Search
- Page Path
- HOME > Search
Original Articles
- Novel upper gastrointestinal bleeding sensor capsule: a first human feasibility and safety trial
- Lukas Bajer, Marvin Ryou, Christopher C. Thompson, Pavel Drastich
- Clin Endosc 2024;57(2):203-208. Published online January 17, 2024
- DOI: https://doi.org/10.5946/ce.2023.111
-
 Graphical Abstract
Graphical Abstract
 Abstract
Abstract
 PDF
PDF PubReader
PubReader ePub
ePub 
- Background
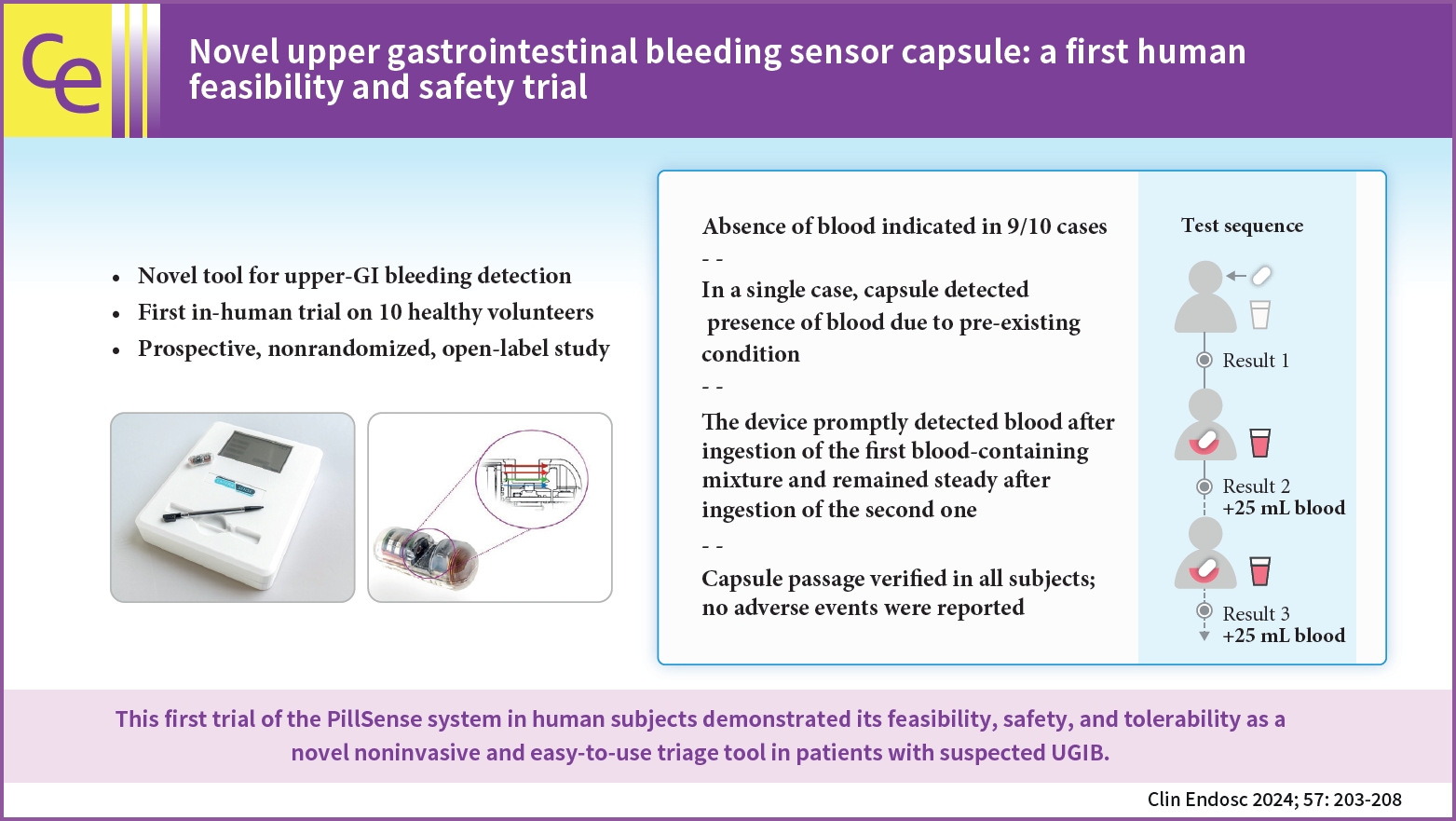
/Aims: Upper gastrointestinal bleeding (UGIB) is the most common GI condition requiring hospitalization, and can be diagnosed by direct visualization. The present study aimed to evaluate the safety and feasibility of using the PillSense system (EnteraSense Ltd.), a novel diagnostic tool designed for the rapid in vivo detection of UGIB, in human volunteers.
Methods
In the present study, 10 volunteers swallowed a PillSense capsule, followed by 2 servings of an autologous blood preparation. Participants were monitored for capsule passage, overall tolerability of the procedure, and adverse events.
Results
The procedure was completed per the protocol established in the present study in 9/10 cases. In 9 of the subjects, after capsule ingestion, the device indicated the absence of blood with sensor output values of 1. After the ingestion of the first blood mixture, the sensor outputs of all devices increased from 2.8 to 4, indicating that each camera detected blood. The sensor output remained within that range after the ingestion of the second mixture; however, in one case, the baseline capsule signal was positive, because of a preexisting condition. The passage of the capsule was verified in all patients, and no adverse events were reported.
Conclusions
The first trial of the PillSense system in human subjects demonstrated the feasibility, safety, and tolerability of utilizing this product as a novel, noninvasive, and easy-to-use triage tool for the diagnosis of patients suspected of having UGIB. -
Citations
Citations to this article as recorded by- Could a bleeding-sensor device be established as a new paradigm for detecting upper gastrointestinal bleeding before performing endoscopy?
Sun Gyo Lim
Clinical Endoscopy.2024; 57(2): 191. CrossRef
- Could a bleeding-sensor device be established as a new paradigm for detecting upper gastrointestinal bleeding before performing endoscopy?
- 2,195 View
- 155 Download
- 1 Crossref

- Preclinical study of a novel ingestible bleeding sensor for upper gastrointestinal bleeding
- Kimberly F. Schuster, Christopher C. Thompson, Marvin Ryou
- Clin Endosc 2024;57(1):73-81. Published online May 31, 2023
- DOI: https://doi.org/10.5946/ce.2022.293
-
 Graphical Abstract
Graphical Abstract
 Abstract
Abstract
 PDF
PDF PubReader
PubReader ePub
ePub 
- Background
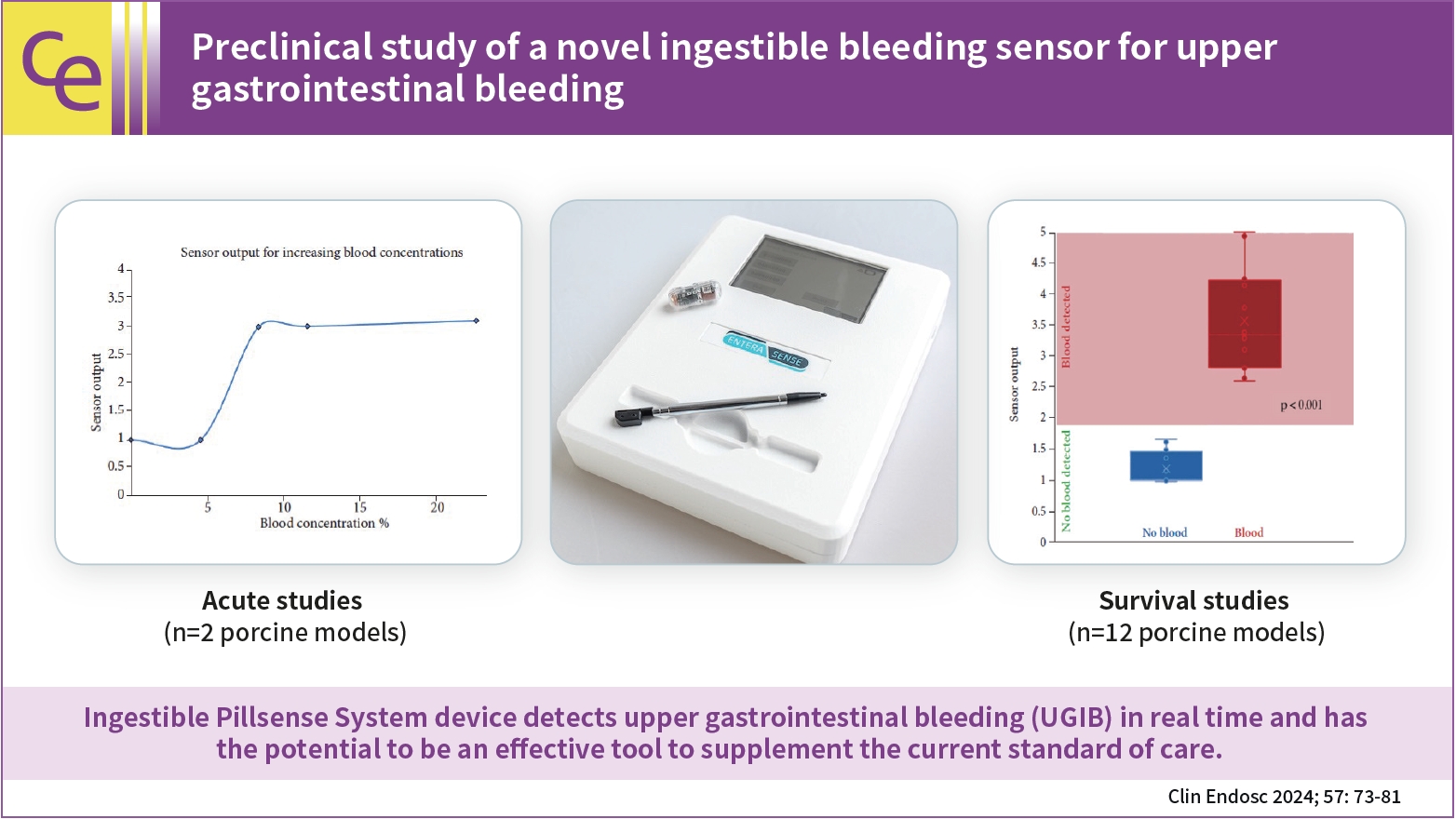
/Aims: Upper gastrointestinal bleeding (UGIB) is a life-threatening condition that necessitates early identification and intervention and is associated with substantial morbidity, mortality, and socioeconomic burden. However, several diagnostic challenges remain regarding risk stratification and the optimal timing of endoscopy. The PillSense System is a noninvasive device developed to detect blood in patients with UGIB in real time. This study aimed to assess the safety and performance characteristics of PillSense using a simulated bleeding model.
