Previous issues
- Page Path
- HOME > Browse Articles > Previous issues
Commentarys
- Unfortunately, a “Back Light System” As a Global Positioning System Failed to Guide the Route in 25-G Fine-Needle Aspiration
- Rungsun Rerknimitr, Phonthep Angsuwatcharakon
- Clin Endosc 2019;52(4):295-296. Published online July 30, 2019
- DOI: https://doi.org/10.5946/ce.2019.104
-
 PDF
PDF PubReader
PubReader ePub
ePub -
Citations
Citations to this article as recorded by- The Association of “GOOP” on Gross Examination of Fine Needle Aspiration Samples and On-Site Adequacy
Nikhil Meena, Thaddeus Bartter, Roshen Mathew, Abhishek Kumar, Winnie Elma Roy, Sunil Kumar Kakadia, Maggie Machiarella
Respiration.2022; 101(1): 63. CrossRef
- The Association of “GOOP” on Gross Examination of Fine Needle Aspiration Samples and On-Site Adequacy
- 3,841 View
- 66 Download
- 1 Web of Science
- 1 Crossref

- Endoscopic Ultrasound-Guided Liver Biopsies: Is the Future Here Yet?
- Ihab I. El Hajj, Mohammad Al-Haddad
- Clin Endosc 2019;52(4):297-298. Published online July 23, 2019
- DOI: https://doi.org/10.5946/ce.2019.126
- 3,976 View
- 80 Download

- Endoscopic Ultrasound-Guided Drainage of Peripancreatic Fluid Collections
- Eun Young Kim, Robert H. Hawes
- Clin Endosc 2019;52(4):299-300. Published online July 30, 2019
- DOI: https://doi.org/10.5946/ce.2019.135
- 4,176 View
- 69 Download

Focused Review Series: Recent Update of Endoscopic Ultrasonographys in Gastrointestinal Subepithelial Tumors
- Current Status of Endoscopic Ultrasonography in Gastrointestinal Subepithelial Tumors
- Sang Gyun Kim, Ji Hyun Song, Joo Ha Hwang
- Clin Endosc 2019;52(4):301-305. Published online July 9, 2019
- DOI: https://doi.org/10.5946/ce.2019.024

-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader ePub
ePub - Gastrointestinal subepithelial tumors (GSTs) are usually detected incidentally on endoscopic or radiologic examinations. In conventional endoscopy, a GST usually presents as a protuberant lesion with an intact mucosal surface. As the lesion is located beneath the mucosal layer of the gastrointestinal tract, conventional biopsy typically does not reveal the pathologic diagnosis. First, a GST should be differentiated from an extrinsic compression through the positional change of the patient during conventional endoscopic examination. In cases of GSTs originating from the gastrointestinal wall, endoscopic ultrasonography (EUS) can be beneficial for narrowing the differential diagnosis through delineation of echo findings and by determining the layer of origin. EUS findings can also help determine the management strategies for GSTs by making a differential diagnosis according to malignant potential.
-
Citations
Citations to this article as recorded by- Endoscopic Resection of Upper Gastrointestinal Subepithelial Tumours: Our Clinical Experience and Results
Mehmet Zeki Buldanlı, Oktay Yener
Prague Medical Report.2022; 123(1): 20. CrossRef - Gastric subepithelial tumor: long-term natural history and risk factors for progression
Bokyung Kim, Seungkyung Kang, Eunwoo Lee, Jinju Choi, Hyunsoo Chung, Soo-Jeong Cho, Sang Gyun Kim
Surgical Endoscopy.2022; 36(7): 5232. CrossRef - Traumatic neuroma of remnant cystic duct mimicking duodenal subepithelial tumor: A case report
Dong-Hwan Kim, Ji-Ho Park, Jin-Kyu Cho, Jung-Wook Yang, Tae-Han Kim, Sang-Ho Jeong, Young-Hye Kim, Young- Joon Lee, Soon-Chan Hong, Eun-Jung Jung, Young-Tae Ju, Chi-Young Jeong, Ju-Yeon Kim
World Journal of Clinical Cases.2020; 8(17): 3821. CrossRef
- Endoscopic Resection of Upper Gastrointestinal Subepithelial Tumours: Our Clinical Experience and Results
- 5,692 View
- 228 Download
- 2 Web of Science
- 3 Crossref

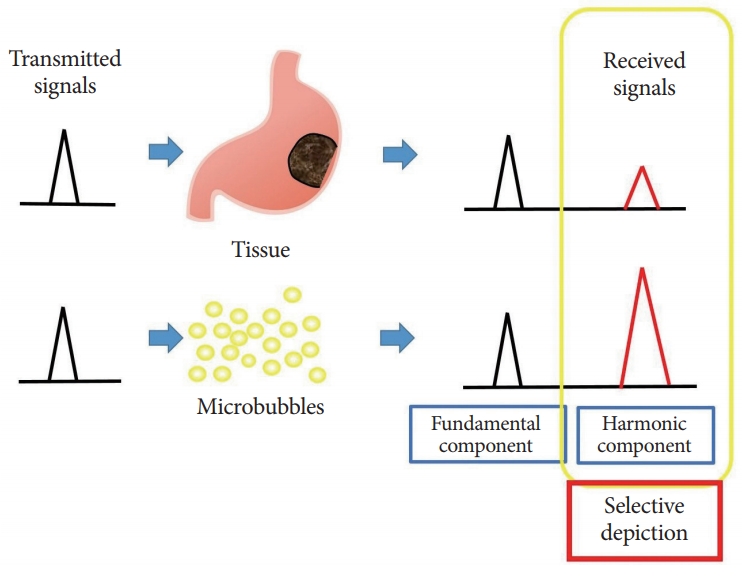
- Contrast Enhanced Endoscopic Ultrasound Imaging for Gastrointestinal Subepithelial Tumors
- Takashi Tamura, Masayuki Kitano
- Clin Endosc 2019;52(4):306-313. Published online July 23, 2019
- DOI: https://doi.org/10.5946/ce.2019.056
-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader ePub
ePub - Subepithelial tumors are divided into benign subepithelial and potentially malignant gastrointestinal stromal tumors. It is difficult to distinguish between these tumor types. Contrast-enhanced harmonic endoscopic ultrasound is reportedly useful for diagnosing subepithelial tumors, can be safely and easily performed by understanding the principle and method, and can be used to distinguish between tumor types with high sensitivity on the basis of differences in contrast effect. The generated image shows a hyperenhancement pattern in gastrointestinal stromal tumors (sensitivity, 78%–100%; specificity, 60%–100%; accuracy, 60%–100%) and hypoenhancement pattern in benign subepithelial tumors. Contrast-enhanced harmonic endoscopic ultrasound can be used to estimate the malignancy potential of gastrointestinal stromal tumors by evaluating the uniformity of the contrast and the blood vessels inside the tumor, with abnormal intra-tumor blood vessels, heterogeneous enhancement, and non-enhancing spots suggesting malignancy. Contrast-enhanced harmonic endoscopic ultrasound has a higher sensitivity than other imaging modalities for the detection of vascularity within gastrointestinal stromal tumors. Additionally, it has been reported that treatment effects can be estimated by evaluating the blood flow in the gastrointestinal stromal tumor before and after treatment with tyrosine kinase inhibitors using contrastenhanced ultrasound. However, there will be subjective-bias and the results depends on the performer’s skill.
