AbstractBackground/AimsHepaticojejunostomy anastomotic stricture (HJAS) is a feared adverse event associated with hepatopancreatobiliary surgery. Although balloon dilation for benign HJAS during endoscopic retrograde cholangiopancreatography with balloon-assisted enteroscopy has been reported to be useful, the treatment strategy remains controversial. Therefore, we evaluated the outcomes and risk factors of recurrent stenosis after balloon dilation alone for benign HJAS.
MethodsWe retrospectively analyzed consecutive patients who underwent balloon-assisted enteroscopyŌĆōendoscopic retrograde cholangiopancreatography for benign HJAS at our institution between July 2014 and December 2020.
ResultsForty-six patients were included, 16 of whom had recurrent HJAS after balloon dilation. The patency rates at 1 and 2 years after balloon dilation were 76.8% and 64.2%, respectively. Presence of a residual balloon notch during balloon dilation was an independent predictor of recurrence (hazard ratio, 2.80; 95% confidence interval, 1.01ŌĆō7.78; p=0.048), whereas HJAS within postoperative 1 year tended to be associated with recurrence (hazard ratio, 2.43; 95% confidence interval, 0.85ŌĆō6.89; p=0.096). The patency rates in patients without a residual balloon notch were 82.1% and 73.1% after 1 and 2 years, respectively.
INTRODUCTIONHepaticojejunostomy is performed in surgeries involving extrahepatic bile duct resection, most commonly for pancreatic cancer and cholangiocarcinoma. Benign hepaticojejunostomy anastomotic stricture (HJAS) occurs in 2.6% to 12.5% of cases.1-3 The incidence of benign HJAS may increase as the postoperative period increases.2 Benign HJAS is expected to increase as improvements in surgical outcomes and perioperative chemotherapy improve postoperative survival.4-9
HJAS can lead to repeated cholangitis, obstructive jaundice, intrahepatic bile duct stones, and liver abscesses, all of which require prompt treatment. Because achieving hepaticojejunostomy anastomosis using conventional endoscopes is difficult, treatment options are traditionally limited to percutaneous transhepatic biliary drainage (PTBD) or surgical reintervention. However, balloon-assisted enteroscopy (BAE) has facilitated endoscopic retrograde cholangiopancreatography (ERCP) for HJAS and achieved favorable results.10-15 BAE-ERCP is now recognized as a useful alternative to PTBD for benign HJAS due to its good outcomes and low invasiveness.
Treatment for benign HJAS includes balloon dilation, temporary biliary stent deployment, radial incision and cutting, and biodegradable stents.16-21 Balloon dilation is favored because of its simplicity. However, studies on treatment outcomes and risk factors for recurrent stenosis are scarce.16,17 Therefore, we conducted a retrospective study to evaluate the treatment outcomes and risk factors for recurrent stenosis after balloon dilation for benign HJAS using BAE-ERCP.
METHODSPatientsWe retrospectively analyzed consecutive patients who underwent BAE-ERCP for benign HJAS at Cancer Institute Hospital of Japanese Foundation for Cancer Research between July 2014 and December 2020. We defined HJAS as a hepaticojejunostomy anastomosis requiring balloon dilation that was associated with cholangitis, jaundice, intrahepatic stones, or elevated liver enzymes. We defined benign HJAS as HJAS without irregular mucosa or rough stenosis on endoscopy, irregular lesions in the bile duct on cholangiography, or findings suggestive of malignancy on computed tomography. Oral anticoagulants were either suspended or replaced with intravenous heparin prior to all procedures. Patients who had previously undergone BAE-ERCP for HJAS and those who required biliary stent deployment in addition to balloon dilation were excluded.
ProcedureBAE-ERCP was performed using single-balloon enteroscopy under moderate sedation with midazolam and analgesia with pethidine. Two types of single-balloon enteroscopy were used during the study period (SIF-Q260, working length: 2,000 mm, channel diameter: 2.8 mm, and SIF-H290S, working length: 1,520 mm, channel diameter: 3.2 mm; Olympus Medical Systems). All procedures were performed by experts or trainees under their guidance.