Methods
A preclinical study was performed using an in vivo porcine model (14 animals). Fourteen PillSense capsules were endoscopically placed in the stomach and blood was injected into the stomach to simulate bleeding. The safety and sensitivity of blood detection and pill excretion were also investigated.
Results
All the sensors successfully detected the presence or absence of blood. The minimum threshold was 9% blood concentration, with additional detection of increasing concentrations of up to 22.5% blood. All the sensors passed naturally through the gastrointestinal tract.
Conclusions
This study demonstrated the ability of the PillSense System sensor to detect UGIB across a wide range of blood concentrations. This ingestible device detects UGIB in real time and has the potential to be an effective tool to supplement the current standard of care. These favorable results will be further investigated in future clinical studies. -
Citations
Citations to this article as recorded by- Miniaturized Capsule System Toward Real‐Time Electrochemical Detection of H2S in the Gastrointestinal Tract
Justin M. Stine, Katie L. Ruland, Luke A. Beardslee, Joshua A. Levy, Hossein Abianeh, Santiago Botasini, Pankaj J. Pasricha, Reza Ghodssi
Advanced Healthcare Materials.2024;[Epub] CrossRef
- Miniaturized Capsule System Toward Real‐Time Electrochemical Detection of H2S in the Gastrointestinal Tract
- 2,187 View
- 141 Download
- 1 Web of Science
- 1 Crossref

- Defining the optimal technique for endoscopic ultrasound shear wave elastography: a combined benchtop and animal model study with comparison to transabdominal shear wave elastography
- Thomas J. Wang, Marvin Ryou
- Clin Endosc 2023;56(2):229-238. Published online February 28, 2023
- DOI: https://doi.org/10.5946/ce.2022.135
-
 Abstract
Abstract
 PDF
PDF Supplementary Material
Supplementary Material PubReader
PubReader ePub
ePub - Background
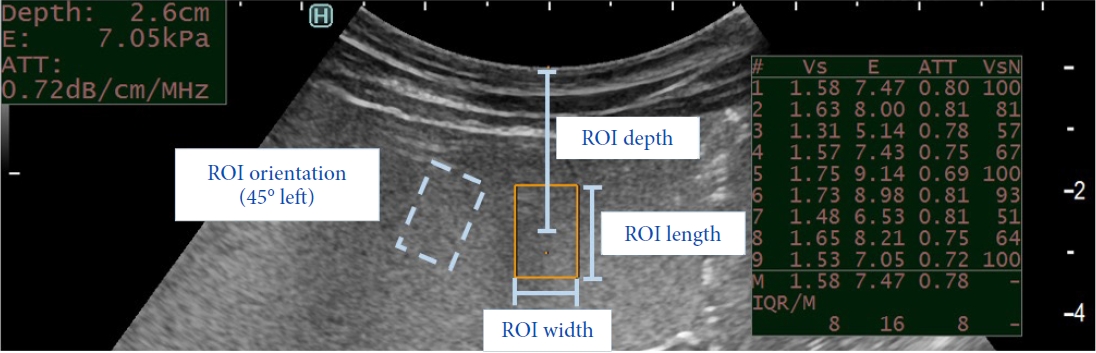
/Aims: Shear wave elastography (SWE) is used for liver fibrosis staging based on stiffness measurements. It can be performed using endoscopic ultrasound (EUS) or a transabdominal approach. Transabdominal accuracy can be limited in patients with obesity because of the thick abdomen. Theoretically, EUS-SWE overcomes this limitation by internally assessing the liver. We aimed to define the optimal technique for EUS-SWE for future research and clinical use and compare its accuracy with that of transabdominal SWE.
Methods
Benchtop study: A standardized phantom model was used. The compared variables included the region of interest (ROI) size, depth, and orientation and transducer pressure. Porcine study: Phantom models with varying stiffness values were surgically implanted between the hepatic lobes.
Results
For EUS-SWE, a larger ROI size of 1.5 cm and a smaller ROI depth of 1 cm demonstrated a significantly higher accuracy. For transabdominal SWE, the ROI size was nonadjustable, and the optimal ROI depth ranged from 2 to 4 cm. The transducer pressure and ROI orientation did not significantly affect the accuracy. There were no significant differences in the accuracy between transabdominal SWE and EUS-SWE in the animal model. The variability among the operators was more pronounced for the higher stiffness values. Small lesion measurements were accurate only when the ROI was entirely situated within the lesion.
Conclusions
We defined the optimal viewing windows for EUS-SWE and transabdominal SWE. The accuracy was comparable in the non-obese porcine model. EUS-SWE may have a higher utility for evaluating small lesions than transabdominal SWE. -
Citations
Citations to this article as recorded by- Endoscopic Ultrasound-based Shear Wave Elastography for Detection of Advanced Liver Disease
Jad AbiMansour, Jerry Yung-Lun Chin, Jyotroop Kaur, Eric J. Vargas, Barham K. Abu Dayyeh, Ryan Law, Vishal Garimella, Michael J. Levy, Andrew C. Storm, Ross Dierkhising, Alina Allen, Sudhakar Venkatesh, Vinay Chandrasekhara
Journal of Clinical Gastroenterology.2024;[Epub] CrossRef - Response
Divyanshoo R. Kohli, Mohammad Shadab Siddiqui
Gastrointestinal Endoscopy.2024; 100(1): 161. CrossRef - Standardization of endoscopic ultrasound shear wave elastography
Julio Iglesias-García, J. Enrique Domínguez-Muñoz
Clinical Endoscopy.2023; 56(2): 185. CrossRef
- Endoscopic Ultrasound-based Shear Wave Elastography for Detection of Advanced Liver Disease
- 2,253 View
- 161 Download
- 1 Web of Science
- 3 Crossref

- Endoscopic Ultrasound-Guided, Percutaneous, and Transjugular Liver Biopsy: A Comparative Systematic Review and Meta-Analysis
- Thomas R. McCarty, Ahmad Najdat Bazarbashi, Basile Njei, Marvin Ryou, Harry R. Aslanian, Thiruvengadam Muniraj
- Clin Endosc 2020;53(5):583-593. Published online September 29, 2020
- DOI: https://doi.org/10.5946/ce.2019.211

-
 Abstract
Abstract
 PDF
PDF Supplementary Material
Supplementary Material PubReader
PubReader ePub
ePub - Background
/Aims: Percutaneous liver biopsy (PCLB) or transjugular liver biopsy (TJLB) have traditionally been performed to obtain a sample of hepatic tissue; however, endoscopic ultrasound-guided liver biopsy (EUSLB) has become an attractive alternative. The aim of this study was to compare the efficacy and safety of EUSLB, PCLB, and TJLB.
