AbstractBackground/AimsFew studies have compared the performances of endoscopic knives. This study aimed to compare the therapeutic outcomes of a novel core knife and the conventional IT knife 2 for endoscopic submucosal dissection (ESD) of gastric mucosal lesions.
MethodsThis prospective, non-inferiority trial included patients diagnosed with gastric adenoma or early-stage adenocarcinoma at Keimyung University Dongsan Hospital between June and November 2020. The patients were randomly assigned to either the core knife or the IT knife 2 group. The operators and assistants scored the knivesŌĆÖ grip convenience and cutting abilities.
ResultsA total of 39 patients were enrolled (core knife group, 20 patients; IT knife 2 group, 19 patients). There were no significant between-group differences in operator-assessed grip convenience (9.600 vs. 9.526, p=0.753), cutting ability (9.600 vs. 9.105, p=0.158), or assistant-assessed grip convenience (9.500 vs. 9.368, p=0.574).
INTRODUCTIONGastric cancer is the fifth most commonly diagnosed malignancy and the fourth leading cause of cancer-related mortality worldwide.1 In South Korea, gastric cancer accounted for 7,624 deaths in 2019.2 The incidence of gastric cancer remains high owing to population growth and aging, making gastric cancer a major public health challenge. Therapeutic procedures and multidisciplinary approaches for gastric cancer have advanced in the last few decades. Endoscopic submucosal dissection (ESD) has become the standard treatment for en bloc and R0 resection regardless of the lesion size, not only for gastric adenoma but also for early-stage adenocarcinoma.3 However, ESD is a more time-consuming and technically demanding procedure than endoscopic mucosal resection (EMR). Furthermore, ESD has a slow learning curve; thus, endoscopic devices and methods have been modified.
A series of endoscopic knives with individual features has been developed to improve the procedural safety and efficiency of ESD. Among previous ESD instruments, insulated-tipped diathermic knives have been widely used.4-6 However, few studies have compared the performance of these endoscopic techniques. Thus, this study aimed to compare the therapeutic outcomes of a novel core knife (CORE-Knife, IC-KNA001; INCORE, Daegu, Korea) with that of a conventional second-generation insulated-tipped knife (IT knife 2 or ITknife2, KD-611L; Olympus, Tokyo, Japan) for the resection of gastric mucosal lesions.
METHODSStudy design and patientsThe sample size calculation indicated that a total of 35.6 patients were needed to perform a non-inferiority test with an alpha of 0.05, 80% power, and margin of 0.15. Considering a dropout rate of 10%, 40 patients newly diagnosed with gastric adenoma or early gastric cancer (EGC) at Keimyung University Dongsan Hospital, were recruited between June and November 2020. The inclusion criteria were as follows: (1) age 18ŌĆō80 years; (2) biopsy-confirmed gastric adenoma or early-stage adenocarcinoma located in the antrum; (3) mucosal lesion Ōēź2 cm without an ulcer; and (4) mucosal lesion Ōēż3 cm with an ulcer. The exclusion criteria were as follows: (1) advanced gastric cancer, (2) recent drug history of anticoagulant or antiplatelet agents within 7 days before the endoscopic procedure, and (3) recurrent gastric cancer after endoscopic or surgical treatment.
The patients were randomly allocated in a 1:1 ratio to either the core knife or IT knife 2 group using a random number table. The study coordinator performed a stratified randomization process using a table of random numbers. Baseline data, including age, sex, height, weight, underlying disease/medications, and American Society of Anesthesiologists (ASA) physical status, were retrieved from the medical records.