After reaching the hepaticojejunostomy anastomotic site, biliary cannulation and cholangiography were performed using a cannulation catheter (MTW Endoskopie Manufaktur, Wesel, Germany) and a 0.025-inch guidewire (Visiglide 2; Olympus Medical Systems). After inserting the guidewire into the bile duct, a catheter was inserted, and cholangiography was performed to measure the diameter of the bile duct above the HJAS. If the HJAS was too narrow for the catheter to pass through, a dilation balloon catheter (CRE PRO or Hurricane RX; Boston Scientific Corp.; REN, KANEKA; MEDIX Corp.) was inserted over the guidewire. If a dilation balloon catheter also failed to pass through the HJAS, a stent retriever (7-Fr screw-type device, Soehendra Stent Retriever; Cook Medical Inc., Bloomington, IN, USA) was used to pre-dilate the HJAS over the guidewire before inserting the balloon catheter.22 The balloon dilator with a balloon diameter of 4 to10 mm (depending on the diameter of the bile duct) was inflated at the HJAS for 30 to 120 seconds (with pressure of up to 810.6 kPa). Balloon dilation was generally conducted only once, although it was repeated up to four times if dilation was deemed insufficient. A residual balloon notch was considered positive if a visible notch remained in the balloon dilator with maximal pressure applied on fluoroscopy immediately before deflation in all dilation attempts. Fluoroscopic images of the absent and present residual balloon notches are presented in Figure 1, respectively.
Any intrahepatic stones were removed using a retrieval balloon catheter and/or basket catheter. An endoscopic nasobiliary drainage tube was placed at the discretion of the physician, generally in patients with concomitant cholangitis or residual intrahepatic stones.
Follow-upPatients were followed-up with laboratory studies every 1 to 3 months after discharge. BAE-ERCP was repeated in patients with findings suggestive of recurrent stenosis, such as jaundice, fever, elevated liver enzymes, and/or a dilated biliary tree on imaging. Balloon dilation with or without stent deployment was performed to treat recurrent stenosis. Follow-up was conducted until November 30, 2021.
EvaluationThis study evaluated technical and clinical success, HJAS patency after balloon dilation, risk factors for recurrent stenosis, and adverse events (AEs). Technical success was defined as endoscopically visible improvement of benign HJAS after balloon dilation. Clinical success was defined as an improvement in clinical symptoms and/or laboratory data within 14 days after the procedure. The final preoperative computed tomography scan was used to determine the preoperative bile duct diameter. We considered whether the surgery involved vascular resection and whether bile leakage occurred postoperatively. The length of the stenosis was measured from the intestinal wall to the lower end of the dilated bile duct using fluoroscopy. The presence or absence of a scar-like appearance at the anastomosis site on endoscopy was evaluated.23 Patency time was defined as the time from the first treatment to the second balloon dilation or to the last follow-up date. According to the presence or absence of recurrent HJAS, the patients were divided into recurrent HJAS group and no recurrence group. AEs were defined as any postoperative event based on a lexicon for endoscopic AEs.24
Statistical analysisContinuous variables are presented as medians (ranges) and compared using the MannŌĆōWhitney U test. Categorical variables are described as absolute numbers (proportions) and were analyzed using the chi-squared or Fisher exact test. A p-value <0.05 was considered statistically significant. Patency time was calculated using the KaplanŌĆōMeier method and compared using the log-rank test. Nine variables were assessed using univariate analysis to identify factors predicting recurrent stenosis as follows: sex, history of hepatectomy, history of preoperative biliary drainage, HJAS occurring within 1 year postoperatively, presence of jaundice, use of a stent retriever, length of stenosis exceeding 3 mm, residual balloon notch, and intrahepatic stone removal. Factors with p-values <0.15 in the univariate analysis were used in the multivariate logistic regression analysis, and hazard ratios (HRs) with a 95% confidence interval (CI) were calculated. All statistical analyses were performed using EZR ver. 1.54 (Saitama Medical Center, Jichi Medical University).25
Ethical statementsThis study was approved by the ethics committee of Cancer Institute Hospital of Japanese Foundation for Cancer Research (approval number: 2021-GA-1017). The requirement of informed consent for study participation was waived owing to the retrospective nature of the study. All the procedures were performed in accordance with the principles of the Declaration of Helsinki.