Methods
Search strategies were developed in accordance with PRISMA and MOOSE guidelines. Major outcomes included the following: adequacy of biopsy specimens (i.e., complete portal triads [CPT], total specimen length [TSL] in mm, and length of longest piece [LLP]) in mm), and rate of adverse events. Only studies comparing all biopsy approaches (i.e., EUSLB, PCLB, and TJLB) were included.
Results
Five studies (EUSLB [n=301]; PCLB [n=176]; and TJLB [n=179]) were included. Biopsy cumulative adequacy rates for EUSLB, PCLB, and TJLB were 93.51%, 98.27%, and 97.61%, respectively. Based on the subgroup analysis limited to EUS biopsy needles in current clinical practice, there was no difference in biopsy adequacy or adverse events for EUSLB compared to PCLB and TJLB (all p>0.050). A comparison of EUSLB and PCLB revealed no difference between specimens regarding both CPT (p=0.079) and LLP (p=0.085); however, a longer TSL (p<0.001) was observed. Compared to TJLB, EUSLB showed no difference in LLP (p=0.351), fewer CPT (p=0.042), and longer TSL (p=0.005).
Conclusions
EUSLB appears to be a safe, minimally invasive procedure that is comparable to PCLB and TJLB regarding biopsy specimens obtained and rate of adverse events associated with each method. -
Citations
Citations to this article as recorded by- Endoscopic procedures in hepatology: Current trends and new developments
Wim Laleman, Emma Vanderschueren, Zain Seyad Mehdi, Reiner Wiest, Andres Cardenas, Jonel Trebicka
Journal of Hepatology.2024; 80(1): 124. CrossRef - Gut-liver axis: Pathophysiological concepts and medical perspective in chronic liver diseases
Susana G. Rodrigues, Schalk van der Merwe, Aleksander Krag, Reiner Wiest
Seminars in Immunology.2024; 71: 101859. CrossRef - Comparison of diagnostic outcomes, safety, and cost of Franseen-tip 19G versus 22G needles for endoscopic ultrasound-guided liver biopsies
Ankit Dalal, Nagesh Kamat, Gaurav Patil, Amol Vadgaonkar, Sanil Parekh, Sehajad Vora, Amit Maydeo
Endoscopy International Open.2024; 12(02): E291. CrossRef - Endoscopic ultrasound-guided liver biopsy in liver transplant recipients: A preliminary experience
Wei Rao, Yue-Ping Jiang, Jin-Zhen Cai, Man Xie
Hepatobiliary & Pancreatic Diseases International.2024;[Epub] CrossRef - Comparison of Diagnostic Accuracy and Diagnostic Adequacy Between Endoscopic Ultrasound-Guided and Percutaneous Liver Biopsies: A Meta-Analysis of Randomized Controlled Trials and Observational Studies
Mansoor Ahmad, Taslova Tahsin Abedin, Faria Khilji, Kinan Obeidat, Lam Vinh Sieu, Sandipkumar S Chaudhari, Divine Besong Arrey Agbor, Danish Allahwala
Cureus.2024;[Epub] CrossRef - Comparative accuracy of endosonographic shear wave elastography and transcutaneous liver stiffness measurement: a pilot study
Divyanshoo R. Kohli, Daniel Mettman, Nevene Andraws, Erin Haer, Jaime Porter, Ozlem Ulusurac, Steven Ullery, Madhav Desai, Mohammad S. Siddiqui, Prateek Sharma
Gastrointestinal Endoscopy.2023; 97(1): 35. CrossRef - EUS-guided versus percutaneous liver biopsy: A comprehensive review and meta-analysis of outcomes
Saurabh Chandan, Smit Deliwala, ShahabR Khan, BabuP Mohan, BanreetS Dhindsa, Jay Bapaye, Hemant Goyal, LenaL Kassab, Faisal Kamal, HarlanR Sayles, GursimranS Kochhar, DouglasG Adler
Endoscopic Ultrasound.2023; 12(2): 171. CrossRef - Advances in Endoscopic Ultrasound (EUS)-Guided Liver Biopsy
Daryl Ramai, Viraaj Pannu, Antonio Facciorusso, Banreet Dhindsa, Joseph Heaton, Andrew Ofosu, Saurabh Chandan, Marcello Maida, Barbara Lattanzi, Eduardo Rodriguez, Vicky H. Bhagat, Jayanta Samanta, Monique T. Barakat
Diagnostics.2023; 13(4): 784. CrossRef - Endoscopic Advances in Hepatology
Emma Vanderschueren, Jonel Trebicka, Wim Laleman
Seminars in Liver Disease.2023; 43(02): 176. CrossRef - Quality of Tissue Samples Obtained by Endoscopic Ultrasound-Guided Liver Biopsy: A Randomized, Controlled Clinical Trial
José Lariño-Noia, Javier Fernández-Castroagudín, Daniel de la Iglesia-García, Héctor Lázare, Laura Nieto, Sol Porto, Nicolau Vallejo-Senra, Esther Molina, Alba San Bruno, Xurxo Martínez-Seara, Julio Iglesias-García, Silvia García-Acuña, J. Enrique Domíngu
American Journal of Gastroenterology.2023; 118(10): 1821. CrossRef - Endo-hepatology: Updates for the clinical hepatologist
Frances Lee, Tarun Rustagi, R. Todd Frederick
Clinical Liver Disease.2023; 22(2): 42. CrossRef - Technical Success, Sample Adequacy, and Complications of Pediatric Transjugular Liver Biopsy: A Systematic Review and Meta-Analysis
Karen Smayra, Shahid Miangul, Nathanael Yap, Ao Shi, Fatma Abdulsalam, Maamoun Adra, Hayato Nakanishi, Jake Ball, Tara A. Betts, Christian A. Than, Aneeta Parthipun
Digestive Diseases and Sciences.2023; 68(10): 3846. CrossRef - Role of endoscopic ultrasound and endoscopic ultrasound-guided tissue acquisition in diagnosing hepatic focal lesions
Hussein Hassan Okasha, Hanane Delsa, Abdelmoneim Alsawaf, Ahmed Morad Hashim, Hani M Khattab, Dalia Abdelfatah, Abeer Abdellatef, Amr Albitar
World Journal of Methodology.2023; 13(4): 287. CrossRef - Chinese expert consensus on multidisciplinary diagnosis and treatment of pancreatic neuroendocrine liver metastases
Yihebali Chi, Liming Jiang, Susheng Shi, Shun He, Chunmei Bai, Dan Cao, Jianqiang Cai, Qichen Chen, Xiao Chen, Yiqiao Deng, Shunda Du, Zhen Huang, Li Huo, Yuan Ji, Jie Li, Wenhui Lou, Jie Luo, Xueying Shi, Lijie Song, Bei Sun, Huangying Tan, Feng Wang, Xu
Journal of Pancreatology.2023; 6(4): 139. CrossRef - Distinct ways to perform a liver biopsy: The core technique setups and updated understanding of these modalities
Chao Sun, Xingliang Zhao, Lei Shi, Xiaofei Fan, Xiaolong Qi
Endoscopic Ultrasound.2023; 12(6): 437. CrossRef - Endo-Hepatology: The Buzz Goes Much beyond Liver Biopsy—A Narrative Review
Rajesh Puri, Zubin Sharma, Swapnil Dhampalwar, Abhishek Kathuria, Bimal Sahu
Journal of Digestive Endoscopy.2023; 14(04): 227. CrossRef - Diagnostic yield of endoscopic ultrasound-guided liver biopsy in comparison to percutaneous liver biopsy: a systematic review and meta-analysis
Antonio Facciorusso, Stefano Francesco Crinò, Daryl Ramai, Carlo Fabbri, Benedetto Mangiavillano, Andrea Lisotti, Nicola Muscatiello, Christian Cotsoglou, Pietro Fusaroli
Expert Review of Gastroenterology & Hepatology.2022; 16(1): 51. CrossRef - Endoscopic Ultrasound-Guided Liver Biopsy Using Newer 19G FNB Needles Compared to Percutaneous and Transjugular Liver Biopsy: A Tertiary Center Experience
Harsh K. Patel, George Therapondos, Gretchen Galliano, Ricardo. Romero, John Evans, Ari Cohen, Muhammad F. Mubarak, Janak N. Shah, Abdul Hamid El Chafic
Techniques and Innovations in Gastrointestinal Endoscopy.2022; 24(2): 127. CrossRef - Role of endoscopic ultrasound-guided liver biopsy: a meta-analysis
Keyu Zeng, Zhenpeng Jiang, Jie Yang, Kefei Chen, Qiang Lu
Scandinavian Journal of Gastroenterology.2022; 57(5): 545. CrossRef - Endoscopic ultrasound guided interventions in the management of pancreatic cancer
Tossapol Kerdsirichairat, Eun Ji Shin
World Journal of Gastrointestinal Endoscopy.2022; 14(4): 191. CrossRef - Endohepatology – current status
Jerome C. Edelson, Natalie E. Mitchell, Don C. Rockey
Current Opinion in Gastroenterology.2022; 38(3): 216. CrossRef - Diagnostic and interventional EUS in hepatology: An updated review
Vaneet Jearth, Sridhar Sundaram, SurinderSingh Rana
Endoscopic Ultrasound.2022; 11(5): 355. CrossRef - A Gene Expression Signature to Select Hepatocellular Carcinoma Patients for Liver Transplantation
Hugo Pinto-Marques, Joana Cardoso, Sílvia Silva, João L. Neto, Maria Gonçalves-Reis, Daniela Proença, Marta Mesquita, André Manso, Sara Carapeta, Mafalda Sobral, Antonio Figueiredo, Clara Rodrigues, Adelaide Milheiro, Ana Carvalho, Rui Perdigoto, Eduardo
Annals of Surgery.2022; 276(5): 868. CrossRef - Endo‐hepatology: The changing paradigm of endoscopic ultrasound in cirrhosis
Achintya Dinesh Singh, Ahmad Najdat Bazarbashi, Christina C. Lindenmeyer
Clinical Liver Disease.2022; 20(6): 209. CrossRef - Feasibility and Safety of Transjugular Liver Biopsy for Japanese Patients with Chronic Liver Diseases
Makoto Iijima, Takahiro Arisaka, Akira Yamamiya, Keiichi Tominaga, Kazunori Nagashima, Akira Kanamori, Satoshi Masuyama, Yuichi Majima, Kenichi Goda, Kazuyuki Ishida, Atsushi Irisawa
Diagnostics.2021; 11(1): 131. CrossRef - Editors' Choice of Noteworthy Clinical Endoscopy Publications in the First Decade
Gwang Ha Kim, Kwang An Kwon, Do Hyun Park, Jimin Han
Clinical Endoscopy.2021; 54(5): 633. CrossRef - Role of Endoscopic Ultrasound in Liver Disease: Where Do We Stand?