Endoscopic submucosal dissectionESD was performed by one of three endoscopists (YJL, JYL, and KBC) with more than 500 cases of experience using both the core knife and IT knife 2. The ESD protocol was as follows: first, an endoscope (GIF-H260 and GIF-Q260; Olympus, Tokyo, Japan) was inserted, the lesion area was thoroughly washed and examined, and the range and depth of the lesion were determined. With an approximately 5-mm margin from the rim of the lesion, the entire perimeter was marked using an electronic needle knife or argon plasma coagulation (forced APC, 20 W, flow 1.8 L/min; ERBE VIO 300D; ERBE, T├╝bingen, Germany). Saline mixed with 0.0025% epinephrine and tinted indigo carmine was injected at an area 2 to 3 mm from the markings to elevate the mucosal layer. The core knife or IT knife 2 was then used for the incision along the entire perimeter (Endocut Q Effect, 3; cut duration, 2; interval, 3). Using the ceramic hemisphere attached to both tools, an induction hole was made in a normal mucosal area 3ŌĆō5 mm away from the marked rim and apical side of the endoscope. Subsequently, a ceramic hemisphere was inserted for the mucosal incision, and the cut was made in a sliding motion. A local submucosal injection was administered to elevate the entire lesion. Next, the knife was placed on the side of the lesion, and submucosal dissection was performed parallel to the muscularis propria. The main electrosurgical unit settings were Endocut Q Effect 3, cut duration 2 to 3, and interval 2 to 3 for dissection and forced coagulation, effect 2, and 80 W for hemostasis of intraoperative hemorrhage with a hot biopsy. After completion of the submucosal dissection, visible vessels on the artificial ulcer base were coagulated using hemostatic forceps, and the site of the deep proper muscle injury was closed using clips (Figs. 1, 2).
Outcome measuresThe primary outcome measures were grip convenience (assessed by both the operator and the assistant) and cutting ability (assessed by the operator). The secondary outcome measures were procedure time, carbonization of the endoscopic knife, en bloc or complete resection rate (defined as an en bloc resection with negative margins), procedure-related complications, hemoglobin change 1 day after the procedure, and pathological results.
Grip convenience and cutting ability were scored on a 10-point visual analog scale, with a score of 0 indicating uncomfortable grip convenience and poor cutting ability and a score of 10 indicating comfortable grip convenience and good cutting ability. The total procedure time was determined as the sum of the time for marking and resection (time A) and hemostasis (time B). En bloc resection was defined as resection of the tumor in one piece, with no endoscopically residual tumor. Complete resection (R0) was defined as tumor resection with no histological evidence of cancer cells on the lateral or vertical margins. Carbonization of the knife tip was scored as grade IŌĆōIV.
Statistical analysisContinuous variables are presented as mean┬▒standard deviation and categorical variables as frequency and proportion. Normally distributed continuous variables were analyzed using the Student t-test. Categorical variables were analyzed using the chi-square test or Fisher exact test, as appropriate. All statistical analyses were performed using IBM SPSS ver. 25 (IBM Corp., Armonk, NY, USA). Statistical significance was set at a two-sided p-value<0.05.
Ethical statementsThis prospective, non-inferiority, randomized trial was approved by the Medical Ethics Committee of Keimyung University Dongsan Hospital (IRB No: 2020-04-076) and was conducted in compliance with the tenets of the Helsinki Declaration. Written informed consent was obtained from all patients. All patient records were anonymized prior to analysis.
RESULTSBaseline characteristicsAfter excluding one patient because the lesion was located close to a previous ESD scar, 39 patients (adenoma, 17; adenocarcinoma, 22) were randomly allocated to the core knife group (20 patients) or the IT knife 2 group (19 patients). Table 1 summarizes the demographic characteristics. The mean age was 63.8┬▒8.9 years, and 31 patients (79.5%) were male. The patientsŌĆÖ mean height and weight were 164.9┬▒8.8 cm and 67.0┬▒10.2 kg, respectively. Two patients in the core knife group and five patients in the IT knife 2 group were taking antiplatelet drugs. The medication was stopped at least 1 week prior to the endoscopic procedure. All patients had good performance status (ASA I, 29 [74.4%]; ASA II, 10 [25.6%]). Nine patients (23.1%) had hypertension, and five (12.8%) had diabetes mellitus. There were no significant differences in age, sex, height, weight, or underlying diseases between the two groups. All lesions were located in the gastric antrum: 14 lesser curvature (35.9%); 7 greater curvature (17.9%); 8 anterior wall (20.5%); and 10 posterior wall (25.6%). Surface erosion of the lesion was detected in three patients (15.0%) in the core knife group and in one patient (5.3%) in the IT knife 2 group. Atrophy was observed in 12 patients (60.0%) in the core group and 11 (57.9%) in the IT knife 2 group.
