INTRODUCTION
Gastroesophageal reflux disease (GERD) is a condition that develops when the reflux of stomach contents causes troublesome symptoms and/or complications.1 Worldwide, the prevalence of GERD is approximately 14% but varies according to region and country (4% in China compared with 22% in Turkey).2 In the United States, a 2015 population-based survey showed that 44% reported GERD symptoms in the past. One in three people reported GERD symptoms in the past week, and one-third of those who experienced GERD symptoms are actively on acid-suppressive therapy.3 GERD is associated with a high disease burden and poor quality of life.4
GERD is highly suspected in patients with typical symptoms, such as heartburn and regurgitation. Objectively, GERD diagnosis is established if an esophageal mucosal injury (erosive esophagitis, peptic stricture, or BarrettŌĆÖs esophagus [BE]) is present during upper endoscopy or the presence of abnormal esophageal acid exposure in patients with normal upper endoscopy. However, there is no gold standard for diagnosing GERD.
In clinical practice, GERD is diagnosed when there is a response (improvement in heartburn and/or regurgitation) to a trial of proton pump inhibitor (PPI) therapy, either in the form of empirical therapy or PPI testing. However, the PPI test is limited by its low specificity of only 45% compared with endoscopy and pH monitoring, as the reference studies show.5
New endoscopic techniques allow for better diagnosis and, thus, more individualized treatments. The latest endoscopic treatments for GERD provide alternatives to anti-reflux surgery and chronic medical treatment. This review summarizes the role of endoscopy in the diagnosis and treatment of GERD (Table 1).6
SEARCH STRATEGY
On May 1, 2023, a search of all published articles was performed using the search terms ŌĆ£gastroesophageal reflux diseaseŌĆØ or ŌĆ£GERDŌĆØ and ŌĆ£endoscopy.ŌĆØ The retrieved articles were manually reviewed for relevance, and their reference lists were examined for additional sources of information. Relevant articles were summarized and used for this review.
Endoscopy as a Diagnostic Tool for GERD
Although current guidelines allow GERD to be diagnosed by the presence of typical symptoms and response to empirical therapy, additional tests such as upper endoscopy and pH monitoring can aid in a confirmatory diagnosis.7 More than two-thirds of treatment-naïve patients with heartburn have normal endoscopic findings, which increase if they receive PPI therapy.8 Therefore, upper endoscopy has high specificity but low sensitivity in diagnosing GERD.9 Presently, several endoscopic techniques are available for the diagnosis of GERD. These techniques include white-light imaging, high-resolution and high-magnification endoscopy, chromoendoscopy, image-enhanced endoscopy (narrow-band imaging [NBI], I-SCAN, etc.), and confocal laser endomicroscopy.
Not all patients with typical symptoms undergo upper endoscopy to diagnose GERD. Generally, endoscopy is reserved for patients with symptoms such as dysphagia, odynophagia, weight loss, anorexia, gastrointestinal (GI) bleeding, and vomiting; in addition, for patients who meet the criteria for BE screening, with refractory (no response) or partial response to PPI or prior endoscopic or surgical anti-reflux intervention.10
Conventional white-light imaging
Conventional upper endoscopy utilizes white light to capture images of the upper GI tract and remains the most commonly used technique in routine endoscopy. White-light imaging helps visualize mucosal breaks, BE, esophageal ulceration, and/or peptic stricture. This simple technique also allows the stratification of erosive esophagitis (EE), using the Los Angeles (LA) classification, into four different grades with increasing severity (LA grades AŌĆōD).11 Otherwise, if no mucosal breaks are visible in patients reporting reflux symptoms, non-erosive reflux disease (NERD) diagnosis is suspected. However, in Japan, NERD is further graded as minimal changes (LA grade M), when identifying erythema without sharp demarcation and/or white turbidity in the very distal esophagus,, and LA grade N, which denotes normal mucosa.12
Additionally, white-light imaging can visualize salmon-colored tongues that may harbor metaplastic epithelium. BE is classified into short and long segments based on PragueŌĆÖs C & M criteria established in 2006.13 The Prague classification remains the standard evaluation of BarrettŌĆÖs mucosa. The lower measurement is bounded by the proximal cardiac notch, and the two upper measurements are marked by the most proximal extent of the circumferential segment (C) and the maximum extent of the longest tongue (M).