Tajana Pavic, Ivana Mikolasevic, Dominik Kralj, Nina Blazevic, Anita Skrtic, Ivan Budimir, Ivan Lerotic, Davor Hrabar
Diagnostics.2021; 11(11): 2021. CrossRef
- Endoscopic procedures in hepatology: Current trends and new developments
- 6,133 View
- 174 Download
- 23 Web of Science
- 27 Crossref

-
Validation of a Novel Endoscopic Retrograde Cholangiopancreatography Cannulation Simulator

- Pichamol Jirapinyo, Andrew C. Thompson, Hiroyuki Aihara, Marvin Ryou, Christopher C. Thompson
- Clin Endosc 2020;53(3):346-354. Published online February 17, 2020
- DOI: https://doi.org/10.5946/ce.2019.105
-
 Abstract
Abstract
 PDF
PDF Supplementary Material
Supplementary Material PubReader
PubReader ePub
ePub - Background
/Aims: Endoscopic retrograde cholangiopancreatography (ERCP) requires a unique skill set. Currently, there is no objective methodology to assess and train a professional to perform ERCP. This study aimed to develop and validate a novel ERCP simulator.
Methods
The simulator consists of papillae presenting different anatomy and positioned in varied locations. Deep cannulation of the pancreatic duct, followed by the bile duct, was performed. The time allotted was 5 minutes. The content validity indexes (CVIs) for realism, relevance, and representativeness were calculated. Correlation between ERCP experience and simulator score was determined.
Results
Twenty-three participants completed the simulation. The CVIs for realism were orientation of duodenoscope to papilla (1.00), angulation of papillotome to achieve cannulation (0.71), and haptic feedback during cannulation (0.80). The CVIs for relevance were use of elevator (1.00), wheels to achieve en face orientation (1.00), and papillotome for selective cannulation (1.00). Regarding CVI for representativeness, the results were as follows: basic cannulation (0.83), papilla locations (0.83), and papilla anatomies (0.80). The novice, intermediate, and experienced groups scored 6.7±8.7, 30.0±16.3, and 74.4±43.9, respectively (p<0.0001). There was a strong correlation between the ERCP experience level and the individual’s simulator score (Pearson value of 0.77, R2 of 0.60).
Conclusions
This simulator appears to be realistic, relevant, and representative of ERCP cannulation techniques. Additionally, it is effective at objectively assessing basic ERCP skills by differentiating scores based on clinical experience. -
Citations
Citations to this article as recorded by- Morfología de la papila de Vater como factor que influye en el éxito en canulación durante el entrenamiento del Residente en Endoscopia Avanzada. Estudio clínico prospectivo
D.E. Benavides-Salgado, R.A. Jiménez-Castillo, J.E. Cuéllar-Monterrubio, J.O. Jáquez-Quintana, A. Garza-Galindo, C. Cortes-Hernández, H.J. Maldonado-Garza, D. García-Compeán, J.A. González-González
Revista de Gastroenterología de México.2024; 89(2): 237. CrossRef - Papilla of Vater morphology as an influencing factor in successful cannulation during resident training in advanced endoscopy. A prospective clinical study
D.E. Benavides-Salgado, R.A. Jiménez-Castillo, J.E. Cuéllar-Monterrubio, J.O. Jáquez-Quintana, A. Garza-Galindo, C. Cortes-Hernández, H.J. Maldonado-Garza, D. García-Compeán, J.A. González-González
Revista de Gastroenterología de México (English Edition).2024; 89(2): 237. CrossRef - Anatomical endoscopic retrograde cholangiopancreatography simulator using moulded meshed silicone: A novel simulator pilot study
Alen Maximillian Brodaric, Ngar Lok Joshua Wong, Jessica Falon, Jean Wong, Kai Cheng, Sarah Whereat, David Storey
ANZ Journal of Surgery.2023; 93(7-8): 1817. CrossRef - The use of simulators to acquire ERCP skills: a systematic review
Konstantinos Georgiou, Kiril T. Atliev, Ninos Oussi, Nikola Boyanov, Gabriel Sandblom, Lars Enochsson
Annals of Medicine & Surgery.2023; 85(6): 2924. CrossRef - There is no royal road: a shortcut for endoscopic submucosal dissection training
Seong Woo Jeon
Clinical Endoscopy.2023; 56(5): 590. CrossRef - Validity of a virtual reality endoscopic retrograde cholangiopancreatography simulator: can it distinguish experts from novices?
Konstantinos Georgiou, Nikola Boyanov, Pantelis Antonakis, Dimitrios Thanasas, Gabriel Sandblom, Lars Enochsson
Frontiers in Surgery.2023;[Epub] CrossRef - Training in endoscopic retrograde cholangio-pancreatography: a critical assessment of the broad scenario of training programs and models
Camilla Gallo, Ivo Boškoski, Maria Valeria Matteo, Beatrice Orlandini, Guido Costamagna
Expert Review of Gastroenterology & Hepatology.2021; 15(6): 675. CrossRef
- Morfología de la papila de Vater como factor que influye en el éxito en canulación durante el entrenamiento del Residente en Endoscopia Avanzada. Estudio clínico prospectivo
- 5,226 View
- 124 Download
- 7 Web of Science
- 7 Crossref

- Endoscopic Ultrasound Fine-Needle Aspiration versus Fine-Needle Biopsy for Lymph Node Diagnosis: A Large Multicenter Comparative Analysis
- Diogo Turiani Hourneaux de Moura, Thomas R. McCarty, Pichamol Jirapinyo, Igor Braga Ribeiro, Galileu Ferreira Ayala Farias, Marvin Ryou, Linda S. Lee, Christopher C. Thompson
- Clin Endosc 2020;53(5):600-610. Published online December 3, 2019
- DOI: https://doi.org/10.5946/ce.2019.170

-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader ePub
ePub - Background
/Aims: Endoscopic ultrasound fine-needle aspiration (EUS-FNA) is preferred for sampling of lymph nodes (LNs) adjacent to the gastrointestinal wall; however, fine-needle biopsy (FNB) may provide improved diagnostic outcomes. This study aimed to evaluate the comparative efficacy and safety of FNA versus FNB for LN sampling.
