Articles
- Page Path
- HOME > Clin Endosc > Volume 46(6); 2013 > Article
- Case Report Endoscopic Treatment of Duodenal Neuroendocrine Tumors
- Sang Ho Kim, Chang Hwan Park, Ho Seok Ki, Chung Hwan Jun, Seon Young Park, Hyun Soo Kim, Sung Kyu Choi, Jong Sun Rew
-
Clinical Endoscopy 2013;46(6):656-661.
DOI: https://doi.org/10.5946/ce.2013.46.6.656
Published online: November 19, 2013
Division of Gastroenterology, Department of Internal Medicine, Chonnam National University Medical School, Gwangju, Korea.
- Correspondence: Chang Hwan Park. Division of Gastroenterology, Department of Internal Medicine, Chonnam National University Medical School, 42 Jebong-ro, Dong-gu, Gwangju 501-757, Korea. Tel: +82-62-220-6296, Fax: +82-62-225-8578, p1052ccy@hanmail.net
• Received: November 7, 2012 • Revised: February 5, 2013 • Accepted: February 13, 2013
Copyright © 2013 Korean Society of Gastrointestinal Endoscopy
This is an Open Access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (http://creativecommons.org/licenses/by-nc/3.0/) which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Abstract
- Duodenal neuroendocrine tumors (NETs) are rare neoplasms. In this study, the medical records of 14 patients with duodenal NETs diagnosed at Chonnam National University Hospital from July 2001 to August 2011 were reviewed and analyzed retrospectively. Four patients were diagnosed in the first 5 years, and 10 patients were diagnosed in the latter 5 years of the study. Ten of 12 patients (83.3%) who underwent endoscopic biopsy were confirmed to have NET before resection. Endoscopic resection was performed in 12 patients, surgical resection in one patient, and regular follow-up in one patient who refused resection. None of the patients showed recurrence or distant metastasis. Duodenal NETs are increasingly observed and are mostly detected during screening upper gastrointestinal endoscopy. Careful endoscopic examination and biopsy can improve the diagnostic yield of NETs. Most well-differentiated, nonfunctional duodenal NETs that are limited to the mucosa/submucosa can be treated effectively with endoscopic resection.
INTRODUCTION
CASE REPORT
DISCUSSION
- 1. Modlin IM, Lye KD, Kidd M. A 5-decade analysis of 13,715 carcinoid tumors. Cancer 2003;97:934–959. 12569593.ArticlePubMed
- 2. Burke AP, Sobin LH, Federspiel BH, Shekitka KM, Helwig EB. Carcinoid tumors of the duodenum. A clinicopathologic study of 99 cases. Arch Pathol Lab Med 1990;114:700–704. 1694655.PubMed
- 3. Kulke MH, Mayer RJ. Carcinoid tumors. N Engl J Med 1999;340:858–868. 10080850.ArticlePubMed
- 4. Oberg K, Astrup L, Eriksson B, et al. Guidelines for the management of gastroenteropancreatic neuroendocrine tumours (including bronchopulmonary and thymic neoplasms). Part I-general overview. Acta Oncol 2004;43:617–625. 15545182.ArticlePubMed
- 5. Hedenbro JL, Ekelund M, Wetterberg P. Endoscopic diagnosis of submucosal gastric lesions. The results after routine endoscopy. Surg Endosc 1991;5:20–23. 1871670.ArticlePubMed
