuda T, et al. Value of cytodiagnosis using endoscopic nasopancreatic drainage for early diagnosis of pancreatic cancer: establishing a new method for the early detection of pancreatic carcinoma in situ. Pancreas 2012;41:523ŌĆō529.
14. Satoh T, Kikuyama M, Kawaguchi S, et al. Acute pancreatitis-onset carcinoma in situ of the pancreas with focal fat replacement diagnosed using serial pancreatic-juice aspiration cytologic examination (SPACE). Clin J Gastroenterol 2017;10:541ŌĆō545.
15. Peters ML, Eckel A, Mueller PP, et al. Progression to pancreatic ductal adenocarcinoma from pancreatic intraepithelial neoplasia: results of a simulation model. Pancreatology 2018;18:928ŌĆō934.
16. Mikata R, Ishihara T, Tada M, et al. Clinical usefulness of repeated pancreatic juice cytology via endoscopic naso-pancreatic drainage tube in patients with pancreatic cancer. J Gastroenterol 2013;48:866ŌĆō873.
17. Mouri T, Sasaki T, Serikawa M, et al. A comparison of 4-Fr with 5-Fr endoscopic nasopancreatic drainage catheters: a randomized, controlled trial. J Gastroenterol Hepatol 2016;31:1783ŌĆō1789.
|
|
|||||||||||||||||||||||||||||||||||||||||||