RESULTSPatient characteristicsFifty-four patients underwent balloon dilation during the first episode of benign HJAS using BAE-ERCP, of whom two patients were excluded because of ERCP failure. Endoscopic ultrasound (EUS)-guided hepaticogastrostomy was performed in one case because the hepaticojejunostomy anastomosis could not be identified. PTBD was performed in another case because the guidewire could not pass through the heavily stenosed HJAS. The technical success rate was 96.3% (52 of 54 cases). Six cases were excluded because biliary stents were placed after balloon dilation (Fig. 2).
Ultimately, the study included 46 patients for whom technical success was achieved. The baseline and perioperative characteristics are summarized in Table 1. Clinical success was achieved in all the cases. Recurrent HJAS was observed in 16 (34.8%) patients. The median follow-up period was 25.5 months (interquartile range, 15.2ŌĆō41.1 months), and no significant difference in the follow-up time was observed between the two groups (26.9 months in the recurrent HJAS group vs. 24.0 months in the no recurrence group, p=0.76). Resected lesions were malignant in 75.0% of the recurrent HJAS group vs. 86.7% of the no recurrence group (p=0.42). Pancreaticoduodenectomy was the most common surgery, while hepatectomy was performed in 25.0% of the recurrent HJAS group vs. 10.0% of the no recurrence group (p=0.22). The most common suture method for hepaticojejunostomy anastomosis was a combination of continuous and discontinuous sutures; continuous sutures were used in only one case. No significant differences in preoperative bile duct diameter and preoperative biliary drainage were observed between the two groups. The median time from surgery to HJAS was shorter in the recurrent stenosis group than that in the no recurrence group (p=0.09).
Treatment outcomes
Table 2 summarizes the characteristics of BAE-ERCP in the patients with and without recurrent stenosis. The presence or absence of a scar-like appearance at the anastomotic site was not associated with recurrent stenosis (56.3% with scar-like appearance vs. 36.7% without scar-like appearance, p=0.23). No significant differences in endoscopes used or use of a stent retriever were observed between the two groups. The length of the anastomotic stricture tended to be longer in the recurrent HJAS group than in the no recurrence group (4.5 mm vs. 2.5 mm, respectively; p=0.07). The median balloon diameter was 8.0 mm. Residual balloon notch was observed significantly more often in the recurrent stenosis group than in the no recurrence group (56.3% vs. 23.3%, p=0.049). Intrahepatic stone removal after balloon dilation of HJAS was performed in 43.8% of the recurrent HJAS group and 60.0% of the no recurrence group (p=0.36).
Figure 3 presents cumulative HJAS patency after balloon dilation. Patency rates after 1 and 2 years were 76.8% and 64.2%, respectively.
Risk factors for recurrent stenosis
Table 3 presents the univariate and multivariate analyses of factors associated with recurrent stenosis after balloon dilation. Residual balloon notch was a significant predictor of recurrent HJAS (p=0.02), while HJAS within 1 year postoperatively (p=0.05) and anastomotic stricture length >3 mm (p=0.10) tended to predict recurrent HJAS in the univariate analysis. Meanwhile, the multivariate logistic regression analysis revealed that residual balloon notch was an independent factor for recurrent stenosis (HR, 2.80; 95% CI, 1.01ŌĆō7.78; p=0.048). HJAS within postoperative 1 year tended to be associated with recurrent stenosis (HR, 2.43; 95% CI, 0.85ŌĆō6.89; p=0.096). The length of the stenosis did not affect the restenosis rate. The patency time of patients with the disappearance of the balloon notch was significantly longer than that of patients with a residual balloon notch (p=0.02, Fig. 4). The patency rates of patients with and without a residual balloon notch were 66.7% and 82.1% after 1 year and 45.7% and 73.1% after 2 years, respectively.
Adverse eventsThree AEs (6.5%) were observed: two cases of mild cholangitis and one case of perforation. No AEs were associated with scope insertion.