Recently, the Lyon consensus proposed a different approach to LA classification.6 The authors suggested that conclusive evidence for GERD only includes any of the following: LA grades C and D, long-segment BE, and esophageal peptic stricture. In contrast, those presenting with LA grades A and B are considered to have borderline or inconclusive evidence of GERD and should undergo further testing to confirm or refute the presence of GERD. However, the decision to consider LA grade B as inconclusive/borderline evidence of GERD remains controversial (Fig. 1, Table 2).6 A recent study comparing pH-impedance monitoring results between LA grades demonstrated that 100% of patients with LA grade B EE had objective GERD.14
High-resolution and high-magnification endoscopy
High-resolution and high-magnification endoscopy improve the ability to identify minimal mucosal changes and enlarge the image, respectively. Both high-resolution and high-magnification influence the quality of endoscopic images, consequently increasing the diagnostic yield for identifying lesions otherwise missed by conventional white-light endoscopy. In a retrospective study that included 500 upper endoscopies, the use of dual high-magnification, high-resolution endoscopy was associated with a higher likelihood of detecting a wide range of significant pathologies, including upper GI mucosal ulceration, stricture formation, biopsy-proven cancer, biopsy-proven BE, or Helicobacter pylori (+)-gastritis (odds ratio, 1.87; 95% confidence interval, 1.11ŌĆō3.12).15
Image-enhanced endoscopy
1) Dye-based chromoendoscopy
Dye-based chromoendoscopy, also known as chromoendoscopy, is an endoscopic technique that utilizes contrast agents (classified as vital and non-vital dyes) to stain tissues for better characterization of the esophageal mucosa. The vital dye is rapidly absorbed by normal squamous epithelial cells of the esophagus. Vital staining dyes commonly used in practice include LugolŌĆÖs solution, methylene blue, Congo red, and toluidine blue. In contrast, non-vital dyes are not absorbed by the epithelial cells but instead fill the mucosal pits and folds of the GI mucosa, highlighting any mucosal irregularities.16 Examples of non-vital dyes include indigo carmine and crystal violet. A meta-analysis of 14 studies demonstrated that dye-based chromoendoscopy improves the diagnostic yield of BE by approximately 35%.17
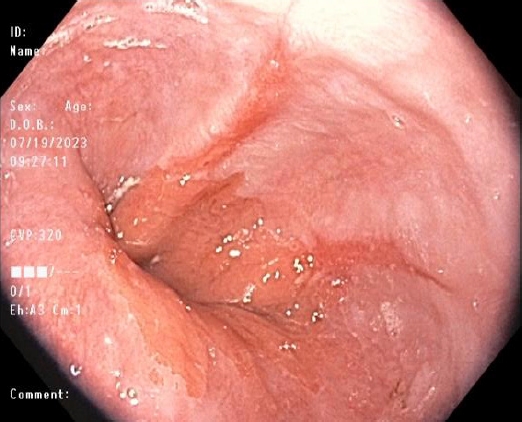
2) Narrow-band imaging
NBI is a widely available and easy-to-use narrow-spectrum endoscopy technique. This image enhancement technique uses only a narrowed spectral filter, mainly ŌĆ£blue light,ŌĆØ to better delineate the tissue based on its underlying histology.18 The depth of light penetration into tissues is proportional to the wavelength emitted by the light and thus allows the identification of minuscule epithelial changes suggesting GERD, such as villous mucosal surface, mucosal islands, microerosions, and increased vascularity in the distal esophagus (Fig. 2), which therefore, allow targeted biopsies to be done. Furthermore, NBI can be utilized to monitor GERD after PPI therapy owing to its ability to detect small inflammatory foci located in the esophagus and better delineate BarrettŌĆÖs mucosa.19 NBI with high magnification had high sensitivity but poor specificity for the diagnosis of high-grade dysplasia in BE.20 NBI-guided targeted biopsies have high diagnostic accuracy and could be a valid substitute for random biopsies, especially for diagnosing dysplasia or neoplasia in BE.21,22
3) I-SCAN
I-SCAN is a postprocessing digital filter-based contrast-enhanced technology that modifies the sharpness, hue, and contrast of images. This technique has three functional modes: surface, contrast, and tone enhancement. Surface enhancement enhances the structure of the mucosa, contrast enhancement adds blue color in relatively darker regions, and tone enhancement constructs a single new color image by modulating the individual red-green-blue components.23 The use of I-SCAN significantly improved the identification of minimal change esophagitis in patients with GERD.23,24 Compared with high-definition endoscopy, I-SCAN helped identify subtle abnormalities and changes in the mucosa of the distal esophagus and esophagogastric junction (EGJ).25
4) Flexible spectral imaging color enhancement
Flexible spectral imaging color enhancement (FICE) is a computed virtual chromoendoscopy technique that reconstructs endoscopy images and helps improve the visualization of mucosal structure and microcirculation by illumination of different wavelengths.26 FICE uses specific wavelengths from digitized data and images reconstructed using only a single selected wavelength. This technology allows the visualization of subtle changes in the epithelium of patients with NERD and is known to have higher sensitivity, negative predictive value, and accuracy (compared to white-light endoscopy) in the detection of ŌĆ£triangular lesions,ŌĆØ indicating minimal esophagitis. These ŌĆ£triangular lesionsŌĆØ are triangular indentations arising from the villiform columnar region of the Z line and extending into the squamous mucosa.
