INTRODUCTION
Subepithelial tumors (SETs) are often detected during diagnostic endoscopy [1]. Most SETs are asymptomatic and many such lesions are clinically unimportant. However, SETs can have malignant potency and it is therefore critical to be able to exclude malignant or premalignant lesions [2].
Among SETs, the malignant potential of gastrointestinal stromal tumors (GISTs) is related to size; however, malignancy can be detected in smaller lesions [3]. While endoscopic ultrasound (EUS) is often used diagnostically, the accuracy of EUS imaging alone is not sufficient. When used in combination with EUS-guided fine-needle aspiration (FNA), diagnostic accuracy increases although diagnostic yields vary by study [4]. Thus, linear-type EUS should be used for biopsy, despite this procedureтs limitations in cases of small SETs. We have previously performed endoscopic biopsy using an endoscopic submucosal dissection (ESD) technique in SETs [5]. In this review, we discuss the role of endoscopic biopsy in the pathologic diagnosis of SETs.
THE LIMITATIONS OF EUS FINDINGS AND EUS-GUIDED BIOPSY IN THE DIAGNOSIS OF SET
EUS enables evaluation of mass characteristics such as size, layer, delineation, and echogenicity [6,7]. Management plans for SETs are determined by algorithm using EUS findings. With this algorithm, endoscopists decide on either regular monitoring or surgical resection of the tumor [8]. However, EUS imaging alone is not sufficiently accurate for diagnosing SETs [9] and large tissue samples are required to increase the accuracy of pathologic diagnosis [10]. For this reason, EUS-guided mass biopsies such as EUS-FNA and trucut biopsies (EUS-TCBs) are currently used. However, EUS-FNA has a relatively low diagnostic accuracy of just 62% and does not consistently provide information upon immunohistochemical staining [11,12]. While EUS-TCB does overcome some of the limitations of EUS-FNA, it too has a diagnostic accuracy of less than 60%. Linear-type EUS and EUS-FNA needles are required for EUS-guided biopsy; however, this technique has some limitations in cases of small SETs. Jumbo forceps biopsies and biteon- bite techniques are generally not able to provide sufficient tissue samples [13].
ROLE OF SIMPLE ENDOSCOPY IN THE DIAGNOSIS AND MANAGEMENT OF SET
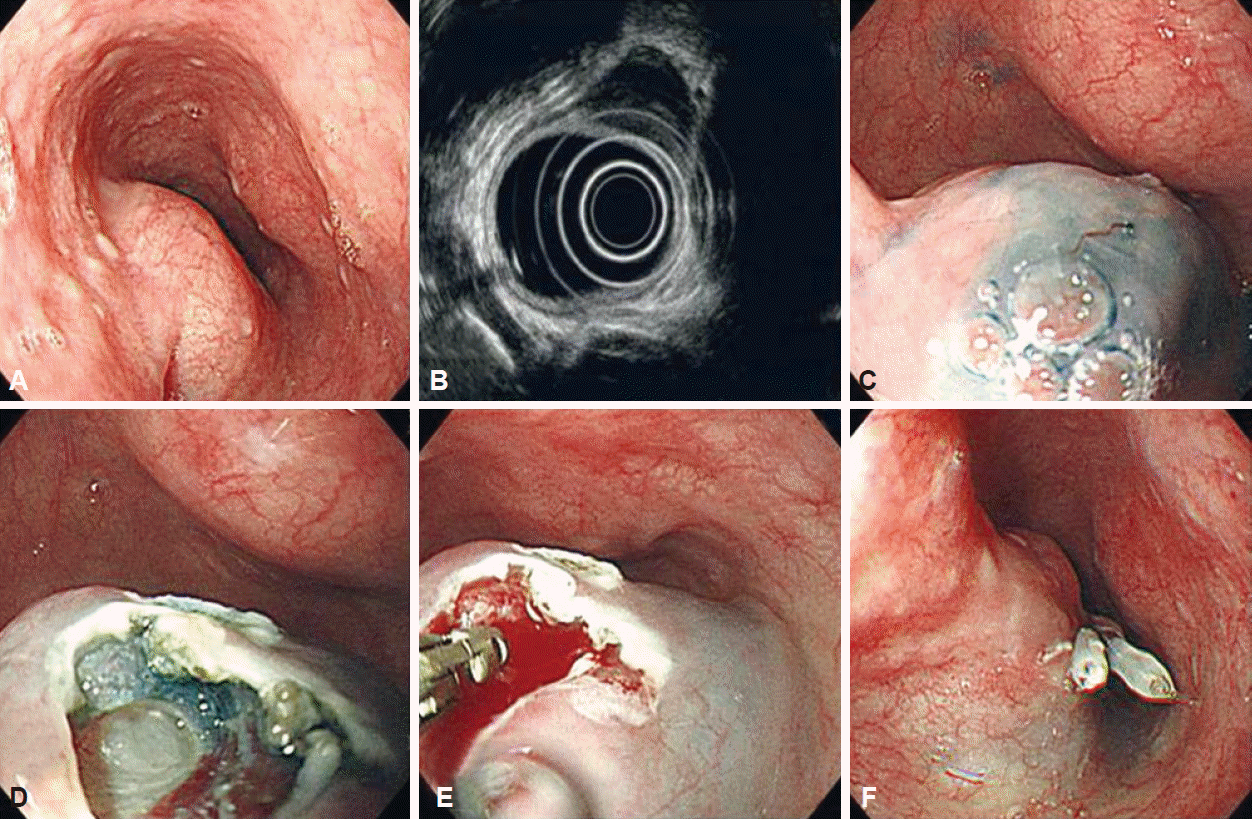
Deep biopsy procedure
Endoscopic biopsies of SETs were performed using a flex knife, IT2-knife, and a standard upper endoscope by a single, expert endoscopist. An epinephrine solution in hypertonic saline was injected into the submucosa, after which an approximately 0.6-cm incision was made using a flex knife. The IT2-knife was introduced through this site and an incision approximately 15 mm in diameter was made in the overlying mucosa. Next, minimal submucosal dissection was performed using the flex or IT2-kinife. After subepithelial mass detection, multiple biopsies were performed where a subepithelial lesion was detected beneath the submucosal layer. After the procedure, the iatrogenic incision was closed by clipping (Fig. 1).
Clinical features of upper GI SETs and deep biopsy results
A total of 52 patients who underwent EUS for upper gastrointestinal tract SET were pathologically diagnosed using a deep biopsy technique. Patients were included based on EUS examination and a lesion size >2 cm in diameter. Lesions were well-circumscribed masses originating in the muscularis propria or submucosal layer of the stomach.