In the perforation cases, HJAS occurred 2.7 months after pancreaticoduodenectomy, and balloon dilation was performed. An 8-mm balloon was selected because the bile duct diameter was 8.8 mm, although a residual balloon notch was observed upon inflation. Three days after the procedure, the patient developed fever that was not accompanied by abdominal pain or jaundice. The patientŌĆÖs C-reactive protein level was elevated, and computed tomography revealed a small amount of free air in the retroperitoneum. The patient was treated with antibiotics, and recovered with conservative treatment alone.
DISCUSSIONIn this study, we retrospectively reviewed postsurgical cases of benign HJAS that underwent balloon dilation. Patency rates after 1 and 2 years were 76.8% and 64.2%, respectively. Our results were consistent with those of a previous report on balloon dilation for benign HJAS, which achieved a clinical success rate of 100% and patency rates of 73% and 55% after 1 and 2 years, respectively.16 We also identified that residual balloon notch was a risk factor for recurrent HJAS and that HJAS within postoperative 1 year tended to predict recurrent HJAS.
To reduce the risk of recurrent HJAS, the effectiveness of balloon dilation combined with plastic stent or fully covered self-expanding metal stent (FC-SEMS) placement has been investigated.17-19 The 1-year patency rate after balloon dilation with plastic stent placement was 89.4% to 94.4%.17,19 Balloon dilation with FC-SEMS placement led to maintained patency in 85.0% of cases after 3 months and recurrent HJAS in 5.9% during a follow-up period of 11.9 months.18 Meanwhile, balloon dilation combined with plastic stent or FC-SEMS placement requires multiple BAE-ERCP procedures and additional admissions for stent removal, leading to higher medical costs.
As balloon dilation alone is sufficient for some cases of benign HJAS, identifying subgroups that benefit from plastic stent or FC-SEMS placement and those that only require balloon dilation is desirable. However, whether early HJAS formation (<1 year postoperatively to HJAS) is a risk factor for recurrent HJAS after balloon dilation alone remains unclear.16,17 In this study, we identified residual balloon notch on fluoroscopy as an independent predictor of recurrent HJAS. Balloon dilation alone may be sufficient if the notch disappears on fluoroscopy, with 1-year and 2-year patency rates of 82.1% and 73.1%, respectively. By contrast, the 1-year and 2-year patency rates decreased to 66.7% and 45.7%, respectively, if a residual balloon notch was present. Plastic stent or FC-SEMS placement may be indicated in the latter subgroup.
To date, no consensus on the treatment strategies for recurrent HJAS has been established. FC-SEMS for HJAS led to 100% stricture resolution in one study, although recurrent HJAS occurred in 25.0% (3/12) of cases.26 Another proposed alternative is radial incision and cutting; however, procedural difficulty and perforation risk present significant hurdles for the procedure.20 PTBD was a common choice for difficult cases in the past; however, it is associated with various AEs including sepsis, parietal pain, hemobilia, and hemorrhage, as well as restrictions in activities of daily living due to the indwelling drainage tube.27,28
Recently, the usefulness of EUS-guided biliary drainage (EUS-BD) for HJAS has been reported.29,30 Despite a high success rate, EUS-BD procedures have problems with a high rate of AEs.31 Many problems remain unresolved, including long-term outcomes, procedural standardization, and development of specialized devices. Furthermore, PTBD and EUS-BD can be difficult in cases without bile duct dilation.32-34 Presently, we consider BAE-ERCP to be the first choice of treatment for benign HJAS.
AEs have been reported to occur in 2.9% to 6.5% of cases after balloon dilation alone.16,17 AEs such as cholangitis, stent migration, bleeding, pancreatitis, and perforation are more frequent after balloon dilation combined with plastic stent or FC-SEMS placement, arising in 5% to 20.7% of cases.17-19,25 However, care is warranted even when performing balloon dilation alone, as AEs such as perforation may occur with overly aggressive balloon dilation.
This study had some limitations. First, this was a retrospective study conducted at a single institution with a limited sample size. Second, balloon diameter and dilation time were left to the discretion of each physician. Third, the median follow-up period was 25.5 months, precluding a thorough evaluation of the long-term results. The long-term results of HJAS treatment with BAE-ERCP require further investigation.
