AbstractBackground/AimsEndoscopic ultrasound (EUS)-guided hepaticogastrostomy (EUS-HGS) performed at the intrahepatic bile duct segment 3 (B3) is widely used for biliary drainage. Although performing post-puncture procedures is easier in the intrahepatic bile duct segment 2 (B2) when using a conventional oblique-viewing (OV) EUS scope, this method may cause transesophageal puncture and severe adverse events. We evaluated the safety and efficacy of B2 puncture using a novel OV-EUS scope.
MethodsIn this single-center retrospective study, we prospectively enrolled and collected data from 45 patients who consecutively underwent EUS-HGS procedures with a novel OV-EUS scope between September 2021 and December 2022 at our cancer center.
INTRODUCTIONRecently, endoscopic ultrasound (EUS)-guided hepaticogastrostomy (EUS-HGS) has been widely performed alongside conventional percutaneous and transpapillary procedures to establish biliary drainage for malignant biliary strictures and gastrointestinal obstruction and following intestinal surgery.1 The advantages of EUS-HGS include a high success rate without the risk of pancreatitis. Hence, it is widely used as an alternative to transpapillary biliary drainage in complex cases and as a first-choice drainage method.2,3
EUS-HGS is also performed for benign biliary strictures.4 Previous studies have reported that the occurrence of adverse events in EUS-guided biliary drainage (EUS-BD) depends on the expertise and experience of the endoscopist.5,6 Therefore, only endoscopists skilled in both endoscopic retrograde cholangiopancreatography (ERCP) and EUS-fine needle aspiration (FNA)-related procedures should perform EUS-BD.1,7,8
In EUS-HGS, anastomosis of the intrahepatic bile duct of segment 2 (B2) is technically more manageable than that of intrahepatic bile duct of segment 3 (B3) during guidewire manipulation, route dilation, and stent placement9,10 because the scope in B3 EUS-HGS must anatomically traverse the portal vein radicle and bent portion, making the procedure challenging.11 In contrast to the B3 route, the B2 route does not have an acutely bent portion; therefore, a B2 puncture is recommended for EUS-guided intrahepatic biliary access.12 We have previously reported the usefulness of B2-EUS-HGS using a forward-viewing (FV) EUS scope. However, FV-EUS despite enabling safe B2 puncture at a high rate has drawbacks such as a narrow 90┬░ scanning range.11 Further, B2 puncture using an oblique-viewing (OV) EUS scope may cause complications due to puncture from a transesophageal route, resulting in serious adverse effects such as mediastinitis13; therefore, B3 puncture is generally preferred and is considered the first choice.14 However, a study has recently reported that B2 puncture using an OV-EUS scope was as useful and safe as B3 puncture for EUS-HGS.10
The EG-740UT (Fujifilm) is a novel OV-EUS scope with features that appear to be favorable for EUS-HGS through the B2 route. Therefore, we aimed to evaluate the safety and efficacy of B2 puncture EUS-HGS using the novel OV-EUS scope.
METHODSWe included 391 patients who underwent EUS-HGS at Aichi Cancer Center Hospital between April 2009 and December 2022. Following its introduction in September 2021, B2-EUS-HGS using the novel OV-EUS scope (EG-740UT) has been performed on 54 consecutive patients. Of these, nine patients were excluded in accordance with a previous report because the diameter of the targeted bile duct was <2.0 mm15; the reaming 45 patients were included in this analysis (Fig. 1). The patients were prospectively enrolled, and their clinical data were retrospectively collected.
New OV-EUS scope
Figures 2 and 3 show a comparison of the EG-740UT and GF-UCT260 (Olympus) scopes and the 22-G FNA needle (EZ shot 3Plus; Olympus). The EG-740UT has a greater up-angle of 150┬░, whereas that of GF-UCT260 is 130┬░. As shown in the EUS images, the EG-740UT scope can puncture at a nearly vertical angle compared with that of the GF-UCT260 scope, which is an advantage in B2 puncture procedure. Another advantage of the EG-740UT scope over GF-UCT260 is its strong guidewire-locking mechanism, which allows for stable device exchange during the procedure.