Methods
This was a multicenter retrospective study of prospectively collected data to evaluate outcomes of EUS-FNA and EUS-FNB for LN sampling. Characteristics analyzed included sensitivity, specificity, accuracy, the number of needle passes, diagnostic adequacy of rapid on-site evaluation (ROSE), cell-block analysis, and adverse events.
Results
A total of 209 patients underwent EUS-guided LN sampling. The mean lesion size was 16.22±8.03 mm, with similar sensitivity and accuracy between FNA and FNB ([67.21% vs. 75.00%, respectively, p=0.216] and [78.80% vs. 83.17%, respectively, p=0.423]). The specificity of FNB was better than that of FNA (100.00% vs. 93.62%, p=0.01). The number of passes required for diagnosis was not different. Abdominal and peri-hepatic LN location demonstrated FNB to have a higher sensitivity (81.08% vs. 64.71%, p=0.031 and 80.95% vs. 58.33%, p=0.023) and accuracy (88.14% vs. 75.29%, p=0.053 and 88.89% vs. 70.49%, p=0.038), respectively. ROSE was a significant predictor for accuracy (odds ratio, 5.16; 95% confidence interval, 1.15–23.08; p=0.032). No adverse events were reported in either cohort.
Conclusions
Both EUS-FNA and EUS-FNB are safe for the diagnosis of LNs. EUS-FNB is preferred for abdominal LN sampling. EUSFNA+ ROSE was similar to EUS-FNB alone, showing better diagnosis for EUS-FNB than traditional FNA. While ROSE remained a significant predictor for accuracy, due to its poor availability in most centers, its use may be limited to cases with previous inconclusive diagnoses. -
Citations
Citations to this article as recorded by- Current perspectives on the diversification of endoscopic ultrasound-guided fine-needle aspiration and biopsy
Shinpei Doi, Takako Adachi, Ayako Watanabe, Nobuhiro Katsukura, Takayuki Tsujikawa
Journal of Medical Ultrasonics.2024; 51(2): 235. CrossRef - Comparing the diagnostic adequacy of 25-Gauge fork-tip versus franseen versus reverse-bevel-type needles in EUS–guided tissue acquisition: A prospective randomized study with a retrospective control
Adam Haig, Andrew St John, Kasturi Vaska, Xuan Banh, Alexander Huelsen
Endoscopic Ultrasound.2024; 13(1): 22. CrossRef - Comparison of 19-gauge conventional and Franseen needles for the diagnosis of lymphadenopathy and classification of malignant lymphoma using endoscopic ultrasound fine-needle aspiration
Mitsuru Okuno, Keisuke Iwata, Tsuyoshi Mukai, Yusuke Kito, Takuji Tanaka, Naoki Watanabe, Senji Kasahara, Yuhei Iwasa, Akihiko Sugiyama, Youichi Nishigaki, Yuhei Shibata, Junichi Kitagawa, Takuji Iwashita, Eiichi Tomita, Masahito Shimizu
Clinical Endoscopy.2024; 57(3): 364. CrossRef - Endoscopic Ultrasound-Guided Fine Needle Biopsy in the Diagnostic Work-Up of Deep-Seated Lymphadenopathies and Spleen Lesions: A Monocentric Experience
Flaminia Bellisario, Fabia Attili, Fabrizia Campana, Federica Borrelli de Andreis, Silvia Bellesi, Elena Maiolo, Eleonora Alma, Rosalia Malafronte, Giuseppe Macis, Luigi Maria Larocca, Salvatore Annunziata, Francesco D’Alò, Stefan Hohaus
Diagnostics.2023; 13(17): 2839. CrossRef - Comparison of Fine-Needle Biopsy (FNB) versus Fine-Needle Aspiration (FNA) Combined with Flow Cytometry in the Diagnosis of Deep-Seated Lymphoma
Yilei Yang, Aruna, Bin Cheng, Dingkun Xiong, Dong Kuang, Haochen Cui, Si Xiong, Xia Mao, Yunlu Feng, Yuchong Zhao
Diagnostics.2023; 13(17): 2777. CrossRef - Managing adverse events after endoscopic ultrasound‐guided drainage of the biliary tract and pancreatic fluid collections: Narrative review (with video)
Mateus Pereira Funari, Igor Braga Ribeiro, Marcos Eduardo Lera dos Santos, Sergio Eiji Matuguma, Eduardo Guimarães Hourneaux de Moura
Digestive Endoscopy.2022; 34(2): 359. CrossRef - Primary Pancreatic Lymphoma Evaluated by Fine-Needle Aspiration
Qiong Gan, Nancy P Caraway, Cady Ding, John M Stewart
American Journal of Clinical Pathology.2022; 158(2): 242. CrossRef - Disseminated tuberculosis following invasive procedures for peripancreatic lymph node tuberculosis with portal vein obstruction: a case report
Aya Kato, Takahisa Mashiba, Yoshinori Tateishi, Rentaro Oda, Hiraku Funakoshi, Keiichi Iwanami, Yasuaki Motomura
Clinical Journal of Gastroenterology.2022; 15(3): 673. CrossRef - Endoscopic ultrasound fine-needle biopsy vs fine-needle aspiration for lymph nodes tissue acquisition: a systematic review and meta-analysis
Antonio Facciorusso, Stefano Francesco Crinò, Paraskevas Gkolfakis, Daryl Ramai, Andrea Lisotti, Ioannis S Papanikolaou, Benedetto Mangiavillano, Ilaria Tarantino, Andrea Anderloni, Carlo Fabbri, Konstantinos Triantafyllou, Pietro Fusaroli
Gastroenterology Report.2022;[Epub] CrossRef - High Diagnostic Accuracy and Safety of Endoscopic Ultrasound-Guided Fine-Needle Aspiration in Malignant Lymph Nodes: A Systematic Review and Meta-Analysis
Linbin Chen, Yin Li, Xiaoyan Gao, Shiyong Lin, Longjun He, Guangyu Luo, Jianjun Li, Chunyu Huang, Guobao Wang, Qing Yang, Hongbo Shan
Digestive Diseases and Sciences.2021; 66(8): 2763. CrossRef - A novel and feasible technique for diagnosis and treatment of small subepithelial tumors
Epifânio Silvino do Monte Junior, Dalton Marques Chaves, Christiano Makoto Sakai, Gustavo de Oliveira Luz, Igor Braga Ribeiro, Vitor Massaro Takamatsu Sagae, Eduardo Guimarães Hourneaux de Moura
Endoscopy.2021; 53(01): E38. CrossRef - Ultrasound-Guided Fine-Needle Aspiration Versus Fine-Needle Capillary Sampling in Evaluation of Lymph Node Metastasis of Thyroid Cancer
Shujun Xia, Yilai Chen, Weiwei Zhan, Wei Zhou
Frontiers in Oncology.2021;[Epub] CrossRef - Endoscopic ultrasound fine needle aspiration vs fine needle biopsy for pancreatic masses, subepithelial lesions, and lymph nodes
Irving Levine, Arvind J Trindade
World Journal of Gastroenterology.2021; 27(26): 4194. CrossRef - Endoscopic Ultrasound Fine-Needle Biopsy versus Fine-Needle Aspiration for Tissue Sampling of Abdominal Lymph Nodes: A Propensity Score Matched Multicenter Comparative Study