In conclusion, this retrospective study revealed that residual balloon notch is an independent risk factor for recurrent stenosis after balloon dilation for benign HJAS. Prospective studies are desirable to determine whether plastic stents or FC-SEMS should be placed in patients with residual balloon notches after balloon dilation.
NOTESAuthor Contributions
Conceptualization: TM, TS, NS; Data curation: TM, TO, TT, CM, YY, TF, AK, MM; Formal analysis: TM, TS; Investigation: TM, TO, TT, CM, YY, TF, AK, MM; Methodology: TM, TS; Project administration: MO, NS; Supervision: MO, NS; WritingŌĆōoriginal draft: TM; WritingŌĆōreview & editing: all authors.
Fig.┬Ā2.Patient flow chart. BAE, balloon-assisted enteroscopy; ERCP, endoscopic retrograde cholangiopancreatography; HJAS, hepaticojejunostomy anastomosis stricture; EUS-BD, endoscopic ultrasound-guided biliary drainage; PTBD, percutaneous transhepatic biliary drainage. 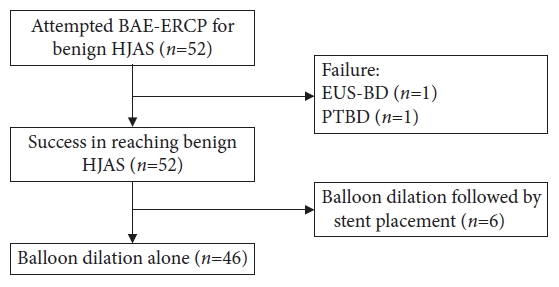
Fig.┬Ā3.Time to recurrent stenosis after first balloon dilation for HJAS. HJAS, hepaticojejunostomy anastomosis stricture; NA, not available; CI, confidence interval. 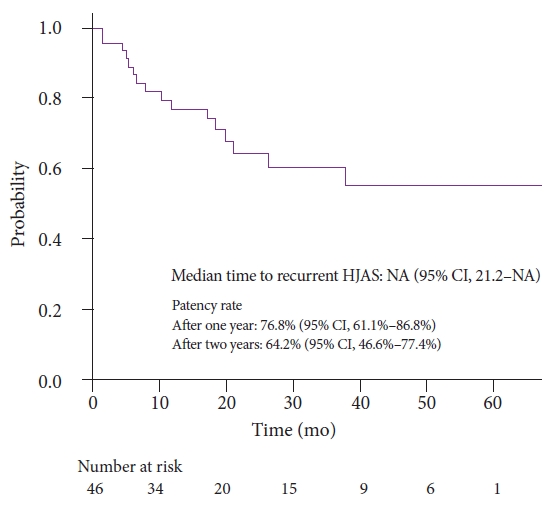
Fig.┬Ā4.Time to recurrent HJAS based on presence of residual balloon notch. HJAS, hepaticojejunostomy anastomosis stricture; NA, not available; CI, confidence interval. 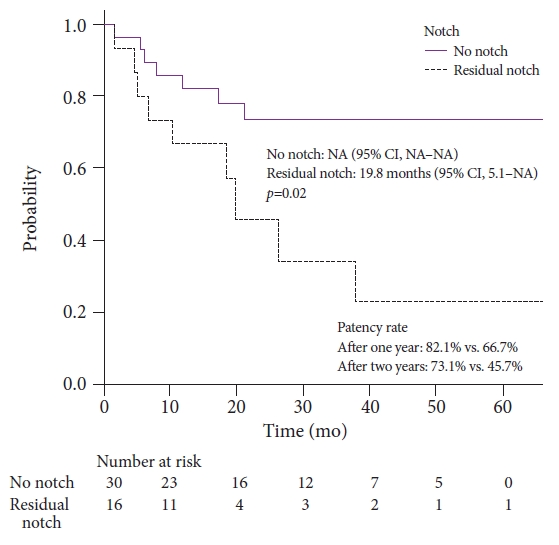
Table┬Ā1.Patient characteristics Table┬Ā2.Treatment-related characteristics Table┬Ā3.The univariate and multivariate analysis of risk factors for recurrent HJAS REFERENCES1. House MG, Cameron JL, Schulick RD, et al. Incidence and outcome of biliary strictures after pancreaticoduodenectomy. Ann Surg 2006;243:571ŌĆō578.