5) Blue laser imaging and linked color imaging
Blue laser imaging (BLI) and linked color imaging (LCI) are newer generations of image-enhanced endoscopy techniques developed in 2013 by Fujifilm. BLI utilizes two monochromatic lasers (410 and 450 nm) to improve the visualization of the vascular microarchitecture of the mucosal surface.27 This newer technology overcomes the limitation of a dark field view in previous narrowed-spectrum technologies. In contrast, LCI uses a specific color-enhancing technology that distinguishes color in red regions of mucosal blood vessels to improve the identification of red and discolored lesions based on differences in mucosal color.28 Compared with conventional white-light imaging and BLI, LCI improved visibility for detecting reflux esophagitis lesions with a better contrast image from the surrounding healthy esophageal mucosa.29
6) Autofluorescence endoscopy
Fluorophores are tissue molecules that emit fluorescent light with longer wavelengths when excited by light with shorter wavelengths. Fluorophores are found in endogenous tissue molecules of the GI tract, such as collagen, flavin, and nicotinamide adenine dinucleotide phosphate. Autofluorescence endoscopy is an Olympus technology based on the principle of fluorescence light detection. Changes in the mucosal thickness, mucosal blood flow, and endogenous tissue molecules influence the autofluorescence signals. Dysplastic and non-dysplastic BE have different autofluorescence characteristics based on the molecule content in the underlying tissue. The sensitivity of autofluorescence endoscopy for the detection of high-grade dysplasia and adenocarcinoma lesions was 42%, although it offers improved diagnostic detection compared to conventional endoscopy. Therefore, autofluorescence endoscopy can supplement the standard four-quadrant biopsy protocol in patients with BE.30
Confocal laser endomicroscopy
Confocal laser endomicroscopy provides high-magnification and high-resolution images of the mucosal layer of the GI tract. This modality works through the illumination of the tissue with a low-power laser, followed by the detection of the reflected fluorescence of light from the tissue captured through a pinhole.31 Fluorescent-aided endomicroscopy has been shown to improve the diagnostic accuracy of neoplastic changes in the EGJ (sensitivities of 90% and 93% and specificities of 94% and 98% for intestinal metaplasia and dysplasia, respectively).32
Esophageal biopsy and histology assessment
Upper endoscopy also allows esophageal biopsies to aid in making a more accurate diagnosis. Ideally, an esophageal biopsy should be performed before administering PPI or other acid-suppressive drugs. The Rome IV criteria for functional esophageal disorders recommend performing esophageal biopsies to rule out other esophagitis, such as eosinophilic or lymphocytic. Eosinophilic esophagitis may present solely with typical symptoms of GERD, especially in the younger population.33 In addition, biopsies play a role in differentiating patients with NERD from those with reflux hypersensitivity or functional heartburn using a structured histopathological scoring system.34,35 However, histopathological findings are often inconclusive of GERD due to overlaps between NERD, reflux hypersensitivity, and functional heartburn phenotypes.
Biopsies can also be used to identify BE and the presence and grade of dysplasia. The current recommendations include conventional white-light endoscopy and/or chromoendoscopy during BE surveillance. The recent American College of Gastroenterology screening guidelines for BE recommend that at least eight esophageal biopsies be performed to exclude the presence of intestinal metaplasia and the use of the Seattle protocol for segments longer than 4 cm.36 However, when eight biopsies are not obtainable in short segments of suspected BE, at least four biopsies are required per centimeter of circumferential BE and one biopsy per centimeter in the tongue of BE.