Details of B2-EUS-HGSNine endoscopists at our facility performed the EUS-HGS procedure; of these, five were beginners who has performed <10 procedures. All patients were sedated with intravenous medication, and prophylactic intravenous antibiotics were administered during the procedure. Ascites drainage was performed before the procedure among the patients who underwent EUS-HGS because patients with severe ascites are at risk for peritonitis.16 Then, an FV endoscope was used to place a landmark clip at the esophagogastric junction for easy identification to prevent esophageal puncture.2
B2-EUS-HGS using the EG-740UT was performed as follows: B2 was visualized using the EG-740UT scope and an ultrasound device (SU-1; Fujifilm). A Y-connector (0.096 Rotating Hemostatic Valve; Abbott) that was pre-inserted with a 0.018-inch guidewire (Fielder 18; Olympus) was filled with the contrast medium and connected to a 22-G needle (Expect Slimline; Boston Scientific, USA). Doppler imaging was used to guide the needle to puncture the B2 segment and avoid any vessels. A connector was used to insert the guidewire to stabilize the needle and prevent air from entering the bile duct. A contrast medium was injected to confirm that the guidewire was located in the bile duct. The puncture route was dilated using a 0.018-inch guidewire-compatible dilator catheter (Tornus ES, Asahi Intec or ES dilator, ZEON Medical). The dilator catheter was then replaced with a biliary catheter (uneven-double lumen catheter, Piolax Medical Devices, Kanagawa, Japan or Tandem XL; Boston Scientific, Japan) to perform bile aspiration and confirm the bile duct branches. Finally, a fully covered self-expandable metal stent (FCSEMS) (6 mm├Ś10 or 12 cm HANARO Benefit; M.I. Tech. or 8 mm├Ś10 or 12 cm Covered Bile Rush Advance, Piolax Medical Devices) was inserted through the stomach wall. Stents longer than 10 cm were used to prevent internal stent migration.17 However, B3 puncture was performed when performing a B2 puncture was difficult. All patients who underwent EUS-HGS were examined using abdominal computed tomography (CT) the following day to detect possible complications such as stent deviation, perforation, intrahepatic hematoma, and bile leakage.
Result definitionsTechnical success was defined as the placement of a biliary stent at the intended location in the left intrahepatic bile duct through the stomach. In patients who achieved technical success, clinical success was defined as a decrease in the serum total bilirubin level to less than half the preprocedure level or an improvement in liver dysfunction or cholangitis within 2 weeks. Procedure time was defined as the time period starting from bile duct puncture to the completion of stent placement. Biliary drainage was classified as primary and salvage drainage. Primary drainage was defined as the first attempt of biliary drainage. Salvage drainage was defined as the conversion of the biliary drainage method to additional drainage approaches such as the percutaneous or transpapillary approach. The severity of ascites was classified into three categories based on the CT images: mildŌĆöconfined to the pouch of Douglas or MorisonŌĆÖs pouch; severeŌĆöcovering the abdominal organs; and moderateŌĆöintermediate between mild and severe. Prior evaluation of the bile duct puncture route using EUS was not performed in all cases. Adverse events associated with the EUS-HGS procedure were classified according to a lexicon for endoscopic adverse events.18 Early adverse events were defined as those that occurred within 2 weeks of the procedure.
RESULTS
Table 1 shows the patient characteristics. The median patient age was 67 years (range, 24ŌĆō83). Malignant diseases accounted for 91.1% (41/45) of the primary diseases with pancreatic cancer having the highest incidence (n=19, 42.2%). The major indications for EUS-HGS were primary biliary drainage in 31 patients (68.9%) and salvage drainage in 14 patients (31.1%). Salvage drainage included changes from other biliary drainage methods such as ERCP for duodenal stenosis and percutaneous biliary drainage. Ascites was observed in 33.3% (15/45) of patients, of whom seven had mild ascites and eight had moderate ascites. One patient (2.2%) underwent ascitic drainage prior to EUS-HGS. The remaining seven patients with moderate ascites did not have sufficient ascites to permit drainage, and ascitic fluid drainage was not performed.
Table 2 summarizes details of the EUS-HGS procedure. The median procedure duration was 13 minutes (range, 5ŌĆō30). An initial B2 puncture was performed in all cases; a subsequent B3 puncture was performed in three cases when B2-EUS-HGS was unsuccessful. EG-740UT was used in all cases and the scope was not changed at any instance.
Table 3 presents the clinical outcomes of the EUS-HGS procedures performed in this study. The technical success rate of B2-EUS-HGS was 93.3% (42/45), and the overall success rate of EUS-HGS was 97.8% (44/45). Moreover, B2-EUS-HGS was successfully performed using a 19-G needle in all 10 patients. In two cases, B3-EUS-HGS was successfully performed after changing from B2 to B3 puncture; the change was made in one case because of difficultly in guidewire manipulation and in the other because cholangiography following B2 puncture showed presence of contrast media in the portal vein instead of in the bile duct. In one case, EUS-HGS was converted to ERCP because of difficulty in inserting a guidewire into either the B2 or B3 duct after a puncture. The overall clinical success rate was 88.9% (40/45 patients). All five cases of clinical failure were due to the progression of malignant disease.