Antonio Facciorusso, Stefano Francesco Crinò, Nicola Muscatiello, Paraskevas Gkolfakis, Jayanta Samanta, Juliana Londoño Castillo, Christian Cotsoglou, Daryl Ramai
Cancers.2021; 13(17): 4298. CrossRef - High Sensitivity of Endoscopic Ultrasound-Guided Fine-Needle Aspiration and Endoscopic Ultrasound-Guided Fine-Needle Biopsy in Lymphadenopathy Caused by Metastatic Disease: A Prospective Comparative Study
Per Hedenström, Vasilis Chatzikyriakos, Roozbeh Shams, Catarina Lewerin, Riadh Sadik
Clinical Endoscopy.2021; 54(5): 722. CrossRef - Editors' Choice of Noteworthy Clinical Endoscopy Publications in the First Decade
Gwang Ha Kim, Kwang An Kwon, Do Hyun Park, Jimin Han
Clinical Endoscopy.2021; 54(5): 633. CrossRef - Endoscopic ultrasound assessment and tissue acquisition of mediastinal and abdominal lymph nodes
Giacomo Tamanini, Anna Cominardi, Nicole Brighi, Pietro Fusaroli, Andrea Lisotti
World Journal of Gastrointestinal Oncology.2021; 13(10): 1475. CrossRef - Current status of newer generation endoscopic ultrasound core needles in the diagnostic evaluation of gastrointestinal lesions
Amin K. Soltani, Kumar Krishnan
Journal of the American Society of Cytopathology.2020; 9(5): 389. CrossRef - Improved diagnostic yield of endoscopic ultrasound-fine needle biopsy with histology specimen processing
Lawrence Ku, Mohammad A Shahshahan, Linda A Hou, Viktor E Eysselein, Sofiya Reicher
World Journal of Gastrointestinal Endoscopy.2020; 12(8): 212. CrossRef - Endoscopic Ultrasound Fine-Needle Biopsy May Contribute to the Diagnosis of Malignant Lymph Nodes
Mamoru Takenaka, Shunsuke Omoto, Masatoshi Kudo
Clinical Endoscopy.2020; 53(5): 508. CrossRef - Usefulness of a target sample check illuminator in the detection of target specimens in endoscopic ultrasound‐guided fine‐needle biopsy samples: Multicenter prospective study
Kazuya Matsumoto, Kazuo Hara, Ichiro Yasuda, Takao Itoi, Hiroki Kurumi, Shimpei Matsumoto, Shinpei Doi, Mitsuyoshi Honjo, Yohei Takeda, Jin Shibuya, Hisashi Noma, Hajime Isomoto
Digestive Endoscopy.2020;[Epub] CrossRef - New Devices for Endoscopic Treatments in Gastroenterology: A Narrative Review
Manuele Furnari, Andrea Telese, Alexander Hann, Andrea Lisotti, Ivo Boškoski, Leonardo Henry Eusebi
Current Drug Metabolism.2020; 21(11): 850. CrossRef
- Current perspectives on the diversification of endoscopic ultrasound-guided fine-needle aspiration and biopsy
- 5,654 View
- 183 Download
- 21 Web of Science
- 22 Crossref

Review
- Endoscopic Ultrasound-Guided Fine Needle Aspiration and Endoscopic Retrograde Cholangiopancreatography-Based Tissue Sampling in Suspected Malignant Biliary Strictures: A Meta-Analysis of Same-Session Procedures
- Diogo Turiani Hourneax de Moura, Marvin Ryou, Eduardo Guimarães Hourneaux de Moura, Igor Braga Ribeiro, Wanderlei Marques Bernardo, Christopher C. Thompson
- Clin Endosc 2020;53(4):417-428. Published online November 5, 2019
- DOI: https://doi.org/10.5946/ce.2019.053
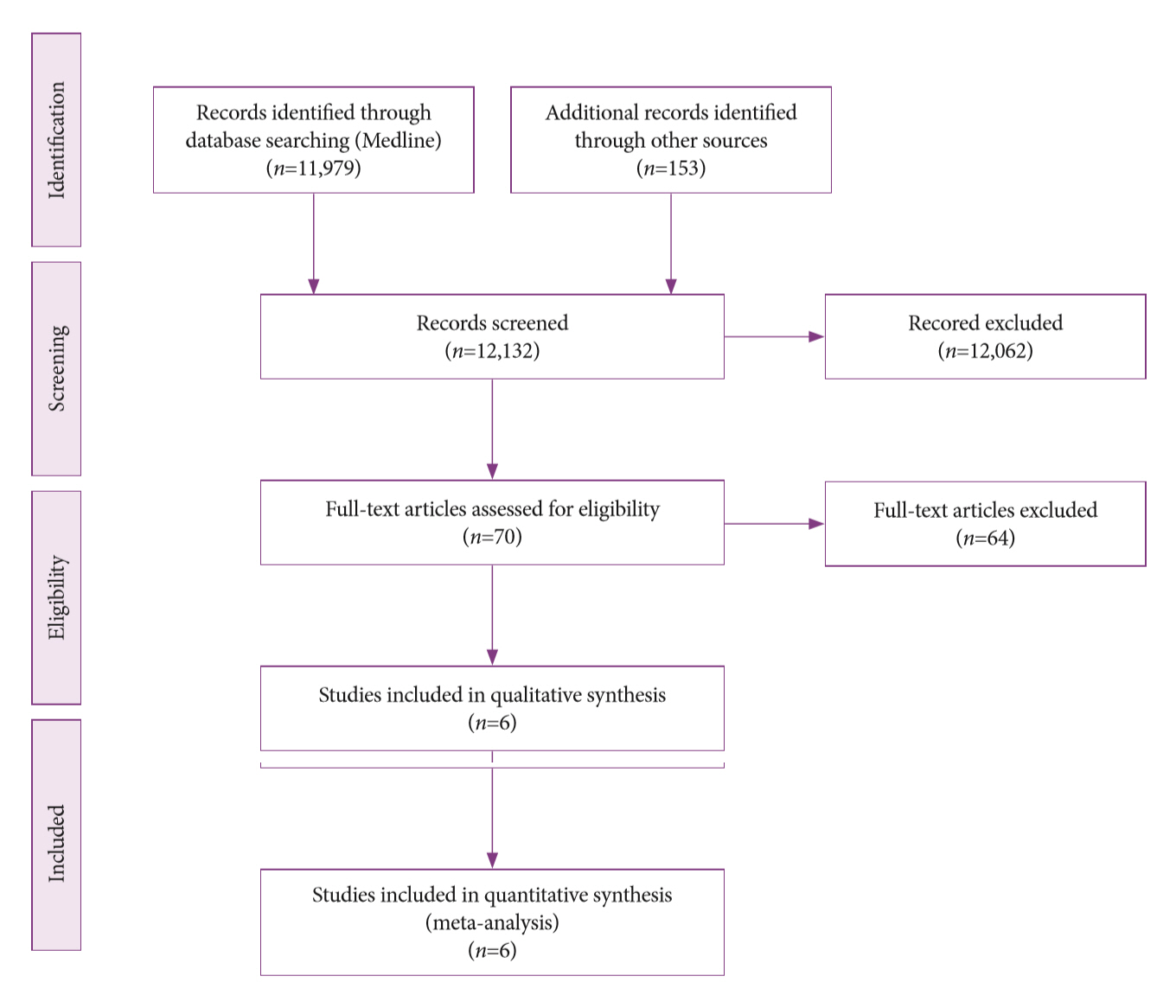
-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader ePub
ePub - Background
/Aims: The diagnosis of biliary strictures can be challenging. There are no systematic reviews studying same-session endoscopic retrograde cholangiopancreatography (ERCP)-based tissue sampling and endoscopic ultrasound-guided fine needle aspiration (EUS-FNA) for the diagnosis of biliary strictures.
Methods
A systematic review was conducted on studies analyzing same-session EUS and ERCP for tissue diagnosis of suspected malignant biliary strictures. The primary outcome was the accuracy of each method individually compared to the two methods combined. The secondary outcome was the accuracy of each method in pancreatic and biliary etiologies. In the meta-analysis, we used Forest plots, summary receiver operating characteristic curves, and estimates of the area under the curve for intention-to-treat analysis.
Results
Of the 12,132 articles identified, six were included, resulting in a total of 497 patients analyzed. The sensitivity, specificity, positive likelihood ratio, negative likelihood ratio, and accuracy of the association between the two methods were: 86%, 98%, 12.50, 0.17, and 96.5%, respectively. For the individual analysis, the sensitivity, specificity and accuracy of EUS-FNA were 76%, 100%, and 94.5%, respectively; for ERCP-based tissue sampling, the sensitivity, specificity, and accuracy were 58%, 98%, and 78.1%, respectively. For pancreatic lesions, EUS-FNA was superior to ERCP-based tissue sampling. However, for biliary lesions, both methods had similar sensitivities.
Conclusions
Same-session EUS-FNA and ERCP-based tissue sampling is superior to either method alone in the diagnosis of suspected malignant biliary strictures. Considering these results, combination sampling should be performed when possible. -
Citations
Citations to this article as recorded by- British Society of Gastroenterology guidelines for the diagnosis and management of cholangiocarcinoma
Simon M Rushbrook, Timothy James Kendall, Yoh Zen, Raneem Albazaz, Prakash Manoharan, Stephen P Pereira, Richard Sturgess, Brian R Davidson, Hassan Z Malik, Derek Manas, Nigel Heaton, K Raj Prasad, John Bridgewater, Juan W Valle, Rebecca Goody, Maria Hawk
Gut.2024; 73(1): 16. CrossRef - Contrast-enhanced guided endoscopic ultrasound procedures
Marcel Ioan Gheorghiu, Andrada Seicean, Cristina Pojoga, Claudia Hagiu, Radu Seicean, Zeno Sparchez
World Journal of Gastroenterology.2024; 30(17): 2311. CrossRef - ACG Clinical Guideline: Diagnosis and Management of Biliary Strictures
B. Joseph Elmunzer, Jennifer L. Maranki, Victoria Gómez, Anna Tavakkoli, Bryan G. Sauer, Berkeley N. Limketkai, Emily A. Brennan, Elaine M. Attridge, Tara J. Brigham, Andrew Y. Wang
American Journal of Gastroenterology.2023; 118(3): 405. CrossRef - Endoscopic Ultrasound in the Diagnosis of Extrahepatic Cholangiocarcinoma: What Do We Know in 2023?