2. Reid-Lombardo KM, Ramos-De la Medina A, Thomsen K, et al. Long-term anastomotic complications after pancreaticoduodenectomy for benign diseases. J Gastrointest Surg 2007;11:1704ŌĆō1711.
3. Dimou FM, Adhikari D, Mehta HB, et al. Incidence of hepaticojejunostomy stricture after hepaticojejunostomy. Surgery 2016;160:691ŌĆō698.
4. Primrose JN, Fox RP, Palmer DH, et al. Capecitabine compared with observation in resected biliary tract cancer (BILCAP): a randomised, controlled, multicentre, phase 3 study. Lancet Oncol 2019;20:663ŌĆō673.
5. Uesaka K, Boku N, Fukutomi A, et al. Adjuvant chemotherapy of S-1 versus gemcitabine for resected pancreatic cancer: a phase 3, open-label, randomised, non-inferiority trial (JASPAC 01). Lancet 2016;388:248ŌĆō257.
6. Conroy T, Hammel P, Hebbar M, et al. FOLFIRINOX or gemcitabine as adjuvant therapy for pancreatic cancer. N Engl J Med 2018;379:2395ŌĆō2406.
7. Motoi F, Kosuge T, Ueno H, et al. Randomized phase II/III trial of neoadjuvant chemotherapy with gemcitabine and S-1 versus upfront surgery for resectable pancreatic cancer (Prep-02/JSAP05). Jpn J Clin Oncol 2019;49:190ŌĆō194.
8. Ikeda M, Nakachi K, Konishi M, et al. Adjuvant S-1 versus observation in curatively resected biliary tract cancer: a phase III trial (JCOG1202: ASCOT). Jpn J Clin Oncol 2022;4_suppl:382.
9. Inoue Y, Saiura A, Oba A, et al. Neoadjuvant gemcitabine and nab-paclitaxel for borderline resectable pancreatic cancers: intention-to-treat analysis compared with upfront surgery. J Hepatobiliary Pancreat Sci 2021;28:143ŌĆō155.
10. Tsutsumi K, Kato H, Yabe S, et al. A comparative evaluation of treatment methods for bile duct stones after hepaticojejunostomy between percutaneous transhepatic cholangioscopy and peroral, short double-balloon enteroscopy. Therap Adv Gastroenterol 2017;10:54ŌĆō67.
11. Shimatani M, Hatanaka H, Kogure H, et al. Diagnostic and therapeutic endoscopic retrograde cholangiography using a short-type double-balloon endoscope in patients with altered gastrointestinal anatomy: a multicenter prospective study in Japan. Am J Gastroenterol 2016;111:1750ŌĆō1758.
12. Tomoda T, Tsutsumi K, Kato H, et al. Outcomes of management for biliary stricture after living donor liver transplantation with hepaticojejunostomy using short-type double-balloon enteroscopy. Surg Endosc 2016;30:5338ŌĆō5344.
13. Itokawa F, Itoi T, Ishii K, et al. Single- and double-balloon enteroscopy-assisted endoscopic retrograde cholangiopancreatography in patients with Roux-en-Y plus hepaticojejunostomy anastomosis and Whipple resection. Dig Endosc 2014;26 Suppl 2:136ŌĆō143.
14. Shimatani M, Matsushita M, Takaoka M, et al. Effective ŌĆ£shortŌĆØ double-balloon enteroscope for diagnostic and therapeutic ERCP in patients with altered gastrointestinal anatomy: a large case series. Endoscopy 2009;41:849ŌĆō854.
15. Tsujino T, Yamada A, Isayama H, et al. Experiences of biliary interventions using short double-balloon enteroscopy in patients with Roux-en-Y anastomosis or hepaticojejunostomy. Dig Endosc 2010;22:211ŌĆō216.