-
Citations
Citations to this article as recorded by- The value of contrast-enhanced harmonic endoscopic ultrasound in differential diagnosis and evaluation of malignant risk of gastrointestinal stromal tumors (<50mm)
Jiali Wu, Mengqi Zhuang, Yubao Zhou, Xiang Zhan, Weiwei Xie
Scandinavian Journal of Gastroenterology.2023; 58(5): 542. CrossRef - Endoscopic Ultrasound Advanced Techniques for Diagnosis of Gastrointestinal Stromal Tumours
Socrate Pallio, Stefano Francesco Crinò, Marcello Maida, Emanuele Sinagra, Vincenzo Francesco Tripodi, Antonio Facciorusso, Andrew Ofosu, Maria Cristina Conti Bellocchi, Endrit Shahini, Giuseppinella Melita
Cancers.2023; 15(4): 1285. CrossRef - EUS-Guided Diagnosis of Gastric Subepithelial Lesions, What Is New?
Thomas Vasilakis, Dimitrios Ziogas, Georgios Tziatzios, Paraskevas Gkolfakis, Eleni Koukoulioti, Christina Kapizioni, Konstantinos Triantafyllou, Antonio Facciorusso, Ioannis S. Papanikolaou
Diagnostics.2023; 13(13): 2176. CrossRef - Rapidly Growing, High-Risk Gastrointestinal Stromal Tumor of the Stomach: A Case Report
Sung Jin Lim, Han Mo Yoo, Seung-Woo Lee, Hae Joung Sul, Dong Soo Lee
The Korean Journal of Helicobacter and Upper Gastrointestinal Research.2023; 23(4): 306. CrossRef - The value of color Doppler ultrasonography combined with serum tumor markers in differential diagnosis of gastric stromal tumor and gastric cancer
Xinyu Cheng, Jianguo Xia, Qi Xu, Huawei Gui
Open Medicine.2023;[Epub] CrossRef - Ultrasound imaging of subepithelial rectal tumors (review)
Y. L. Trubacheva, E. M. Bogdanova, A. E. Pershina
Koloproktologia.2022; 21(1): 107. CrossRef - The Asian Federation of Societies for Ultrasound in Medicine and Biology (AFSUMB) Guidelines for Contrast-Enhanced Endoscopic Ultrasound
Masayuki Kitano, Yasunobu Yamashita, Ken Kamata, Tiing Leong Ang, Hiroo Imazu, Eizaburo Ohno, Yoshiki Hirooka, Pietro Fusaroli, Dong-Wan Seo, Bertrand Napoléon, Anthony Yuen Bun Teoh, Tae Hyeon Kim, Christoph F. Dietrich, Hsiu-Po Wang, Masatoshi Kudo
Ultrasound in Medicine & Biology.2021; 47(6): 1433. CrossRef - Efficacy and Safety of Endoscopic Treatment for Gastrointestinal Stromal Tumors in the Upper Gastrointestinal Tract
Cicilia Marcella, Shakeel Sarwar, Hui Ye, Rui Hua Shi
Clinical Endoscopy.2020; 53(4): 458. CrossRef - Contrast Harmonic-Enhanced Endoscopic Ultrasound (EUS) Is the Perfect Companion of EUS-Guided Tumor Ablation
Gianmarco Marocchi, Andrea Lisotti, Pietro Fusaroli
Gut and Liver.2020; 14(5): 669. CrossRef
- The value of contrast-enhanced harmonic endoscopic ultrasound in differential diagnosis and evaluation of malignant risk of gastrointestinal stromal tumors (<50mm)
- 7,249 View
- 184 Download
- 7 Web of Science
- 9 Crossref

- Endoscopic Ultrasound-Guided Fine Needle Aspiration and Biopsy in Gastrointestinal Subepithelial Tumors
- Gyu Young Pih, Do Hoon Kim
- Clin Endosc 2019;52(4):314-320. Published online July 30, 2019
- DOI: https://doi.org/10.5946/ce.2019.100
-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader ePub
ePub - The incidence of asymptomatic and incidentally found upper gastrointestinal subepithelial tumors (SETs) is increasing with the implementation of national cancer screening and the development of high-resolution endoscopy in Korea. However, endoscopy alone cannot be used to determine whether SETs are benign or malignant. Endoscopic ultrasound (EUS) is used to further characterize these lesions through the examination of their layered structure, internal echogenicity, size, and relationship to the extramural structure. These provide additional information on whether the lesion is benign or malignant. Nevertheless, the sensitivity and specificity of EUS alone in predicting malignancy is unsatisfactory. Recent guidelines have recommended deciding the timing of EUS-fine needle aspiration and biopsy (EUS-FNA/B) for SETs based on tumor size, malignant features on endoscopy, and high-risk features on EUS. The diagnostic accuracy of EUS-FNA/B is reportedly influenced by factors including needle size, number of needle passes, use of suction, use of a stylet in the needle assembly, fanning technique, availability of an on-site cytopathologist, and experience of the endosonographer. Therefore, according to the characteristics of the SETs, various subsequent methods and techniques should be appropriately employed to improve the diagnostic yield of EUS-FNA/B.