Rares Ilie Orzan, Cristina Pojoga, Renata Agoston, Radu Seicean, Andrada Seicean
Diagnostics.2023; 13(6): 1023. CrossRef - Endoscopic evaluation of indeterminate biliary strictures: Cholangioscopy, endoscopic ultrasound, or both?
Raymond S. Y. Tang
Digestive Endoscopy.2023;[Epub] CrossRef - Brush Cytology, Forceps Biopsy, or Endoscopic Ultrasound-Guided Sampling for Diagnosis of Bile Duct Cancer: A Meta-Analysis
Seung Bae Yoon, Sung-Hoon Moon, Sung Woo Ko, Hyun Lim, Ho Suk Kang, Jong Hyeok Kim
Digestive Diseases and Sciences.2022; 67(7): 3284. CrossRef - Managing adverse events after endoscopic ultrasound‐guided drainage of the biliary tract and pancreatic fluid collections: Narrative review (with video)
Mateus Pereira Funari, Igor Braga Ribeiro, Marcos Eduardo Lera dos Santos, Sergio Eiji Matuguma, Eduardo Guimarães Hourneaux de Moura
Digestive Endoscopy.2022; 34(2): 359. CrossRef - Endoscopic Ultrasound for the Diagnosis and Staging of Biliary Malignancy
Martin Coronel, Jeffrey H. Lee, Emmanuel Coronel
Clinics in Liver Disease.2022; 26(1): 115. CrossRef - Endoscopic Management of Pancreatobiliary Malignancies
Dong Wook Lee, Eun Young Kim
Digestive Diseases and Sciences.2022; 67(5): 1635. CrossRef - IgG4-related sclerosing cholangitis involving the gallbladder mimicking a hilar cholangiocarcinoma
Yun Chae Lee, Hyung Ku Chon, Keum Ha Choi
Endoscopy.2022; 54(12): E739. CrossRef - Promising Genomic Testing for Biliary Tract Cancer Using Endoscopic Ultrasound-Guided Fine-Needle Aspiration/Biopsy Specimens
Masaki Kuwatani, Kazumichi Kawakubo, Naoya Sakamoto
Diagnostics.2022; 12(4): 900. CrossRef - Endoscopic Ultrasound Plus Endoscopic Retrograde Cholangiopancreatography Based Tissue Sampling for Diagnosis of Proximal and Distal Biliary Stenosis Due to Cholangiocarcinoma: Results from a Retrospective Single-Center Study
Edoardo Troncone, Fabio Gadaleta, Omero Alessandro Paoluzi, Cristina Maria Gesuale, Vincenzo Formica, Cristina Morelli, Mario Roselli, Luca Savino, Giampiero Palmieri, Giovanni Monteleone, Giovanna Del Vecchio Blanco
Cancers.2022; 14(7): 1730. CrossRef - The Role of Cholangioscopy and EUS in the Evaluation of Indeterminate Biliary Strictures
Wilson Siu, Raymond S. Y. Tang
Gastroenterology Insights.2022; 13(2): 192. CrossRef - Current endoscopic approaches to biliary strictures
Tatsuya Sato, Yousuke Nakai, Mitsuhiro Fujishiro
Current Opinion in Gastroenterology.2022; 38(5): 450. CrossRef - Acute cholecystitis caused by gallbladder metastasis from non-small cell lung cancer: a case report
Kouki Imaoka, Daisuke Satoh, Ko Oshita, Takuya Yano, Tetsushi Kubota, Michihiro Ishida, Yasuhiro Choda, Masanori Yoshimitsu, Kanyu Nakano, Masao Harano, Hiroyoshi Matsukawa, Hitoshi Idani, Shigehiro Shiozaki, Masazumi Okajima
Clinical Journal of Gastroenterology.2021; 14(1): 351. CrossRef - Current Status and Research Progress of ERCP in the Diagnosis and Treatment of Biliary and Pancreatic System Diseases
跃华 李
Advances in Clinical Medicine.2021; 11(07): 3123. CrossRef - Same day endoscopic retrograde cholangio-pancreatography immediately after endoscopic ultrasound for choledocholithiasis is feasible, safe and cost-effective
Wisam Sbeit, Anas Kadah, Amir Shahin, Tawfik Khoury
Scandinavian Journal of Gastroenterology.2021; 56(10): 1243. CrossRef - Editors' Choice of Noteworthy Clinical Endoscopy Publications in the First Decade
Gwang Ha Kim, Kwang An Kwon, Do Hyun Park, Jimin Han
Clinical Endoscopy.2021; 54(5): 633. CrossRef - Tips and tricks for the diagnosis and management of biliary stenosis-state of the art review
Giovanna Del Vecchio Blanco, Michelangela Mossa, Edoardo Troncone, Renato Argirò, Andrea Anderloni, Alessandro Repici, Omero Alessandro Paoluzi, Giovanni Monteleone
World Journal of Gastrointestinal Endoscopy.2021; 13(10): 473. CrossRef - Stent versus Balloon Dilation for the Treatment of Dominant Strictures in Primary Sclerosing Cholangitis: A Systematic Review and Meta-Analysis
Marina Tucci Gammaro Baldavira Ferreira, Igor Braga Ribeiro, Diogo Turiani Hourneaux de Moura, Thomas R. McCarty, Alberto Machado da Ponte Neto, Galileu Ferreira Ayala Farias, Antônio Afonso de Miranda Neto, Pedro Victor Aniz Gomes de Oliveira, Wanderley
Clinical Endoscopy.2021; 54(6): 833. CrossRef - Endoscopic ultrasound fine needle aspiration vs fine needle biopsy in solid lesions: A multi-center analysis
Diogo Turiani Hourneaux Moura, Thomas R McCarty, Pichamol Jirapinyo, Igor Braga Ribeiro, Galileu Ferreira Ayala Farias, Antonio Coutinho Madruga-Neto, Marvin Ryou, Christopher C Thompson
World Journal of Clinical Cases.2021; 9(34): 10507. CrossRef - Efficacy of digital single-operator cholangioscopy in the visual interpretation of indeterminate biliary strictures: a systematic review and meta-analysis
Pedro Victor Aniz Gomes de Oliveira, Diogo Turiani Hourneaux de Moura, Igor Braga Ribeiro, Ahmad Najdat Bazarbashi, Tomazo Antonio Prince Franzini, Marcos Eduardo Lera dos Santos, Wanderley Marques Bernardo, Eduardo Guimarães Hourneaux de Moura
Surgical Endoscopy.2020; 34(8): 3321. CrossRef - Role of pancreatography in the endoscopic management of encapsulated pancreatic collections – review and new proposed classification
Igor Mendonça Proença, Marcos Eduardo Lera dos Santos, Diogo Turiani Hourneaux de Moura, Igor Braga Ribeiro, Sergio Eiji Matuguma, Spencer Cheng, Thomas R McCarty, Epifanio Silvino do Monte Junior, Paulo Sakai, Eduardo Guimarães Hourneaux de Moura
World Journal of Gastroenterology.2020; 26(45): 7104. CrossRef
- British Society of Gastroenterology guidelines for the diagnosis and management of cholangiocarcinoma
- 5,822 View
- 205 Download
- 21 Web of Science
- 23 Crossref

Brief Report
- Multicenter Implementation of a New Electronic Medical Record System Leads to Longer Procedure Times and Poor Staff Satisfaction
- Andrew C. Storm, Marvin Ryou, Christopher C. Thompson
- Clin Endosc 2019;52(1):87-89. Published online August 21, 2018
- DOI: https://doi.org/10.5946/ce.2018.080

-
 PDF
PDF PubReader
PubReader ePub
ePub -
Citations
Citations to this article as recorded by- American Society for Gastrointestinal Endoscopy guideline on informed consent for GI endoscopic procedures
Andrew C. Storm, Douglas S. Fishman, James L. Buxbaum, Nayantara Coelho-Prabhu, Mohammad A. Al-Haddad, Stuart K. Amateau, Audrey H. Calderwood, Christopher J. DiMaio, Sherif E. Elhanafi, Nauzer Forbes, Larissa L. Fujii-Lau, Terry L. Jue, Divyanshoo R. Koh
Gastrointestinal Endoscopy.2022; 95(2): 207. CrossRef
- American Society for Gastrointestinal Endoscopy guideline on informed consent for GI endoscopic procedures
- 4,402 View
- 81 Download
- 1 Web of Science
- 1 Crossref

Original Article
- A Prospective Blinded Study of Endoscopic Ultrasound Elastography in Liver Disease: Towards a Virtual Biopsy
- Allison R. Schulman, Ming V. Lin, Anna Rutherford, Walter W. Chan, Marvin Ryou
- Clin Endosc 2018;51(2):181-185. Published online March 23, 2018
- DOI: https://doi.org/10.5946/ce.2017.095
-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader ePub
ePub - Background
/Aims: Liver biopsy has traditionally been used for determining the degree of fibrosis, however there are several limitations. Endoscopic ultrasound (EUS) real-time elastography (RTE) is a novel technology that uses image enhancement to display differences in tissue compressibility. We sought to assess whether liver fibrosis index (LFI) can distinguish normal, fatty, and cirrhotic liver tissue.