Early adverse events occurred in 8.9% of the patients (4/45) including one case each of mild fever, biloma, stent deviation, and moderate-severity focal cholangitis. One patient developed fever; hence, antibiotics were administered, and the patient was treated conservatively. Biloma was diagnosed using CT and treated with EUS-guided abscess drainage using an FCSEMS (6 mm├Ś12 cm HANARO Benefit, M.I. Tech.) Subsequently, the patientŌĆÖs symptoms improved. In a case where scope interference occurred immediately after the procedure, causing the stent to deviate into the stomach, ERCP was performed immediately, and the patient was discharged without further complications. The patient with focal cholangitis developed fever 12 days after EUS-HGS was performed. Therefore, a stent exchange was performed, and the patientŌĆÖs condition improved. No transesophageal punctures were observed.
DISCUSSIONWe aimed to evaluate the technical success and clinical outcomes of B2-EUS-HGS using the new OV-EUS scope EG-740UT. The new scope has a shorter bending radius and wider puncture width than does the conventional OV-EUS scope and allows for more vertical punctures, which are advantageous for B2-EUS-HGS. We demonstrated that B2-EUS-HGS using EG-740UT could be safely performed with a high success rate. To the best of our knowledge, this is the first study to validate the utility of EG-740UT for B2-EUS-HGS. The technical success rate of B2-EUS-HGS in this study was 93.3% and that of EUS-HGS was 97.8%. A 22-G needle and 0.018 guidewire combination was the primary technique used in this study. The 22-G needle being less stiff than the 19-G needle allowed the scope to bend more strongly and the puncture needle to be inserted at a nearly vertical angle. Therefore, the combination of 22-G needle and 0.018 guidewire is more suitable for B2 puncture. However, as shown in Figures 2 and 3, the novel OV-EUS had a stronger scope flexion than that of the previous OV-EUS, and the puncture needle could be placed at a near-vertical angle. Additionally, B2-EUS-HGS using a 19-G needle was successfully performed in all 10 patients. Therefore, the novel OV-EUS has a better possibility of successful B2-EUS-HGS, even with a 19-G needle. A previous report on B2-EUS-HGS using an OV scope reported a 100% technical success rate; however, that report only accounted for cases where B2 puncture was possible, and the success rate of all EUS-HGS procedures in which B2 puncture was attempted is unknown.10 In our previous study, B2-EUS-HGS using an FV-EUS scope was possible in 88.5% of cases.11 The FV scope has a wide 180┬░ up-angle range and a short rigid portion, making B2-EUS-HGS possible without a transesophageal puncture. However, the FV scope has a narrow 90┬░ observation range and no elevator; therefore, handling it may be difficult for beginners.19 In contrast, the OV-EUS scope does not have this limitation. In fact, the EG-740UT has a solid guidewire-locking mechanism, possibly making the B2 puncture safer and easier to perform than with earlier models.
Our study has some inherent limitations being a retrospective, single-arm, and a single center study. Therefore, a comparative multicenter study is required to validate the findings of this study.
In conclusion, B2-EUS-HGS was performed safely using the new EUS scope, EG-740UT, with a high success rate and uncomplicated by transesophageal puncture. This novel scope has the advantages of a wide 150┬║ scope bending range, vertical puncture, and a solid guidewire-locking mechanism. A B2 puncture using this new scope has the potential to become the standard method for EUS-HGS.
NOTESConflicts of Interest
Nobumasa Mizuno has received grants or contracts for their institution from Novartis, MSD, Incyte, Ono Pharmaceutical, Seagen, and Dainippon Sumitomo Pharma; has received payments or honoraria for lectures, presentations, speakersŌĆÖ bureaus, manuscript writing, or educational events from Yakult Honsha, AstraZeneca, Novartis, Fujifilm Toyama Chemical, MSD, and Taiho Pharmaceutical; and has participated in a Data Safety Monitoring Board and Advisory Board for AstraZeneca. The other authors have no potential conflicts of interest.