-
Citations
Citations to this article as recorded by- Outcomes of Endoscopic Ultrasound-guided Fine Needle Biopsy Using a Novel Hydrostatic Stylet Tissue Acquisition Technique
Patrick T. Magahis, Donevan Westerveld, Malorie Simons, David L. Carr-Locke, Kartik Sampath, Reem Z. Sharaiha, SriHari Mahadev
Journal of Clinical Gastroenterology.2024; 58(4): 407. CrossRef - The role of endoscopic ultrasound in assessment of physiological cardia insufficiency during diagnosis of hiatal hernia
B.F. Shevchenko, O.M. Babii, N.V. Prolom, M.V. Titova, S.O. Tarabarov, S.V. Ushchina
GASTROENTEROLOGY.2024; 58(1): 50. CrossRef - Spectrum of endoscopic gastric subepithelial lesions encountered on EUS-FNA: A single center experience
Poojan Agarwal, Pooja Bakshi, Kusum Verma, Vikas Singla, Anil Arora
Indian Journal of Pathology and Microbiology.2024; 67(2): 374. CrossRef - Ultrasound-Enhanced Fine-Needle Biopsy Improves Yield in Human Epithelial and Lymphoid Tissue
Yohann Le Bourlout, Minna Rehell, Jetta Kelppe, Jaana Rautava, Emanuele Perra, Jouni Rantanen, Gösta Ehnholm, Nick Hayward, Kristofer Nyman, Kenneth P.H. Pritzker, Jussi Tarkkanen, Timo Atula, Katri Aro, Heikki J. Nieminen
Ultrasound in Medicine & Biology.2024;[Epub] CrossRef - Endoscopic Mucosal Resection of Pancreatic Rest Presenting as a Sub-epithelial Nodule in the Gastric Antrum
Janak Bahirwani, Rodrigo Duarte-Chavez, Lisa Stoll, Ayaz Matin
Cureus.2023;[Epub] CrossRef - Lesiones subepiteliales gástricas únicas. ¿Existen factores predictores de tumores del estroma gastrointestinal que eviten la biopsia?
José Ruiz Pardo, Elisabet Vidaña Márquez, Pedro Antonio Sánchez Fuentes, Iñigo Gorostiaga Altuna, Ricardo Belda Lozano, Ángel Reina Duarte
Gastroenterología y Hepatología.2023; 46(1): 54. CrossRef - Single gastric subepithelial lesions. Are there predictors of gastrointestinal stromal tumors that prevent biopsy?
José Ruiz Pardo, Elisabet Vidaña Márquez, Pedro Antonio Sánchez Fuentes, Iñigo Gorostiaga Altuna, Ricardo Belda Lozano, Ángel Reina Duarte
Gastroenterología y Hepatología (English Edition).2023; 46(1): 54. CrossRef - Endoscopic Submucosal Dissection for Subepithelial Tumor Treatment in the Upper Digestive Tract: A Western, Multicenter Study
Raffaele Manta, Francesco Paolo Zito, Francesco Pugliese, Angelo Caruso, Santi Mangiafico, Alessandra D’Alessandro, Danilo Castellani, Ugo Germani, Massimiliano Mutignani, Rita Luisa Conigliaro, Luca Reggiani Bonetti, Takahisa Matsuda, Vincenzo De Frances
GE - Portuguese Journal of Gastroenterology.2023; 30(2): 115. CrossRef - Endoscopic ultrasonography in diagnosis of digestive diseases. Review of clinical cases
Yu.M. Stepanov, N.V. Prolom, S.O. Tarabarov, M.V. Titova, I.M. Adamska, O.V. Zeleniuk
GASTROENTEROLOGY.2023; 57(4): 234. CrossRef - A Novel Biopsy Method Based on Bipolar Radiofrequency Biopsy Needles
Huiyang Wang, Haiwei Bao, Lan Yue, Tian’an Jiang
Frontiers in Oncology.2022;[Epub] CrossRef - Diagnostic ability of EUS-FNB with a novel fork-tip needle for upper gastrointestinal subepithelial tumors
Kei Ushikubo, Yuto Shimamura, Mai Fukuda, Raina Fujiyoshi, Hiroyuki Watanabe, Yuusuke Fujiyoshi, Jin Tanaka, Yohei Nishikawa, Haruo Ikeda, Manabu Onimaru, Haruhiro Inoue
Progress of Digestive Endoscopy.2022; 100(1): 67. CrossRef - Underwater endoscopic mucosal resection of upper gastrointestinal subepithelial tumors: A case series pilot study (with video)
Su Jin Kim, Tae Un Kim, Cheol Woong Choi, Hyung Wook Kim, Su Bum Park, Dae Gon Ryu
Medicine.2022; 101(41): e31072. CrossRef - AGA Clinical Practice Update on Management of Subepithelial Lesions Encountered During Routine Endoscopy: Expert Review
Kaveh Sharzehi, Amrita Sethi, Thomas Savides
Clinical Gastroenterology and Hepatology.2022; 20(11): 2435. CrossRef - Comparison of diagnostic performances of slow-pull suction and standard suction in endoscopic ultrasound-guided fine needle biopsy for gastrointestinal subepithelial tumors
Joon Seop Lee, Chang Min Cho, Yong Hwan Kwon, An Na Seo, Han Ik Bae, Man-Hoon Han
Clinical Endoscopy.2022; 55(5): 637. CrossRef - The role of endoscopic ultrasound investigation in the diagnosis of submucosal neoplasms of the stomach and duodenum (literature review and our clinical observations)