Endoscopic histologic diagnosis of SETs was made after the ESD technique was performed. Of these patients, the mean age was 52.03ТБ13.35 years and 22 were male. The mean diameter of the SETs was 24.15ТБ6.0 mm and the diagnostic yield was 96.15%. Of the 52 SETs, 45 were located in the stomach, four in the esophagus, and three in the duodenum. Their pathologic diagnoses were as follows: 17 leiomyomas, 13 GISTs, 11 ectopic pancreases, two carcinomas, two inflammatory fibroid polyps, two Brunnerтs gland hyperplasias, two lipomas, and one glomus tumor; with two cases that remained undiagnosed. Cases were classified as benign or malignant and just 16 cases were found to be malignant or malignant- related lesions. The mean procedure time was 13.44ТБ2.41 minutes. There were three complications associated with this procedure. In the esophageal SET patient, a pneumomediastium developed after the procedure; however, the patientтs condition improved with conservative treatment. The glomus tumor patient exhibited major active bleeding during the procedure and endoscopic hemostasis was performed without issue. One patient presented with hematemesis 2 days after the procedure. A work-up revealed minimal bleeding at the procedure site that stopped naturally without further endoscopic hemostasis. Surgical resection was performed in eight cases and all other patients are undergoing regular follow-ups.
Prospective study comparing the endoscopy and surgery groups
We performed a prospective study to investigate the effectiveness of pathologic diagnosis of SETs. A total 68 patients with SETs in the upper gastrointestinal tract were assigned to one of two groups. The first group (40 patients) underwent EUS and endoscopic deep biopsy using the ESD technique and the second group (28 patients) underwent surgical resection after EUS without pathologic confirmation, in accordance with management algorithms. The results of deep biopsy caused a change to the treatment plans in 14 of 40 patients (35%). One patient with lymphoepithelial carcinoma was scheduled for surgical resection and 13 patients with benign SETs of diameters тЅ2 cm avoided surgery. Of the 28 patients who underwent surgical resection without pathologic confirmation, 12 (42.9%) were confirmed to have benign SETs [14]. Our research suggests that deep biopsy is a safe, highyield diagnostic method in patients with SETs. Pathologic diagnosis could improve clinical decision making in the management of patients with SETs and avoid unnecessary surgical resection.
CONCLUSIONS
Deep biopsy technique is a safe diagnostic method of high diagnostic yield when compared with EUS-guided biopsy for confirming the histopathologic diagnosis of SETs. Sufficient tissue sampling is possible using this method regardless of SET location and linear-type EUS is not required. In cases of SET, pathologic confirmation is more important than findings by EUS, as pathologic confirmation improves clinical decision making for the management of SETs. This diagnostic method should be considered for SETs before determining whether tumors should undergo long-term monitoring or surgical resection.