Assessment of the endoscopic knives
Table 2 and Figure 3 show the operator convenience and procedure times, respectively, between the two groups. There was no significant difference in the operator-assessed grip convenience score (9.600 vs. 9.526, p=0.753) or cutting ability score (9.600 vs. 9.105, p=0.158) between the core knife group and the IT knife 2 group. Similarly, the assistant-assessed grip convenience score was not significantly different between the two groups (9.500 vs. 9.368, p=0.574). Furthermore, although the total procedure time was longer in the core knife group, the difference was not significant (24.2 minutes vs. 19.3 minutes, p=0.278).
Complete resection was achieved in 39 patients. En bloc resection was performed in all but one patient in the core knife group who underwent piecemeal resection due to submucosal fibrosis. The core knife was replaced in one patient because of carbonization of the knife tip. However, the level of carbonization was not significantly different between the two groups (p=0.513).
Post-procedural complications and pathologyNo patient developed post-ESD complications such as hemorrhage or perforation (Table 3). There was no difference in the hemoglobin level changes between the groups on the day after the procedure (ŌĆō0.1 vs. 1.0 g/dL; p=0.962). For histology, adenoma was more common in the IT knife 2 group (52.6 vs. 35.0%; p=0.431), while adenocarcinoma was more common in the core knife group (65.0 vs. 47.4%; p=0.431), but the differences were not significant. There were also no significant between-group differences in terms of the size of the resected specimen, tumor size, or depth of invasion.
DISCUSSIONESD is widely used as a minimally invasive treatment for gastric mucosal lesions.3,7 First introduced in the 1990s, ESD has since been regarded as an established technique of endoscopic resection and is considered a curative option for the treatment of early neoplasia, with continuous increased use not only in Asia but also in the US and Europe, where ESD has been published in regional and national guidelines.6,8-10 In ESD of EGC, en bloc resection reduces the risk of residual cancer with a low probability of lymph node metastasis. Nevertheless, ESD has limitations such as a long operation time, difficulty in technical acquisition, and a relatively high incidence of postoperative complications such as hemorrhage and perforation.11 In terms of the apparatus types themselves, data on the performance of endoscopic knives are limited, as there have been few comparative studies.
ESD consists of two steps: (1) mucosal incision along the lesion perimeter and (2) submucosal dissection based on the lesionŌĆÖs elevation, which uniquely distinguishes ESD from EMR.5 However, despite significant utility in the marking of the first stage of the area surrounding the lesion in ESD, the previous tip-cutting needle knife has a serious drawback of increasing the risk of hemorrhage and perforation in submucosal dissection.12 Thus, the latest ESD method uses a specialized knife, with the most well-known example being the insulated-tipped knife.12 The prototype was developed in the 1990s to resolve the drawback of EMR; that is, complete resection would be possible only for small lesions. Hosokawa et al. reported the first therapeutic outcomes of this new method in 1995.4
The second-generation IT knife 2 (KD-611L; Olympus) is currently widely used.4,6,13,14 This standard knife has a 2.2-mm ceramic hemisphere insulator at the tip of the knife that prevents perforation and allows for circumferential incision and submucosal dissection. The hemispherical shape ensures submucosal dissection during resection while also reducing unintended tissue damage via attachment to the apical side. It also acts as a pivot upon movement in order to aid in the tilt or swing of the knife. Meanwhile, the core knife used in this study has an apical ceramic hemisphere structure similar to that of IT knife 2. The convex tip that comes in contact with the tissue is fully polished, not only acting as a guide during incision but also simultaneously preventing any unnecessary tissue damage during the procedure. The movement can be well maintained, even if the part being dissected is slightly outside the visual field.
Notably, difficulties can arise when the incision of lesions places the knife on a perpendicular side, while determining the direction of the incision would also be challenging in a horizontal incision in ESD.15 In the case of esophagogastric junction, fundus, or pyloric ring, where the incision is restricted almost vertically or horizontally, or in the case of gastric angles where the distance from the device to mucosa increases during air insufflation, it has been proven that the procedure time tends to be prolonged.16
Our findings showed a comparable result of convenience and effectiveness between the novel core knife and the conventional IT knife 2 in antral lesions. The novel core knife has a multi-step structure as the apical coil channel for enhanced hardness, through which a more refined resection focused on the necessary area is possible. Furthermore, this design can provide a high level of grip strength during ESD with a sufficient level of stability, allowing an easier approach in areas where the planes of the incisions may be perpendicular, as in the gastric cardia or upper body greater curvature, and enabling direct transfer of force exerted by the operator with a higher level of hardness on the axis.17-19 This feature can also be useful in cases requiring a stronger force to be exerted because of the longer distance between the endoscope and lesion.