Newer endoscopic techniques allow for targeted biopsy sampling instead of random biopsies. However, more randomized studies are needed to determine whether these targeted biopsies can replace standard random biopsy techniques.
ENDOSCOPY AS A THERAPEUTIC TOOL FOR GERD
The endoscopic techniques used to treat GERD generally augment the anti-reflux barrier of the EGJ. These minimally invasive techniques serve as alternative therapeutic approaches for GERD patients not interested in surgical intervention or long-term medical treatment. The endoscopic techniques mentioned in this review include the radiofrequency/Stretta procedure (Restech Reflux Solutions), transoral incisionless fundoplication (TIF; EndoGastric Solutions Inc.), endoscopic full-thickness plication/OverStitch (Apollo Endosurgery Inc.), and other novel endoscopic anti-reflux techniques.
Patients with GERD who are candidates for endoscopic therapy include those with typical GERD symptoms (heartburn and regurgitation), mild/moderate EE (LA grade A/B) or NERD, hiatal hernia <3 cm, and complete or partial response to PPI treatment. In general, these procedures are offered to patients who are not candidates for or are unwilling to undergo anti-reflux surgery or long-term medical treatment.37
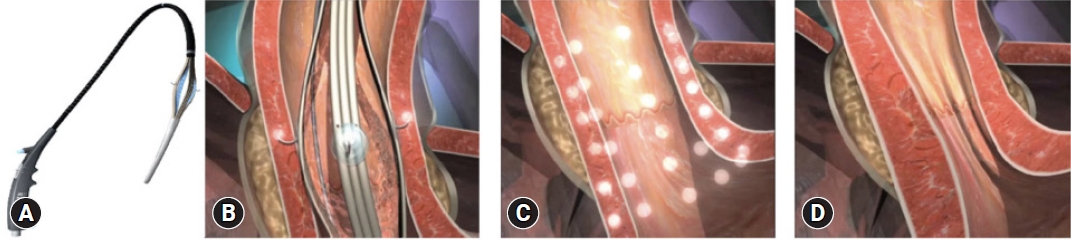
Endoscopic radiofrequency/Stretta procedure
The Stretta procedure was initially approved by the United States Food and Drug Administration in 2000 but has undergone several reiterations since then (Fig. 3A). The Society of American Gastrointestinal and Endoscopic Surgeons currently recommends the Stretta procedure for the treatment of GERD.38 Stretta works by delivering low-power, temperature-controlled radiofrequency energy to the EGJ region and cardia (Fig. 3BŌĆōD).39,40 Consequently, there is an increase in EGJ thickness due to the remodulation of the local musculature. The procedure reduces the transient lower esophageal sphincter relaxation rate, bolsters the EGJ, and prevents proximal migration of gastroesophageal reflux in the esophagus.41 Stretta is safe and effective for the treatment of GERD, with a significant improvement in GERD health-related quality of life (GERD-HRQL), heartburn symptoms, healing of EE, and reduction of esophageal acid exposure.42
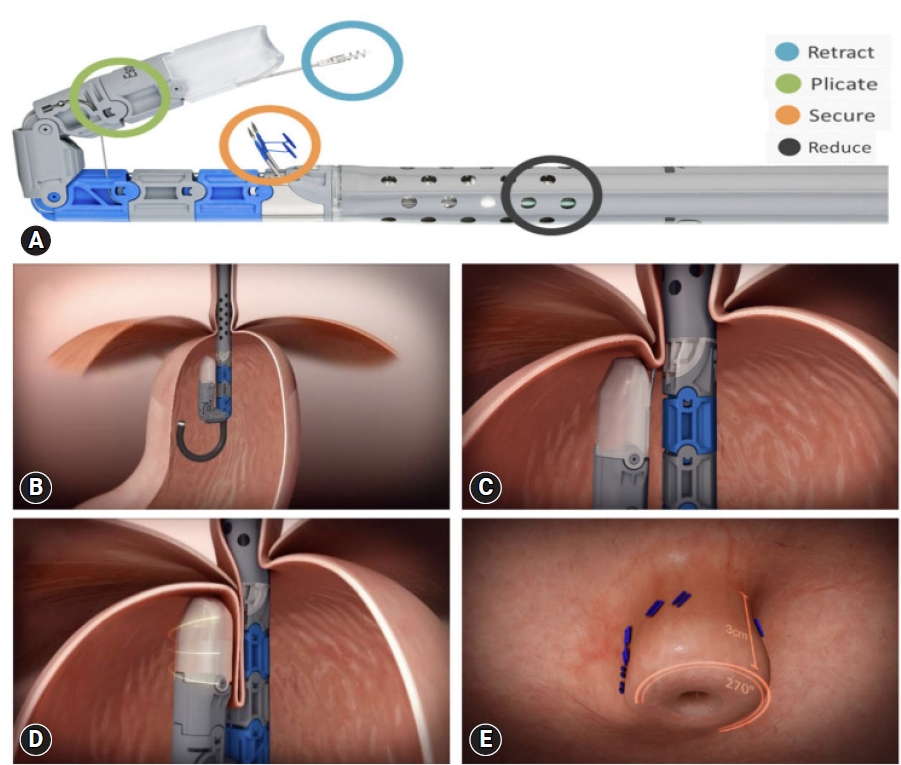
Transoral incisionless fundoplication
TIF (EsophyX2 device) is a technique that reconstructs the lower esophageal sphincter to restore the angle of His and thus augments the gastroesophageal flap valve (Fig. 4A).43 This technique is referred to as TIF 2.0, an improved version of the original Food and Drug Administration-approved TIF 1.0. The main difference is the increase in wrap ability in TIF 2.0, which reaches 270┬░ to 320┬░ compared with the range in TIF 1.0, which provided 250┬░ to 300┬░ (Fig. 4BŌĆōE).40,44 TIF has similar indications as the Stretta procedure, including patients with refractory GERD demonstrating partial response to PPI treatment.45 However, the procedure cannot be performed in patients with post-surgical sleeve procedure.46 Patients with large hiatal hernia (>3 cm) can undergo laparoscopic hiatal hernia repair concomitantly with TIF (c-TIF). The RESPECT47 and TEMPO48 trials demonstrated a higher proportion of patients with GERD who underwent the TIF procedure reported complete elimination of troublesome regurgitation than those who underwent PPI therapy. The TIF effect was maintained even after 5 years of follow-up.49 An ongoing trial (NCT04457193) is aimed to elucidate the efficacy of TIF in the prevention of relapse of intestinal metaplasia and dysplasia in patients with endoscopically ablated BE.50