Fig.┬Ā1.Flowchart of patient selection. B2, bile duct segment 2; EUS-HGS, endoscopic ultrasound-guided hepaticogastrostomy; OV, oblique-viewing. 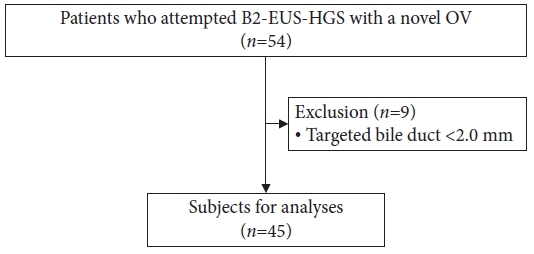
Fig.┬Ā2.Comparison of EG-740UT (Fujifilm) and GF-UCT260 (Olympus) scopes. (A) Full-up angulations of EG-740UT (front) and GF-UCT260 (back). (B) Full-up angulation without the use of the EG-740UT and GF-UCT260 elevators with a 22-G fine needle aspiration (FNA) needle. (C) Full-up angulation and maximum elevation of the EG-740UT and GF-UCT260 elevators using a 22-G FNA needle. 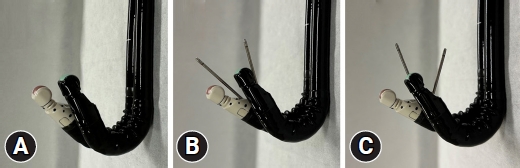
Fig.┬Ā3.Comparison of EG-740UT (Fujifilm) and GF-UCT260 (Olympus) scopes using EUS images. (A) Without using the EG-740UT elevator. (B) With maximum elevation of the EG-740UT elevator. (C) Without using the GF-UCT260 elevator. (D) With maximum elevation of the GF-UCT260 elevator. EUS, endoscopic ultrasound. 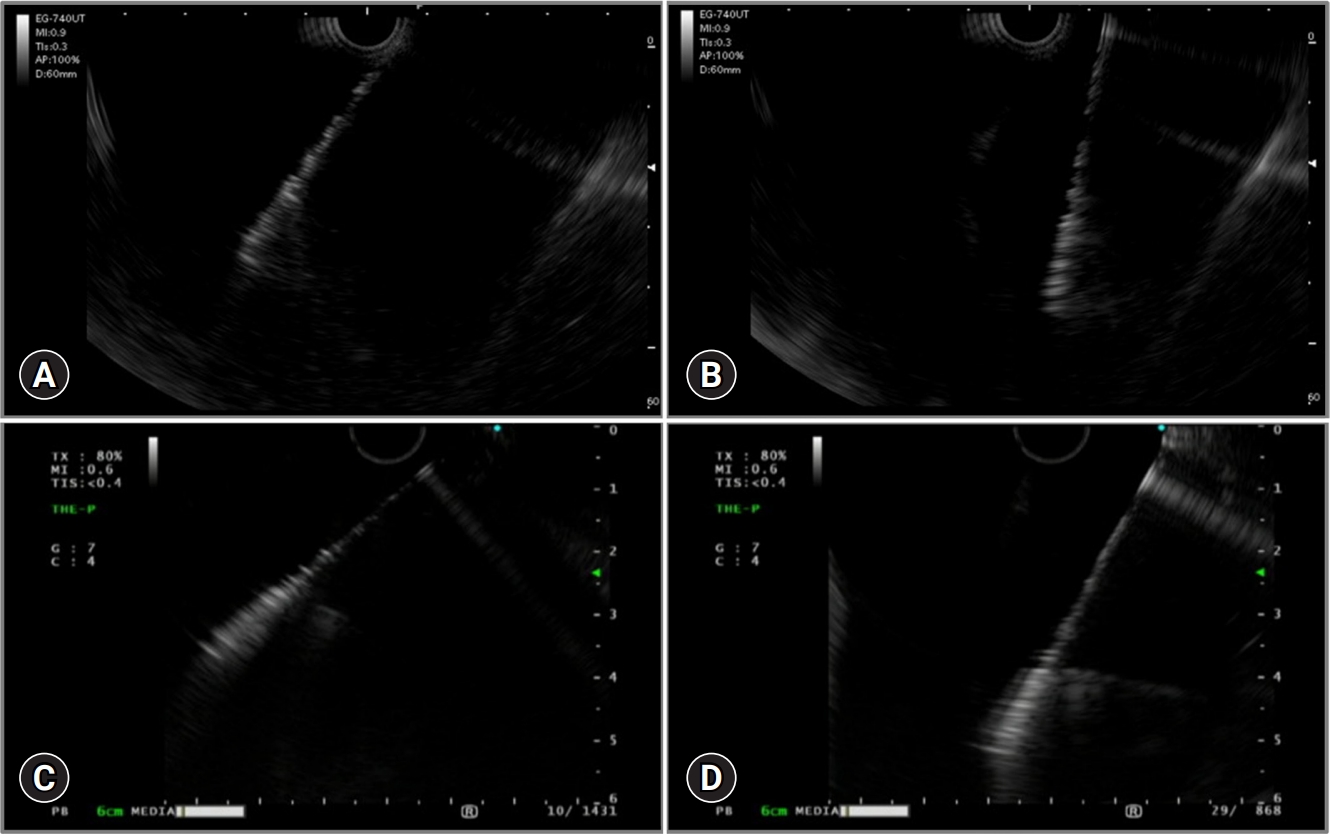
Table┬Ā1.Patient characteristics Table┬Ā2.Details of EUS-HGS procedure Table┬Ā3.Clinical outcomes of EUS-HGS REFERENCES1. Hara K, Yamao K, Mizuno N, et al. Endoscopic ultrasonography-guided biliary drainage: who, when, which, and how? World J Gastroenterol 2016;22:1297ŌĆō1303.