This study had some limitations. The physicians who participated in the evaluation were experts with sufficient experience; no beginners were included. Considering the above-described characteristics of the apparatus, it should be considered that, in beginners, an uncontrolled force transmitted to the incision might cause damage to the deeper muscle layer. Furthermore, this study measured and reported the difference in the hardness of the knife axis based on the direct experience and opinions of the endoscopists. A quantitative study of the device characteristics via in vitro comparisons should be conducted.
In conclusion, the novel core knife achieved non-inferior results in the evaluation of grip convenience and cutting ability scales, took the equivalent treatment time, and demonstrated comparable therapeutic outcomes to the IT knife 2 in ESD of gastric mucosal lesions. Further investigations may provide a better knife choice for endoscopists who specialize in the ESD procedure.
NOTESFunding
This work was supported by the supporting project to evaluate new domestic medical devices in hospitals funded by the Korea Health Industry Development Institute (KHIDI) and INCORE Co. Ltd (Daegu, Korea).
Author Contributions
Conceptualization: KBC; Data curation: MP, DWS; Formal analysis: MP, DWS; Funding acquisition: KBC; Investigation: YJL, JYL, KBC; Methodology: JWL, DWS, JK, YJL, JYL, KBC; Project administration: KBC; Resources: KBC; Software: DWS; Supervision: KBC; Validation: DWS, KBC; Visualization: DWS; WritingŌĆōoriginal draft: MP; WritingŌĆōreview & editing: all authors.
Fig.┬Ā1.Knives used in endoscopic submucosal dissection. (A) Core knife (CORE-Knife, IC-KNA001; INCORE, Daegu, Korea). (B) IT knife 2 (IT knife2, KD-611L; Olympus). 
Fig.┬Ā2.Endoscopic submucosal dissection using a novel core knife. (A) Marking: the tip of the knife is used for marking the margin for resection. (B) Incision: an incision is made around the circumference of the lesion. (CŌĆōE) Submucosal dissection: the submucosa is dissected with care to avoid perforation. (F) Excised specimen. 
Fig.┬Ā3.Comparison of outcome measures between the two knives. (A) Operator and assistant convenience. (B) Procedure time (time A, time from marking to resection; time B, time spent on hemostasis; total procedure time=time A+time B). 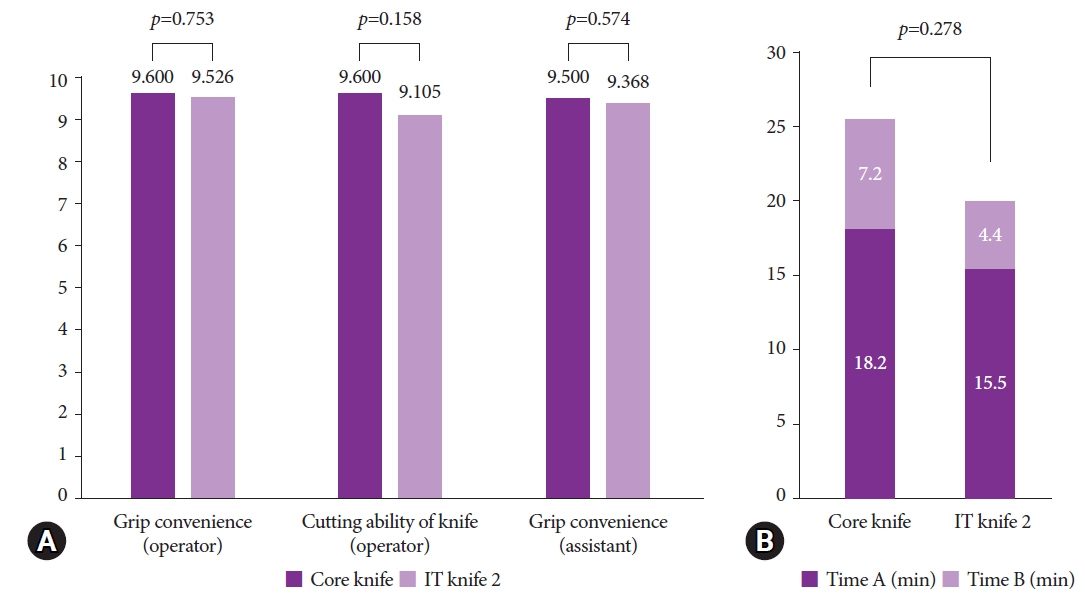
Table┬Ā1.Baseline patient characteristics Table┬Ā2.Comparison between procedure time and convenience Table┬Ā3.Comparison of pathology and complications following endoscopic submucosal resection REFERENCES1. Bray F, Ferlay J, Soerjomataram I, et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2018;68:394ŌĆō424.