GERD-X
GERD-X (G-SURG GmbH) allows full-thickness plication, which is known as the endoscopic full-thickness fundoplication technique (Fig. 5).40 In a randomized, sham-controlled trial comprising 70 patients, this technique significantly increased GERD-HRQL at 3 months, with 62.8% of patients being off PPI at 12 months compared with 11.4% in the sham group.51 These clinical improvements were demonstrated with no major procedure-related adverse events.
Medigus ultrasonic surgical endostapler
The Medigus ultrasonic surgical endostapler (MUSE) (Medigus) is a transoral device that utilizes ultrasound guidance to staple the gastric fundus to create an anterior fundoplication (Fig. 6).40,52 In two prospective single-arm studies, this technique demonstrated therapeutic efficacy (improved GERD-HRQL and discontinuation of daily PPI) with a relatively safe profile at 6 months.52,53 In another study comprising 34 patients with GERD, of the 20 subjects who underwent the MUSE procedure and completed follow-up, 70% experienced significant improvement (50% reduction in GERD-related symptom scores) in GERD-HRQL, 65% in heartburn symptoms, 75% in regurgitation symptoms, and 65% stopped PPI therapy at 12 months post-procedure.54
Endoscopic full-thickness suturing
Endoscopic full-thickness suturing using an OverStitch device applied full-thickness sutures to the distal esophagus to strengthen the valve mechanism of the EGJ. In a preliminary study that included 10 patients with GERD symptoms, this novel endoscopic suturing technique demonstrated feasibility and safety, as well as improved short-term symptoms of GERD, as shown by a reduction in the median GERD-HRQL from 20 (range, 11ŌĆō45) pre-procedure to 6 (range, 3ŌĆō25) post-procedure (p=0.001).55 A recent modification of the technique introduced mucosal ablation with argon plasma coagulation before suture placement to improve the durability of the sutures. This technique was developed because, in the first version of the technique, sutures would easily cut through the mucosa, loosening the plication. A study using a modified technique demonstrated that 59% and 14% of patients (n=29) could discontinue PPI use and reduce their daily dosage post-intervention, respectively.45
Other endoscopic anti-reflux therapies
1) Anti-reflux mucosectomy
Anti-reflux mucosectomy (ARMS) was initially introduced by Inoue et al.56 in patients with short-segment BE with high-grade dysplasia. Although originally performed using endoscopic submucosal dissection, the two commonly used techniques include endoscopic mucosal resection with cap (ARMS-C) or band-technique (ARMS-B).57 ARMS helps create mucosal defects in approximately 2/3 to 4/5 of the circumference on the lesser curvature of the cardiac mucosa, leading to scarring and, consequently, narrowing of the EGJ opening. ARMS-C was shown to be effective and safe for GERD treatment at 6 months; 63% of the patients discontinued PPI, while 30% of the patients reduced their PPI intake. Overall, there was a significant improvement in the GERD symptom questionnaire and DeMeester score.58 Additionally, a retrospective analysis of 19 patients from a prospectively collected database demonstrated that 68% had improved symptoms even when discontinuing PPI.59 Furthermore, for refractory GERD, a meta-analysis that included 10 studies demonstrated the efficacy of ARMS by improving GERD-HRQL and an acceptable safety profile, regardless of the technique used.60