- 6. Modlin IM, Shapiro MD, Kidd M, Eick G. Siegfried oberndorfer and the evolution of carcinoid disease. Arch Surg 2007;142:187–197. 17309971.ArticlePubMed
- 7. Modlin IM, Oberg K, Chung DC, et al. Gastroenteropancreatic neuroendocrine tumours. Lancet Oncol 2008;9:61–72. 18177818.ArticlePubMed
- 8. Klöppel G, Heitz PU, Capella C, Solcia E. Pathology and nomenclature of human gastrointestinal neuroendocrine (carcinoid) tumors and related lesions. World J Surg 1996;20:132–141. 8661808.ArticlePubMed
- 9. Klöppel G, Rindi G, Anlauf M, Perren A, Komminoth P. Site-specific biology and pathology of gastroenteropancreatic neuroendocrine tumors. Virchows Arch 2007;451(Suppl 1):S9–S27. 17684761.ArticlePubMed
- 10. Rindi G, Klöppel G, Alhman H, et al. TNM staging of foregut (neuro)endocrine tumors: a consensus proposal including a grading system. Virchows Arch 2006;449:395–401. 16967267.ArticlePubMedPMC
- 11. Hoffmann KM, Furukawa M, Jensen RT. Duodenal neuroendocrine tumors: classification, functional syndromes, diagnosis and medical treatment. Best Pract Res Clin Gastroenterol 2005;19:675–697. 16253893.ArticlePubMed
- 12. Kang BS, Kim JW. Gastrointestinal carcinoid tumor: clinical review of 36 cases. J Korean Surg Soc 2009;76:1–6.Article
- 13. Zimmer T, Scherübl H, Faiss S, Stölzel U, Riecken EO, Wiedenmann B. Endoscopic ultrasonography of neuroendocrine tumours. Digestion 2000;62(Suppl 1):45–50. 10940687.ArticlePubMed
- 14. Shimizu N, Kaminishi M. Management of patients with neuroendocrine tumors of the esophagus, stomach, and duodenum. Nihon Geka Gakkai Zasshi 2008;109:147–151. 18536318.PubMed
- 15. Deacon AC. The measurement of 5-hydroxyindoleacetic acid in urine. Ann Clin Biochem 1994;31(Pt 3):215–232. 7520678.ArticlePubMed
- 16. Eriksson B, Klöppel G, Krenning E, et al. Consensus guidelines for the management of patients with digestive neuroendocrine tumors: well-differentiated jejunal-ileal tumor/carcinoma. Neuroendocrinology 2008;87:8–19. 18097129.ArticlePubMed
- 17. Jensen RT, Niederle B, Mitry E, et al. Gastrinoma (duodenal and pancreatic). Neuroendocrinology 2006;84:173–182. 17312377.ArticlePubMed
REFERENCES
Fig. 1(A-D) Morphologic findings and resection techniques for duodenal neuroendocrine tumors (A, patient 6; B, patient 7; C, patient 9; D, patient 10). (Ac) Endoscopic mucosal resection (EMR) with circumferential precutting (EMR with precutting): after submucosal injection and circumferential mucosal incision, snaring was performed. (Bc) Endoscopic submucosal dissection: after submucosal injection, circumferential mucosal incision was performed. (Cc) EMR with ligation: band ligation was performed. (Dc) EMR: snaring was performed.
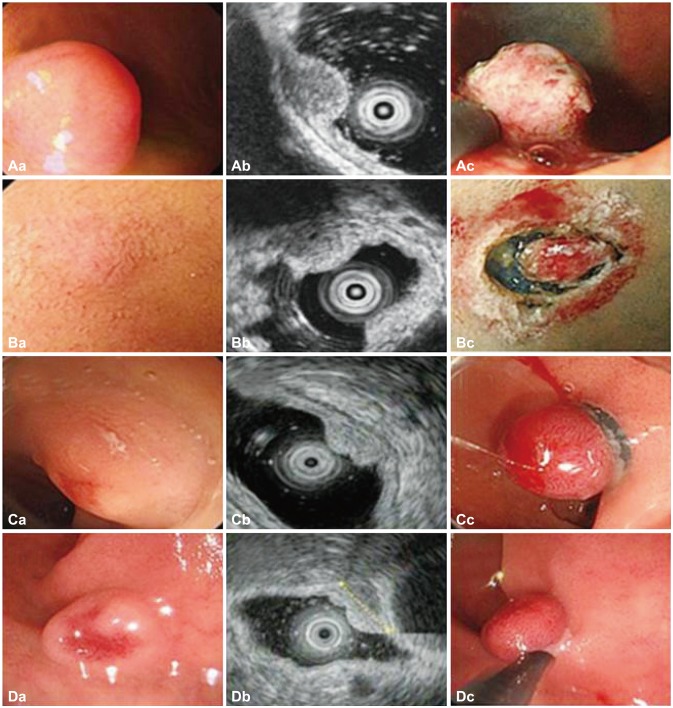

Fig. 2(A-D) Endoscopic findings and coronal computed tomography (CT) scans before and after endoscopic resection (patient 4). (A, C) Endoscopy and CT image show a submucosal mass (black arrows). (B, D) Follow-up endoscopic and coronal CT images show no evidence of recurrence 62 months after resection.