Yu.M. Stepanov, N.V. Prolom, I.S. Konenko, S.O. Tarabarov, N.P. Dementii, I.M. Adamska
GASTROENTEROLOGY.2022; 55(4): 270. CrossRef - Peroral endoscopic tumor resection (POET) with preserved mucosa technique for management of upper gastrointestinal tract subepithelial tumors
Chen-Shuan Chung, Kuo-Hsin Chen, Kuan-Chih Chen, Chiung-Yu Chen, Tzong-Hsi Lee, Cheng-Kuan Lin, Jiann-Ming Wu
Surgical Endoscopy.2021; 35(7): 3753. CrossRef - Gastric Angiolipoma Resected with Endoscopic Submucosal Dissection
Sang Myung Yeo, Jae Kwang Lee, Hyun Soo Kim, Chang Geun Park, Jae Kwon Jung, Dae Jin Kim, Yun Jin Chung, Han Jun Ryu
Clinical Endoscopy.2021; 54(3): 432. CrossRef - Subepithelial Tumor-like Gastric Cancer
Kyoungwon Jung, Moo In Park
The Korean Journal of Helicobacter and Upper Gastrointestinal Research.2021; 21(2): 106. CrossRef - Convolutional neural network‐based object detection model to identify gastrointestinal stromal tumors in endoscopic ultrasound images
Chang Kyo Oh, Taewan Kim, Yu Kyung Cho, Dae Young Cheung, Bo‐In Lee, Young‐Seok Cho, Jin Il Kim, Myung‐Gyu Choi, Han Hee Lee, Seungchul Lee
Journal of Gastroenterology and Hepatology.2021; 36(12): 3387. CrossRef - Fine needle aspiration cytology of primary and metastatic gastrointestinal stromal tumour
Gargi Kapatia, Nalini Gupta, Uma Nahar Saikia, Parikshaa Gupta, Manish Rohilla, Ojas Gupta, Radhika Srinivasan, Arvind Rajwanshi, Pranab Dey
Cytopathology.2020; 31(2): 136. CrossRef - Thoracoscopic surgery combined with endoscopic creation of a submucosal tunnel for a large complicated esophageal leiomyoma
Koki Oyama, Kenoki Ohuchida, Koji Shindo, Taiki Moriyama, Yoshitaka Hata, Masafumi Wada, Eikichi Ihara, Shuntaro Nagai, Takao Ohtsuka, Masafumi Nakamura
Surgical Case Reports.2020;[Epub] CrossRef - Mucosal Incision-Assisted Endoscopic Biopsy as an Alternative to Endoscopic Ultrasound-Guided Fine-Needle Aspiration/Biopsy for Gastric Subepithelial Tumor
Cheol Woong Choi, Joo Ha Hwang
Clinical Endoscopy.2020; 53(5): 505. CrossRef - Endoscopic diagnosis and management of gastric subepithelial lesions
Thomas R. McCarty, Marvin Ryou
Current Opinion in Gastroenterology.2020; 36(6): 530. CrossRef - Endoscopic submucosal dissection as alternative to surgery for complicated gastric heterotopic pancreas
Jin Hee Noh, Do Hoon Kim, So-Woon Kim, Young Soo Park, Hee Kyong Na, Ji Yong Ahn, Kee Wook Jung, Jeong Hoon Lee, Kee Don Choi, Ho June Song, Gin Hyug Lee, Hwoon-Yong Jung
World Journal of Clinical Cases.2020; 8(20): 4708. CrossRef
- Outcomes of Endoscopic Ultrasound-guided Fine Needle Biopsy Using a Novel Hydrostatic Stylet Tissue Acquisition Technique
- 6,972 View
- 185 Download
- 17 Web of Science
- 24 Crossref

Reviews
- Assessment of Endoscopic Gastric Atrophy according to the Kimura-Takemoto Classification and Its Potential Application in Daily Practice
- Duc Trong Quach, Toru Hiyama
- Clin Endosc 2019;52(4):321-327. Published online July 22, 2019
- DOI: https://doi.org/10.5946/ce.2019.072

-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader ePub
ePub - The assessment of endoscopic gastric atrophy (EGA) according to the Kimura-Takemoto classification has been reported to correlate well with histological assessment. Although agreement among beginner endoscopists was less than that among experienced endoscopists, it has been shown that agreement level could markedly improve and remained stable after proper training. Several cohort studies have consistently shown that the severity of EGA at baseline is significantly associated with the presence of advanced precancerous gastric lesions and gastric cancer, as well as the development of gastric cancer in future. Patients with moderate-to-severe EGA still have high risk of gastric cancer even after successful Helicobacter pylori eradication and should be candidates for gastric cancer surveillance. The assessment of EGA, therefore, could be used as a preliminary tool to identify individuals at high risk for gastric cancer. In this paper, we review the agreement on mucosal atrophy assessment between the Kimura-Takemoto classification and histology as well as the potential application of this endoscopic classification to identify precancerous gastric lesions and gastric cancer in daily practice.