Methods
A total of 50 patients undergoing EUS were prospectively enrolled. RTE of the liver was performed to synthesize the LFI in each patient. Univariate and multivariable analyses were performed. Chi-square and t-tests were performed for categorical and continuous variables, respectively. A p-value of <0.05 was considered significant.
Results
Abdominal imaging prior to endoscopic evaluation suggested normal tissue, fatty liver, and cirrhosis in 26, 16, and 8 patients, respectively. Patients with cirrhosis had significantly increased mean LFI compared to the fatty liver (3.2 vs. 1.7, p<0.001) and normal (3.2 vs. 0.8, p<0.001) groups. The fatty liver group showed significantly increased LFI compared to the normal group (3.8 vs. 1.4, p<0.001). Multivariable regression analysis suggested that LFI was an independent predictor of group features (p<0.001).
Conclusions
LFI computed from RTE images significantly correlates with abdominal imaging and can distinguish normal, fatty, and cirrhotic-appearing livers; therefore, LFI may play an important role in patients with chronic liver disease. -
Citations
Citations to this article as recorded by- Endohepatology: The endoscopic armamentarium in the hand of the hepatologist
Ahmed Alwassief, Said Al-Busafi, Qasim L. Abbas, Khalid Al Shamusi, Sarto C. Paquin, Anand V. Sahai
Saudi Journal of Gastroenterology.2024; 30(1): 4. CrossRef - Preliminary Study on the Evaluation of Liver Fibrosis Using Endoscopic Ultrasound Elastography and Transabdominal Ultrasound Transient Elastography Combined with Strain Ratio
Dun-Wei Yao, Hai-Xing Jiang, Shan-Yu Qin
Hepatitis Monthly.2024;[Epub] CrossRef - Endoscopic Ultrasound-based Shear Wave Elastography for Detection of Advanced Liver Disease
Jad AbiMansour, Jerry Yung-Lun Chin, Jyotroop Kaur, Eric J. Vargas, Barham K. Abu Dayyeh, Ryan Law, Vishal Garimella, Michael J. Levy, Andrew C. Storm, Ross Dierkhising, Alina Allen, Sudhakar Venkatesh, Vinay Chandrasekhara
Journal of Clinical Gastroenterology.2024;[Epub] CrossRef - Endoscopic ultrasound-guided tissue acquisition for the diagnosis of focal liver lesion
Alina Tantău, Cosmina Sutac, Anamaria Pop, Marcel Tantău
World Journal of Radiology.2024; 16(4): 72. CrossRef - Comparative accuracy of endosonographic shear wave elastography and transcutaneous liver stiffness measurement: a pilot study
Divyanshoo R. Kohli, Daniel Mettman, Nevene Andraws, Erin Haer, Jaime Porter, Ozlem Ulusurac, Steven Ullery, Madhav Desai, Mohammad S. Siddiqui, Prateek Sharma
Gastrointestinal Endoscopy.2023; 97(1): 35. CrossRef - The Role of Endoscopic Ultrasound in Hepatology
Saleh A. Alqahtani, Floriane Ausloos, Ji Seok Park, Sunguk Jang
Gut and Liver.2023; 17(2): 204. CrossRef - Distinct ways to perform a liver biopsy: The core technique setups and updated understanding of these modalities
Chao Sun, Xingliang Zhao, Lei Shi, Xiaofei Fan, Xiaolong Qi
Endoscopic Ultrasound.2023; 12(6): 437. CrossRef - Future Directions in EndoHepatology
Ahmad Najdat Bazarbashi, Lolwa Al-Obaid, Marvin Ryou
Techniques and Innovations in Gastrointestinal Endoscopy.2022; 24(1): 98. CrossRef - The role of endoscopic ultrasound for portal hypertension in liver cirrhosis
Cosmas Rinaldi Adithya Lesmana, Maria Satya Paramitha, Rino A. Gani, Laurentius A. Lesmana
Journal of Medical Ultrasonics.2022; 49(3): 359. CrossRef - Endoscopic Ultrasound-Guided Liver Biopsy: Where Do We Stand?
Enad Dawod, Jose Nieto, Sammy Saab
American Journal of Gastroenterology.2022; 117(2): 205. CrossRef - Utilidad del índice de fibrosis hepática (IFH) medido durante la ultrasonografía endoscópica en la evaluación del parénquima pancreático
Martín Alonso Gómez Zuleta, Oscar Fernando Ruíz Morales, Eddy Johanna Buitrago Laguado
Revista colombiana de Gastroenterología.2022; 37(1): 10. CrossRef - Endohepatology – current status
Jerome C. Edelson, Natalie E. Mitchell, Don C. Rockey
Current Opinion in Gastroenterology.2022; 38(3): 216. CrossRef - The expanding role of endoscopic ultrasound elastography
Jahnvi Dhar, Jayanta Samanta
Clinical Journal of Gastroenterology.2022; 15(5): 841. CrossRef - Diagnostic and interventional EUS in hepatology: An updated review
Vaneet Jearth, Sridhar Sundaram, SurinderSingh Rana
Endoscopic Ultrasound.2022; 11(5): 355. CrossRef - Enhanced EUS imaging (with videos)
Kumar Krishnan, Manoop S. Bhutani, Harry R. Aslanian, Joshua Melson, Udayakumar Navaneethan, Rahul Pannala, Mansour A. Parsi, Allison R. Schulman, Amrita Sethi, Shelby Sullivan, Guru Trikudanathan, Arvind J. Trindade, Rabindra R. Watson, John T. Maple, Da
Gastrointestinal Endoscopy.2021; 93(2): 323. CrossRef - Real-Time Tissue Elastography to Evaluate Hepatic Hypoxic-Ischemic Injury Caused by Brain Death
Guoying Zhang, Ying Tang, Huimin Yu, Weina Kong, Yun Chen, Yang Liu, Jingwen Zhao
Ultrasound Quarterly.2021; 37(2): 138. CrossRef - Role of Endoscopic Ultrasound in Liver Disease: Where Do We Stand?
Tajana Pavic, Ivana Mikolasevic, Dominik Kralj, Nina Blazevic, Anita Skrtic, Ivan Budimir, Ivan Lerotic, Davor Hrabar
Diagnostics.2021; 11(11): 2021. CrossRef - Role of endoscopic ultrasound in the field of hepatology: Recent advances and future trends
Jahnvi Dhar, Jayanta Samanta
World Journal of Hepatology.2021; 13(11): 1459. CrossRef - Endoscopic ultrasound in chronic liver disease
Brian M Fung, Alexander P Abadir, Armen Eskandari, Michael J Levy, James H Tabibian
World Journal of Hepatology.2020; 12(6): 262. CrossRef - A State-of-the-Art Review on the Evolving Utility of Endoscopic Ultrasound in Liver Diseases Diagnosis
Wisam Sbeit, Anas Kadah, Mahmud Mahamid, Rinaldo Pellicano, Amir Mari, Tawfik Khoury
Diagnostics.2020; 10(8): 512. CrossRef - Comparison of EUS-guided versus percutaneous and transjugular approaches for the performance of liver biopsies
Asim Shuja, Ahmad Alkhasawneh, Andre Fialho, Andrea Fialho, Amal Shukri, Ciel Harris, Carmen Smotherman, Miguel Malespin, Silvio W. de Melo
Digestive and Liver Disease.2019; 51(6): 826. CrossRef - Endoscopic Ultrasound Real-Time Elastography in Liver Disease
Jeong Eun Song, Dong Wook Lee, Eun Young Kim
Clinical Endoscopy.2018; 51(2): 118. CrossRef
- Endohepatology: The endoscopic armamentarium in the hand of the hepatologist
- 6,772 View
- 145 Download
- 22 Web of Science
- 22 Crossref


 KSGE
KSGE


 First
First Prev
Prev