2. Okuno N, Hara K, Mizuno N, et al. Efficacy of the 6-mm fully covered self-expandable metal stent during endoscopic ultrasound-guided hepaticogastrostomy as a primary biliary drainage for the cases estimated difficult endoscopic retrograde cholangiopancreatography: a prospective clinical study. J Gastroenterol Hepatol 2018;33:1413ŌĆō1421.
3. Matsubara S, Nakagawa K, Suda K, et al. Practical tips for safe and successful endoscopic ultrasound-guided hepaticogastrostomy: a state-of-the-art technical review. J Clin Med 2022;11:1591.
4. Pawa R, Pleasant T, Tom C, et al. Endoscopic ultrasound-guided biliary drainage: are we there yet? World J Gastrointest Endosc 2021;13:302ŌĆō318.
5. Vila JJ, P├®rez-Miranda M, Vazquez-Sequeiros E, et al. Initial experience with EUS-guided cholangiopancreatography for biliary and pancreatic duct drainage: a Spanish national survey. Gastrointest Endosc 2012;76:1133ŌĆō1141.
6. Oh D, Park DH, Song TJ, et al. Optimal biliary access point and learning curve for endoscopic ultrasound-guided hepaticogastrostomy with transmural stenting. Therap Adv Gastroenterol 2017;10:42ŌĆō53.
7. Isayama H, Nakai Y, Itoi T, et al. Clinical practice guidelines for safe performance of endoscopic ultrasound/ultrasonography-guided biliary drainage: 2018. J Hepatobiliary Pancreat Sci 2019;26:249ŌĆō269.
8. Matsumoto S, Hara K, Mizuno N, et al. Risk factor analysis for adverse events and stent dysfunction of endoscopic ultrasound-guided choledochoduodenostomy. Dig Endosc 2020;32:957ŌĆō966.
9. Hara K, Okuno N, Haba S, et al. How to perform EUS-guided hepaticogastrostomy easier and safer. J Hepatobiliary Pancreat Sci 2020;27:563ŌĆō564.
10. Sekine M, Hashimoto Y, Shibuki T, et al. A retrospective multicenter study comparing the punctures to B2 and B3 in endoscopic ultrasound-guided hepaticogastrostomy. DEN Open 2023;3:e201.
11. Okuno N, Hara K, Mizuno N, et al. B2 puncture with forward-viewing EUS simplifies EUS-guided hepaticogastrostomy (with video). Endosc Ultrasound 2022;11:319ŌĆō324.
12. Tsuchiya T, Itoi T, Sofuni A, et al. Endoscopic ultrasonography-guided rendezvous technique. Dig Endosc 2016;28 Suppl 1:96ŌĆō101.
13. Okuno N, Hara K, Mizuno N, et al. Risks of transesophageal endoscopic ultrasonography-guided biliary drainage. Gastrointest Interv 2017;6:82ŌĆō84.
14. Ogura T, Itoi T. Technical tips and recent development of endoscopic ultrasound-guided choledochoduodenostomy. DEN Open 2021;1:e8.
15. Samanta J, Sundaram S, Dhar J, et al. EUS-guided biliary drainage in patients with moderate-severe cholangitis is safe and effective: a multi-center experience. Surg Endosc 2023;37:298ŌĆō308.
16. Okuno N, Hara K, Mizuno N, et al. Infectious peritonitis after endoscopic ultrasound-guided biliary drainage in a patient with ascites. Gastrointest Interv 2018;7:40ŌĆō43.
17. Okuno N, Hara K, Mizuno N, et al. Stent migration into the peritoneal cavity following endoscopic ultrasound-guided hepaticogastrostomy. Endoscopy 2015;47 Suppl 1 UCTN:E311.
|
|