-
Citations
Citations to this article as recorded by- Endoscopic diagnosis and prevalence of early gastric cancer in India: A prospective study
Ashutosh Mohapatra, Sonmoon Mohapatra, Shruti Mahawar, Krushna Chandra Pani, Nachiketa Mohapatra, Mohan Ramchandani, Nageshwar Reddy, Mahesh K. Goenka, Noriya Uedo
DEN Open.2024;[Epub] CrossRef - Clinical and morphological characteristics of patients with chronic gastritis and high risk of gastric cancer
A. S. Tertychnyy, D. D. Protsenko, N. V. Pachuashvili, D. P. Nagornaya, P. V. Pavlov, A. P. Kiruhin, A. A. Fedorenko
Experimental and Clinical Gastroenterology.2024; (9): 107. CrossRef - Comparison between the GastroPanel test and the serum pepsinogen assay interpreted with the ABC method—A prospective study
Sun‐Young Lee, Yeon‐Sun Ahn, Hee‐Won Moon
Helicobacter.2024;[Epub] CrossRef - The value of LCI-based modified Kyoto classification risk scoring system in predicting the risk of early gastric cancer
Chao Gao, Guanpo Zhang, Jin Zheng, Yunmeng Zheng, Wulian Lin, Guilin Xu, Yixiang You, Dazhou Li, Wen Wang
Scandinavian Journal of Gastroenterology.2024; : 1. CrossRef - Identification of serum microRNAs as potential diagnostic biomarkers for detecting precancerous lesions of gastric cancer
Hajime Otsu, Sho Nambara, Qingjiang Hu, Yuichi Hisamatsu, Takeo Toshima, Kazuki Takeishi, Yusuke Yonemura, Takaaki Masuda, Eiji Oki, Koshi Mimori
Annals of Gastroenterological Surgery.2023; 7(1): 63. CrossRef - Evolution of the Correa's cascade steps: A long-term endoscopic surveillance among non-ulcer dyspepsia and gastric ulcer after H. pylori eradication
Hsiu-Chi Cheng, Yao-Jong Yang, Hsiao-Bai Yang, Yu-Ching Tsai, Wei-Lun Chang, Chung-Tai Wu, Hsin-Yu Kuo, Yu-Ting Yu, Er-Hsiang Yang, Wei-Chun Cheng, Wei-Ying Chen, Bor-Shyang Sheu
Journal of the Formosan Medical Association.2023; 122(5): 400. CrossRef - Vietnam Association of Gastroenterology (VNAGE) consensus on the management of Helicobacter pylori infection
Duc Trong Quach, Bang Hong Mai, Mien Kieu Tran, Long Van Dao, Huy Van Tran, Khanh Truong Vu, Khien Van Vu, Ho Thi-Thu Pham, Hoang Huu Bui, Dung Dang-Quy Ho, Dung Tuan Trinh, Vinh Thuy Nguyen, Thai Hong Duong, Tuong Thi-Khanh Tran, Ha Thi-Viet Nguyen, Thin
Frontiers in Medicine.2023;[Epub] CrossRef - Endoscopic Screening for Missed Lesions of Synchronous Multiple Early Gastric Cancer during Endoscopic Submucosal Dissection
Jiangnan Wan, Yi Fang, Haizhong Jiang, Bujiang Wang, Lei Xu, Chunjiu Hu, Honghui Chen, Xiaoyun Ding, Tatsuya Toyokawa
Gastroenterology Research and Practice.2023; 2023: 1. CrossRef - Morphometric features of gastric mucosa in atrophic gastritis: A different pattern between corpus and antrum
Xue-Mei Lin, Li Wang, Chun-Hui Xi, Jun Wang, Xian-Fei Wang, Qiong Wang, Cong Yuan
Medicine.2023; 102(14): e33480. CrossRef - Predicting reflux symptom recurrence: The impact of gastroesophageal junction indicators and body mass index among outpatients
Qing Wang, Junhui Lu, Yue Sui, Jing Fan, Jinnan Ren, Zhenzhen Wang, Xing Chen
Experimental and Therapeutic Medicine.2023;[Epub] CrossRef -
Helicobacter pylori intragastric colonization and migration: Endoscopic manifestations and potential mechanisms
Tong Mu, Zhi-Ming Lu, Wen-Wen Wang, Hua Feng, Yan Jin, Qian Ding, Li-Fen Wang
World Journal of Gastroenterology.2023; 29(30): 4616. CrossRef - Factors associated with heterochronic gastric cancer development post-endoscopic mucosal dissection in early gastric cancer patients
Bing Xie, Yun Xia, Xia Wang, Yan Xiong, Shao-Bo Chen, Jie Zhang, Wei-Wei He
World Journal of Gastrointestinal Oncology.2023; 15(9): 1644. CrossRef - Kimura–Takemoto Classification: A Tool to Predict Gastric Intestinal Metaplasia Progression to Advanced Gastric Neoplasia
Leyla Maric, Daniel Castaneda, Harjinder Singh, Pablo Bejarano, Brenda Jimenez Cantisano, Fernando J. Castro