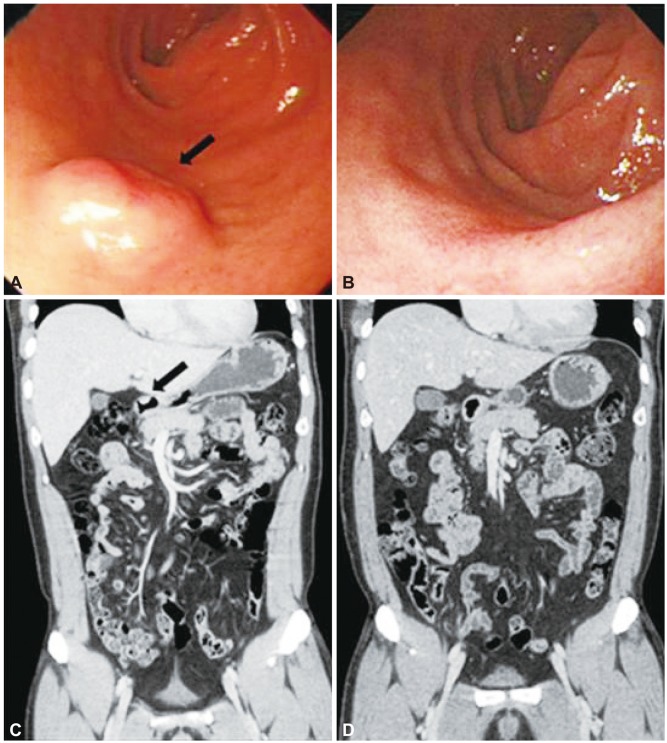

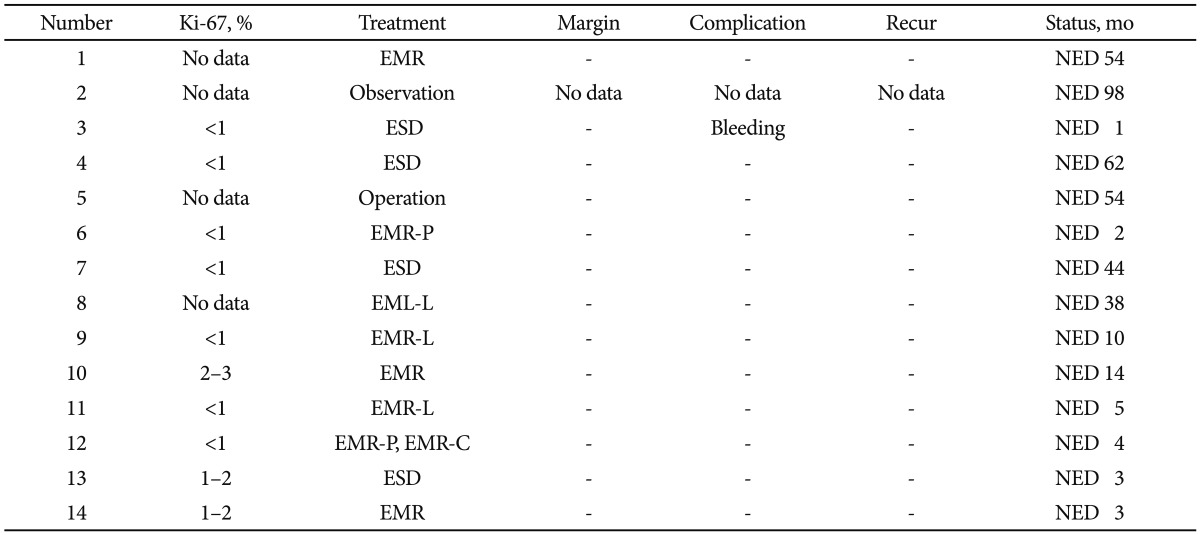
Table 1Survey of Endoscopic Features and Clinical Characteristics of the Patients with Duodenal Neuroendocrine Tumor
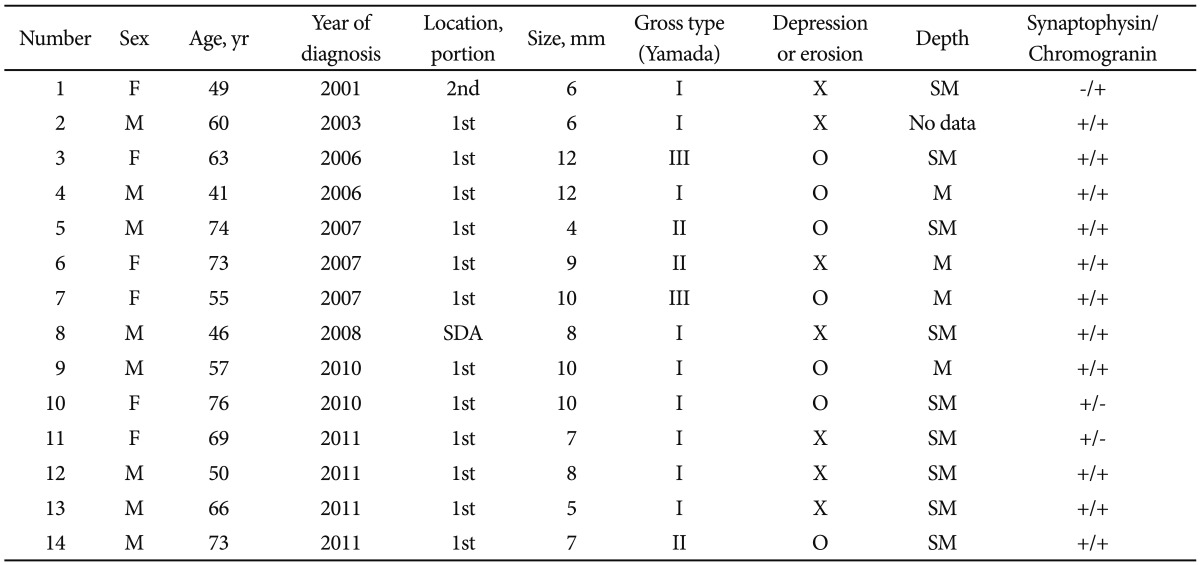

Figure & Data
REFERENCES
Citations
Citations to this article as recorded by 

- Endoscopic management of gastric, duodenal and rectal NETs: Position paper from the Italian Association for Neuroendocrine Tumors (Itanet), Italian Society of Gastroenterology (SIGE), Italian Society of Digestive Endoscopy (SIED)
Francesco Panzuto, Maria Caterina Parodi, Gianluca Esposito, Sara Massironi, Alberto Fantin, Renato Cannizzaro, Massimo Milione, Claudio Giovanni De Angelis, Bruno Annibale
Digestive and Liver Disease.2024; 56(4): 589. CrossRef - Conventional endoscopic mucosal resection versus modified endoscopic mucosal resection for duodenal neuroendocrine tumor
Jin Hee Noh, Do Hoon Kim, Kwangbeom Park, Hee Kyong Na, Ji Yong Ahn, Jeong Hoon Lee, Kee Wook Jung, Kee Don Choi, Ho June Song, Gin Hyug Lee, Hwoon-Yong Jung
Surgical Endoscopy.2023; 37(5): 3884. CrossRef - The meaning of R1 resection after endoscopic removal of gastric, duodenal and rectal neuroendocrine tumors
Gianluca Esposito, Elisabetta Dell’Unto, Irene Ligato, Matteo Marasco, Francesco Panzuto
Expert Review of Gastroenterology & Hepatology.2023; 17(8): 785. CrossRef - Duodenal neuroendocrine tumors: Short-term outcomes of endoscopic submucosal dissection performed in the Western setting
Sunil Gupta, Puja Kumar, Rocio Chacchi, Alberto Murino, Edward J Despott, Arnaud Lemmers, Mathieu Pioche, Michael J. Bourke
Endoscopy International Open.2023; 11(11): E1099. CrossRef - Management of Duodenal Neuroendocrine Tumors: Surgical versus Endoscopic Mucosal Resection