2) Peroral endoscopic cardial constriction
Peroral endoscopic cardial constriction (PECC) is a novel endoscopic technique that aims to narrow the cardia and increase lower esophageal sphincter pressure. These anatomical changes bolster the barrier between the esophagus and stomach.61 Briefly, this technique works by ligating the mucosa and part of the muscularis mucosa, which induces necrosis and scarring of the lower esophageal sphincter. A preliminary study of 13 patients showed a significant improvement in the GERD-HRQL and DeMeester scores, with no serious adverse events. Additionally, these findings were further replicated in a larger sample of 68 patients who demonstrated a significant reduction in symptom scores at 12 months compared with baseline, with 78% of the patients achieving complete independence from anti-reflux medications.62
3) Endoscopic submucosal dissection
This new endoscopic technique was developed using endoscopic submucosal dissection (ESD-G). This technique narrows the hiatal opening to reduce gastroesophageal reflux by creating a scar at the EGJ using ESD.63 Of the 13 patients with refractory GERD who underwent ESD-G, 12 had a significant improvement in GERD symptoms, five had an improvement in EE healing, and three patients each were able to stop or reduce the PPI dose. In a follow-up analysis of 35 patients by the same research group, ESD-G was shown to significantly improve GERD symptoms and EE healing, although the number of reflux episodes did not decrease.64 However, this technique was ineffective in patients with a history of distal gastrectomy.
4) Endoscopic band ligation
Endoscopic band ligation (EBL) is commonly used to treat esophageal varices in patients with cirrhosis. This technique induces scarring of the mucosa, subsequently reducing varice size. Based on this effect, EBL was used to treat patients hoping to narrow and strengthen the EGJ. In one randomized controlled trial of 150 refractory GERD patients, subjects were randomized to either the EBL (banding at four quadrants of the EGJ) or the control group (PPI only).65 Patients in the EBL group demonstrated improved GERD-HRQL score (14.7┬▒3.9 vs. 33.0┬▒5.4) and a significant reduction in reflux episodes (14.1┬▒2.3 vs. 213.3┬▒47.3) and symptom index (0.3┬▒0.2 vs. 0.7┬▒0.1) compared with the control group. Adverse events were comparable between the two groups. This novel technique has shown promising results in patients with refractory GERD.
CONCLUSIONS
Endoscopy is important in the diagnosis and therapeutic management of GERD. Although the diagnosis of GERD is commonly established based on typical symptoms or response to PPI treatment at the clinical level, those who present with alarm symptoms, have chronic GERD at risk of BE, and have an inade┬Łquate symptomatic response to PPI, will require an upper GI endoscopy for further management. White-light imaging is the standard endoscopic technique used for diagnosing GERD. However, newer endoscopic techniques such as high-resolution and high-magnification endoscopy, chromoendoscopy, image-enhanced endoscopy (NBI, I-SCAN, FICE, BLI, and LCI), and confocal laser endomicroscopy have demonstrated promising improvements in GERD diagnosis, especially in patients with NERD. Several therapeutic endoscopic techniques for GERD are currently available, including Stretta, TIF, GERD-X, MUSE, and other novel techniques (ARMS, PECC, ESD-G, and EBL). These minimally invasive endoscopic techniques provide an important alternative to chronic PPI treatment and surgical intervention for GERD.