Digestive Diseases and Sciences.2022; 67(8): 4092. CrossRef - Consistency between the endoscopic Kyoto classification and pathological updated Sydney system for gastritis: A cross‐sectional study
Osamu Toyoshima, Toshihiro Nishizawa, Shuntaro Yoshida, Tatsuya Matsuno, Nariaki Odawara, Akira Toyoshima, Kosuke Sakitani, Hidenobu Watanabe, Mitsuhiro Fujishiro, Hidekazu Suzuki
Journal of Gastroenterology and Hepatology.2022; 37(2): 291. CrossRef - Diagnostic Accuracy of H. pylori Status by Conventional Endoscopy: Time-Trend Change After Eradication and Impact of Endoscopic Image Quality
Duc Trong Quach, Rika Aoki, Akiko Iga, Quang Dinh Le, Toru Kawamura, Ken Yamashita, Shinji Tanaka, Masaharu Yoshihara, Toru Hiyama
Frontiers in Medicine.2022;[Epub] CrossRef - Relevance of pepsinogen, gastrin, and endoscopic atrophy in the diagnosis of autoimmune gastritis
Hiroshi Kishikawa, Kenji Nakamura, Keisuke Ojiro, Tadashi Katayama, Kyoko Arahata, Sakiko Takarabe, Aya Sasaki, Soichiro Miura, Yukie Hayashi, Hitomi Hoshi, Takanori Kanai, Jiro Nishida
Scientific Reports.2022;[Epub] CrossRef - Tauroursodeoxycholic Acid Inhibits Nuclear Factor Kappa B Signaling in Gastric Epithelial Cells and Ameliorates Gastric Mucosal Damage in Mice
Su Hwan Kim, Ji Won Kim, Seong-Joon Koh, Sang Gyun Kim, Jeong Mo Bae, Jung Ho Kim, Jeong Hwan Park, Mee Soo Chang, Kee Don Choi, Hyoun Woo Kang, Byeong Gwan Kim, Kook Lae Lee
The Korean Journal of Gastroenterology.2022; 79(4): 161. CrossRef - Serum pepsinogen: A potential non-invasive screening method for moderate and severe atrophic gastritis among an asian population
Cong Long Nguyen, Tran Tien Dao, Thi-Thuy Ngan Phi, The Phuong Nguyen, Van Tuyen Pham, Truong Khanh Vu
Annals of Medicine and Surgery.2022; 78: 103844. CrossRef - Risk factors for early gastric cancer: focus on Helicobacter pylori gastritis
Hee Seok Moon
Journal of the Korean Medical Association.2022; 65(5): 259. CrossRef - Current status of the gastric cancer screening program in Korea
Young-Il Kim, Il Ju Choi
Journal of the Korean Medical Association.2022; 65(5): 250. CrossRef - Endoscopic diagnosis of early gastric cancer
Dong Chan Joo, Gwang Ha Kim
Journal of the Korean Medical Association.2022; 65(5): 267. CrossRef - Current Evidence for a Paradigm Shift in Gastric Cancer Prevention From Endoscopic Screening toHelicobacter pyloriEradication in Korea
Young-Il Kim, Il Ju Choi
Journal of Gastric Cancer.2022; 22(3): 169. CrossRef - Need for improvement in the evaluation of pre‐malignant upper gastro‐intestinal lesions in India: Results of a nationwide survey
Deepak Madhu, Veeraraghavan Krishnamurthy, Thirumoorthi Natarajan, Sundeep Lakhtakia
Journal of Gastroenterology and Hepatology.2022; 37(11): 2113. CrossRef - Usefulness of the Kyoto Classification Score for Prediction of Current Helicobacter pylori Infection
Heejun Kang, Chul-Hyun Lim, Sukil Kim, Arum Choi, Jung-Hwan Oh
The Korean Journal of Helicobacter and Upper Gastrointestinal Research.2022; 22(4): 281. CrossRef - The simplified Kyoto classification score is consistent with the ABC method of classification as a grading system for endoscopic gastritis
Toshihiro Nishizawa, Osamu Toyoshima, Ryo Kondo, Kazuma Sekiba, Yosuke Tsuji, Hirotoshi Ebinuma, Hidekazu Suzuki, Chizu Tanikawa, Koichi Matsuda, Kazuhiko Koike
Journal of Clinical Biochemistry and Nutrition.2021; 68(1): 101. CrossRef - Endoscopic grading of gastric atrophy on risk assessment of gastric neoplasia: A systematic review and meta‐analysis
Shiyu Xiao, Yihan Fan, Zhihao Yin, Liya Zhou
Journal of Gastroenterology and Hepatology.2021; 36(1): 55. CrossRef - Gastritis: The clinico-pathological spectrum
Massimo Rugge, Edoardo Savarino, Marta Sbaraglia, Ludovica Bricca, Peter Malfertheiner
Digestive and Liver Disease.2021; 53(10): 1237. CrossRef - Efficacy of Seven-Day Potassium-Competitive Acid Blocker-Based First-LineHelicobacter PyloriEradication Therapy Administered with Bismuth