Catherine G. Tran, Scott K. Sherman, Mohammed O. Suraju, Apoorve Nayyar, Henning Gerke, Rami G. El Abiad, Chandrikha Chandrasekharan, Po Hien Ear, Thomas M. O’Dorisio, Joseph S. Dillon, Andrew M. Bellizzi, James R. Howe
Annals of Surgical Oncology.2022; 29(1): 75. CrossRef - Endoscopic mucosal resection using a ligation device for duodenal neuroendocrine tumors: a simple method
Yasuhiro Inokuchi, Kei Hayashi, Yoshihiro Kaneta, Yoichiro Okubo, Mamoru Watanabe, Mitsuhiro Furuta, Nozomu Machida, Shin Maeda
Therapeutic Advances in Gastrointestinal Endoscopy.2022; 15: 263177452211037. CrossRef - Survival and Disease Recurrence in Patients with Duodenal Neuroendocrine Tumours—A Single Centre Cohort
Oddry Folkestad, Hans H. Wasmuth, Patricia Mjønes, Reidun Fougner, Øyvind Hauso, Reidar Fossmark
Cancers.2021; 13(16): 3985. CrossRef - Comparison of endoscopic resection techniques for duodenal neuroendocrine tumors: systematic review
Helcio Pedrosa Brito, Isabela Trindade Torres, Karine Corcione Turke, Artur Adolfo Parada, Jaques Waisberg, Ricardo Vieira Botelho
Endoscopy International Open.2021; 09(08): E1214. CrossRef - The Role of Endoscopy in Small Bowel Neuroendocrine Tumors
Ji Yoon Yoon, Nikhil A. Kumta, Michelle Kang Kim
Clinical Endoscopy.2021; 54(6): 818. CrossRef - Systematic review: management of localised low‐grade upper gastrointestinal neuroendocrine tumours
Klaire Exarchou, Nathan Howes, David Mark Pritchard
Alimentary Pharmacology & Therapeutics.2020; 51(12): 1247. CrossRef - Duodenal Neuroendocrine Tumour Resection with a New Duodenal Full-Thickness Resection Device