2. Korean Statistical Information Service. Causes of Death Statistics in Korea 2019 [Internet]. Daejeon: Korean Statistical Information Service; 2020 [cited 2020 Sep 21]. Available from: http://kostat.go.kr/portal/korea/kor_nw/1/1/index.board?bmode=download&bSeq=&aSeq=385219&ord=2.
3. Park CH, Yang DH, Kim JW, et al. Clinical practice guideline for endoscopic resection of early gastrointestinal cancer. Clin Endosc 2020;53:142ŌĆō166.
4. Ko BM. History and development of accessories for endoscopic submucosal dissection. Clin Endosc 2017;50:219ŌĆō223.
5. Song JH, Kim SG. Endoscopic submucosal dissection for gastric neoplasia. In: Chun HJ, Yang SK, Choi MG, editors. Therapeutic gastrointestinal endoscopy: a comprehensive atlas. Singapore: Springer; 2019. p. 125ŌĆō141.
6. Sumiyama K, Tajiri H. History of ESD. In: Fukami N, editor. Endoscopic submucosal dissection: principles and practice. New York: Springer; 2015. p. 3ŌĆō8.
7. Gotoda T, Iwasaki M, Kusano C, et al. Endoscopic resection of early gastric cancer treated by guideline and expanded National Cancer Centre criteria. Br J Surg 2010;97:868ŌĆō871.
8. Kamiya S, Rouvelas I, Lindblad M, et al. Current trends in gastric cancer treatment in Europe. J Cancer Metastasis Treat 2018;4:35.
9. Draganov PV, Wang AY, Othman MO, et al. AGA Institute Clinical Practice Update: endoscopic submucosal dissection in the United States. Clin Gastroenterol Hepatol 2019;17:16ŌĆō25.
10. Fukami N. Endoscopic submucosal dissection: principles and practice. New York: Springer; 2015.
11. Oda I, Suzuki H, Nonaka S, et al. Complications of gastric endoscopic submucosal dissection. Dig Endosc 2013;25(Suppl 1):71ŌĆō78.
12. Choi HS, Chun HJ. Accessory devices frequently used for endoscopic submucosal dissection. Clin Endosc 2017;50:224ŌĆō233.
13. Fukami N. Appendix: commonly used ESD knives. In: Fukami N, editor. Endoscopic submucosal dissection: principles and practice. New York: Springer; 2015. p. 257ŌĆō260.
14. Hosokawa K, Yoshida S. Recent advances in endoscopic mucosal resection for early gastric cancer. Gan To Kagaku Ryoho 1998;25:476ŌĆō483.
15. Cho JY, Cho WY. The current status of endoscopic submucosal dissection. Korean J Gastrointest Endosc 2008;37:317ŌĆō320.
16. Konuma H, Matsumoto K, Ueyama H, et al. Procedure time for gastric endoscopic submucosal dissection according to location, considering both mucosal circumferential incision and submucosal dissection. Gastroenterol Res Pract 2016;2016:9183793.
17. Kim KO, Kim SJ, Kim TH, et al. Do you have what it takes for challenging endoscopic submucosal dissection cases? World J Gastroenterol 2011;17:3580ŌĆō3584.
18. Lee SG, Cho KB, Hong YS, et al. Nonsurgical treatment of gastric perforation complicated by endoscopic mucosal resection and endoscopic submucosal dissection. Korean J Gastrointest Endosc 2008;37:97ŌĆō104.
19. Park YS, Park SW, Song SY, et al. Endoscopic mucosal resection using insulated-tip electrosurgical knife. Korean J Gastrointest Endosc 2003;26:397ŌĆō404.
|
|
|||||||||||||||||||||||||||||||||||||