Ji Yeon Kim, Sun-Young Lee, Hyobin Kim, Jeong Hwan Kim, In-Kyung Sung, Hyung Seok Park
Yonsei Medical Journal.2021; 62(8): 708. CrossRef - Second-Line Bismuth-Containing Quadruple Therapy for Helicobacterpylori Infection: A 12-Year Study of Annual Eradication Rates
Kiwon Shin, Min-Jae Cho, Jung-Hwan Oh, Chul-Hyun Lim
Journal of Clinical Medicine.2021; 10(15): 3273. CrossRef - Predictive Significance of Promoter DNA Methylation of Cysteine Dioxygenase Type 1 (CDO1) in Metachronous Gastric Cancer
Yo Kubota, Satoshi Tanabe, Mizutomo Azuma, Kazue Horio, Yoshiki Fujiyama, Takafumi Soeno, Yasuaki Furue, Takuya Wada, Akinori Watanabe, Kenji Ishido, Chikatoshi Katada, Keishi Yamashita, Wasaburo Koizumi, Chika Kusano
Journal of Gastric Cancer.2021; 21(4): 379. CrossRef - Gastrointestinal Microbiota Changes in Patients With Gastric Precancerous Lesions
Dehua Liu, Si Chen, Yawen Gou, Wenyong Yu, Hangcheng Zhou, Rutong Zhang, Jinghao Wang, Fei Ye, Yingling Liu, Baolin Sun, Kaiguang Zhang
Frontiers in Cellular and Infection Microbiology.2021;[Epub] CrossRef - Use of endoscopic assessment of gastric atrophy for gastric cancer risk stratification to reduce the need for gastric mapping
Duc Trong Quach, Toru Hiyama, Huy Minh Le, Trung Sao Nguyen, Takuji Gotoda
Scandinavian Journal of Gastroenterology.2020; 55(4): 402. CrossRef - Influence of hypergastrinemia secondary to long-term proton pump inhibitor treatment on ECL cell tumorigenesis in human gastric mucosa
Atsushi Tatsuguchi, Shintaro Hoshino, Noriyuki Kawami, Katya Gudis, Tsutomu Nomura, Akira Shimizu, Katsuhiko Iwakiri
Pathology - Research and Practice.2020; 216(10): 153113. CrossRef - Chronic atrophic gastritis detection with a convolutional neural network considering stomach regions
Misaki Kanai, Ren Togo, Takahiro Ogawa, Miki Haseyama
World Journal of Gastroenterology.2020; 26(25): 3650. CrossRef - Naiv Helicobacter pylori pozitif ve negatif hastaların klinik, demografik ve endoskopik karakteristikleri: Retrospektif analiz
Muhammet AYDIN
Endoskopi Gastrointestinal.2020; 28(2): 39. CrossRef - Gastrointestinal Microbiota Changes in Patients With Gastric Precancerous Lesions
Dehua Liu, Si Chen, Yawen Gou, Wenyong Yu, Hangcheng Zhou, Rutong Zhang, Jinghao Wang, Fei Ye, Yingling Liu, Baolin Sun, Kaiguang Zhang
SSRN Electronic Journal .2020;[Epub] CrossRef
- Endoscopic diagnosis and prevalence of early gastric cancer in India: A prospective study
- 10,729 View
- 465 Download
- 34 Web of Science
- 36 Crossref

- Recent Development of Computer Vision Technology to Improve Capsule Endoscopy
- Junseok Park, Youngbae Hwang, Ju-Hong Yoon, Min-Gyu Park, Jungho Kim, Yun Jeong Lim, Hoon Jai Chun
- Clin Endosc 2019;52(4):328-333. Published online February 21, 2019
- DOI: https://doi.org/10.5946/ce.2018.172
-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader ePub
ePub - Capsule endoscopy (CE) is a preferred diagnostic method for analyzing small bowel diseases. However, capsule endoscopes capture a sparse number of images because of their mechanical limitations. Post-procedural management using computational methods can enhance image quality. Additional information, including depth, can be obtained by using recently developed computer vision techniques. It is possible to measure the size of lesions and track the trajectory of capsule endoscopes using the computer vision technology, without requiring additional equipment. Moreover, the computational analysis of CE images can help detect lesions more accurately within a shorter time. Newly introduced deep leaning-based methods have shown more remarkable results over traditional computerized approaches. A large-scale standard dataset should be prepared to develop an optimal algorithms for improving the diagnostic yield of CE. The close collaboration between information technology and medical professionals is needed.