João Cortez-Pinto, Susana Mão de Ferro, Joana Castela, Isabel Claro, Paula Chaves, António Dias Pereira
GE - Portuguese Journal of Gastroenterology.2020; 27(4): 290. CrossRef - Endoscopic Resection of Duodenal Carcinoid Tumors
Nadim Mahmud, Yutaka Tomizawa, Kristen Stashek, Bryson W. Katona, Gregory G. Ginsberg, David C. Metz
Pancreas.2019; 48(1): 60. CrossRef - Neuroendocrine Carcinoma of Duodenum—an Uncommon Tumour at an Unusual Site
Palki Dewan, Shubha P. Bhat, H. L. Kishan Prasad, Rajesh Ballal, K. Sajitha
Indian Journal of Surgical Oncology.2019; 10(1): 199. CrossRef - Non-functional duodenal neuroendocrine carcinoma: a rare cause of diabetes mellitus
Chad Bisambar, Andrew Collier, Fraser Duthie, Carron Meney
Endocrinology, Diabetes & Metabolism Case Reports.2018;[Epub] CrossRef
Endoscopic Treatment of Duodenal Neuroendocrine Tumors


Fig. 1 (A-D) Morphologic findings and resection techniques for duodenal neuroendocrine tumors (A, patient 6; B, patient 7; C, patient 9; D, patient 10). (Ac) Endoscopic mucosal resection (EMR) with circumferential precutting (EMR with precutting): after submucosal injection and circumferential mucosal incision, snaring was performed. (Bc) Endoscopic submucosal dissection: after submucosal injection, circumferential mucosal incision was performed. (Cc) EMR with ligation: band ligation was performed. (Dc) EMR: snaring was performed.
Fig. 2 (A-D) Endoscopic findings and coronal computed tomography (CT) scans before and after endoscopic resection (patient 4). (A, C) Endoscopy and CT image show a submucosal mass (black arrows). (B, D) Follow-up endoscopic and coronal CT images show no evidence of recurrence 62 months after resection.

 KSGE
KSGE

 PubReader
PubReader ePub Link
ePub Link Cite
Cite