16. Mizukawa S, Tsutsumi K, Kato H, et al. Endoscopic balloon dilatation for benign hepaticojejunostomy anastomotic stricture using short double-balloon enteroscopy in patients with a prior WhippleŌĆÖs procedure: a retrospective study. BMC Gastroenterol 2018;18:14.
17. Tomoda T, Kato H, Miyamoto K, et al. Comparison between endoscopic biliary stenting combined with balloon dilation and balloon dilation alone for the treatment of benign hepaticojejunostomy anastomotic stricture. J Gastrointest Surg 2020;24:1352ŌĆō1358.
18. Sato T, Kogure H, Nakai Y, et al. Endoscopic treatment of hepaticojejunostomy anastomotic strictures using fully-covered metal stents. Dig Endosc 2021;33:451ŌĆō457.
19. Tomoda T, Kato H, Ueki T, et al. Efficacy of double-balloon enteroscopy-assisted endoscopic balloon dilatation combined with stent deployment for hepaticojejunostomy anastomotic stricture. Dig Endosc 2022;34:604ŌĆō611.
20. Inoue T, Ibusuki M, Kitano R, et al. Balloon enteroscopy-assisted radial incision and cutting method for refractory hepaticojejunostomy anastomotic strictures. Endoscopy 2019;51:566ŌĆō570.
21. Lindstr├Čm O, Udd M, Rainio M, et al. Benign biliary strictures treated with biodegradable stents in patients with surgically altered anatomy using double balloon enteroscopy. Scand J Gastroenterol 2020;55:1225ŌĆō1233.
22. Tsutsumi K, Kato H, Sakakihara I, et al. Dilation of a severe bilioenteric or pancreatoenteric anastomotic stricture using a Soehendra Stent Retriever. World J Gastrointest Endosc 2013;5:412ŌĆō416.
23. Sato T, Kogure H, Nakai Y, et al. Double-balloon endoscopy-assisted treatment of hepaticojejunostomy anastomotic strictures and predictive factors for treatment success. Surg Endosc 2020;34:1612ŌĆō1620.
24. Cotton PB, Eisen GM, Aabakken L, et al. A lexicon for endoscopic adverse events: report of an ASGE workshop. Gastrointest Endosc 2010;71:446ŌĆō454.
25. Kanda Y. Investigation of the freely available easy-to-use software ŌĆśEZRŌĆÖ for medical statistics. Bone Marrow Transplant 2013;48:452ŌĆō458.
26. Sato T, Kogure H, Nakai Y, et al. A prospective study of fully covered metal stents for different types of refractory benign biliary strictures. Endoscopy 2020;52:368ŌĆō376.
27. Mukund A, Rajesh S, Agrawal N, et al. Percutaneous management of resistant biliary-enteric anastomotic strictures with the use of a combined cutting and conventional balloon cholangioplasty protocol: a single-center experience. J Vasc Interv Radiol 2015;26:560ŌĆō565.
28. Winick AB, Waybill PN, Venbrux AC. Complications of percutaneous transhepatic biliary interventions. Tech Vasc Interv Radiol 2001;4:200ŌĆō206.
29. Ogura T, Okuda A, Higuchi K. Endoscopic ultrasound-guided hepaticogastrostomy for hepaticojejunostomy stricture using a one-step stent deployment technique (with video). J Hepatobiliary Pancreat Sci 2021;28:e34ŌĆōe35.
30. Pawa R, Jiang D, Gilliam J, et al. EUS-guided hepaticogastrostomy for management of cholangitis, hepatolithiasis, and anastomotic stricture after Roux-en-Y hepaticojejunostomy. VideoGIE 2021;6:225ŌĆō227.
31. Katanuma A, Hayashi T, Kin T, et al. Interventional endoscopic ultrasonography in patients with surgically altered anatomy: techniques and literature review. Dig Endosc 2020;32:263ŌĆō274.
32. K├╝hn JP, Busemann A, Lerch MM, et al. Percutaneous biliary drainage in patients with nondilated intrahepatic bile ducts compared with patients with dilated intrahepatic bile ducts. AJR Am J Roentgenol 2010;195:851ŌĆō857.
|
|
|||||||||||||||||||||||||||||||||||||||