-
Citations
Citations to this article as recorded by- Real‐time small bowel visualization quality assessment in wireless capsule endoscopy images using different lightweight embeddable models
Vahid Sadeghi, Alireza Mehridehnavi, Yasaman Sanahmadi, Sajed Rakhshani, Mina Omrani, Mohsen Sharifi
International Journal of Imaging Systems and Technology.2024;[Epub] CrossRef - A Novel Computer-Aided Detection/Diagnosis System for Detection and Classification of Polyps in Colonoscopy
Chia-Pei Tang, Hong-Yi Chang, Wei-Chun Wang, Wei-Xuan Hu
Diagnostics.2023; 13(2): 170. CrossRef - Revealing the Boundaries of Selected Gastro-Intestinal (GI) Organs by Implementing CNNs in Endoscopic Capsule Images
Sofia A. Athanasiou, Eleftheria S. Sergaki, Andreas A. Polydorou, Alexios A. Polydorou, George S. Stavrakakis, Nikolaos M. Afentakis, Ioannis O. Vardiambasis, Michail E. Zervakis
Diagnostics.2023; 13(5): 865. CrossRef - A Review of Biomedical Devices: Classification, Regulatory Guidelines, Human Factors, Software as a Medical Device, and Cybersecurity
Felix Tettey, Santosh Kumar Parupelli, Salil Desai
Biomedical Materials & Devices.2023;[Epub] CrossRef - Transformer with Hybrid Attention Mechanism for Stereo Endoscopic Video Super Resolution
Tianyi Zhang, Jie Yang
Symmetry.2023; 15(10): 1947. CrossRef - KAPSUL ENDOSKOPİYASI İLƏ İNCƏ BAĞIRSAQ MÜAYİNƏSİNDƏ MÖVCUD VƏZİYYƏT VƏ GƏLƏCƏK PERSPEKTİVLİYİ
Həbib Həsənzadə, Amalya Həsənova Həbib Həsənzadə, Amalya Həsənova
PAHTEI-Procedings of Azerbaijan High Technical Educational Institutions.2023; 34(11): 105. CrossRef - Review: Colon Capsule Endoscopy in Inflammatory Bowel Disease
Writaja Halder, Faidon-Marios Laskaratos, Hanan El-Mileik, Sergio Coda, Stevan Fox, Saswata Banerjee, Owen Epstein
Diagnostics.2022; 12(1): 149. CrossRef - Small Bowel Detection for Wireless Capsule Endoscopy Using Convolutional Neural Networks with Temporal Filtering
Geonhui Son, Taejoon Eo, Jiwoong An, Dong Oh, Yejee Shin, Hyenogseop Rha, You Kim, Yun Lim, Dosik Hwang
Diagnostics.2022; 12(8): 1858. CrossRef - Dynamic Depth-Aware Network for Endoscopy Super-Resolution
Wenting Chen, Yifan Liu, Jiancong Hu, Yixuan Yuan
IEEE Journal of Biomedical and Health Informatics.2022; 26(10): 5189. CrossRef - X-ray Imaging for Gastrointestinal Tracking of Microscale Oral Drug Delivery Devices
Rolf Bech Kjeldsen, Maja Nørgaard Kristensen, Carsten Gundlach, Lasse Højlund Eklund Thamdrup, Anette Müllertz, Thomas Rades, Line Hagner Nielsen, Kinga Zór, Anja Boisen
ACS Biomaterials Science & Engineering.2021; 7(6): 2538. CrossRef - VR-Caps: A Virtual Environment for Capsule Endoscopy
Kağan İncetan, Ibrahim Omer Celik, Abdulhamid Obeid, Guliz Irem Gokceler, Kutsev Bengisu Ozyoruk, Yasin Almalioglu, Richard J. Chen, Faisal Mahmood, Hunter Gilbert, Nicholas J. Durr, Mehmet Turan
Medical Image Analysis.2021; 70: 101990. CrossRef - Development of a deep learning-based software for calculating cleansing score in small bowel capsule endoscopy
Ji Hyung Nam, Youngbae Hwang, Dong Jun Oh, Junseok Park, Ki Bae Kim, Min Kyu Jung, Yun Jeong Lim
Scientific Reports.2021;[Epub] CrossRef - Kvasir-Capsule, a video capsule endoscopy dataset
Pia H. Smedsrud, Vajira Thambawita, Steven A. Hicks, Henrik Gjestang, Oda Olsen Nedrejord, Espen Næss, Hanna Borgli, Debesh Jha, Tor Jan Derek Berstad, Sigrun L. Eskeland, Mathias Lux, Håvard Espeland, Andreas Petlund, Duc Tien Dang Nguyen, Enrique Garcia
Scientific Data.2021;[Epub] CrossRef - Development and Verification of a Deep Learning Algorithm to Evaluate Small-Bowel Preparation Quality
Ji Hyung Nam, Dong Jun Oh, Sumin Lee, Hyun Joo Song, Yun Jeong Lim
Diagnostics.2021; 11(6): 1127. CrossRef - Role of Artificial Intelligence in Video Capsule Endoscopy
Ioannis Tziortziotis, Faidon-Marios Laskaratos, Sergio Coda
Diagnostics.2021; 11(7): 1192. CrossRef - Design and Research of Interactive Animation of Immersive Space Scene Based on Computer Vision Technology
Shan Wu, Hubin Liu, Qi Xu, Yulong Liu, Sang-Bing Tsai
Mathematical Problems in Engineering.2021; 2021: 1. CrossRef - Efficacy of a comprehensive binary classification model using a deep convolutional neural network for wireless capsule endoscopy
Sang Hoon Kim, Youngbae Hwang, Dong Jun Oh, Ji Hyung Nam, Ki Bae Kim, Junseok Park, Hyun Joo Song, Yun Jeong Lim
Scientific Reports.2021;[Epub] CrossRef - Artificial intelligence that determines the clinical significance of capsule endoscopy images can increase the efficiency of reading
Junseok Park, Youngbae Hwang, Ji Hyung Nam, Dong Jun Oh, Ki Bae Kim, Hyun Joo Song, Su Hwan Kim, Sun Hyung Kang, Min Kyu Jung, Yun Jeong Lim, Sudipta Roy
PLOS ONE.2020; 15(10): e0241474. CrossRef - EndoL2H: Deep Super-Resolution for Capsule Endoscopy
Yasin Almalioglu, Kutsev Bengisu Ozyoruk, Abdulkadir Gokce, Kagan Incetan, Guliz Irem Gokceler, Muhammed Ali Simsek, Kivanc Ararat, Richard J. Chen, Nicholas J. Durr, Faisal Mahmood, Mehmet Turan
IEEE Transactions on Medical Imaging.2020; 39(12): 4297. CrossRef
- Real‐time small bowel visualization quality assessment in wireless capsule endoscopy images using different lightweight embeddable models
- 6,149 View
- 232 Download
- 19 Web of Science
- 19 Crossref

Original Articles
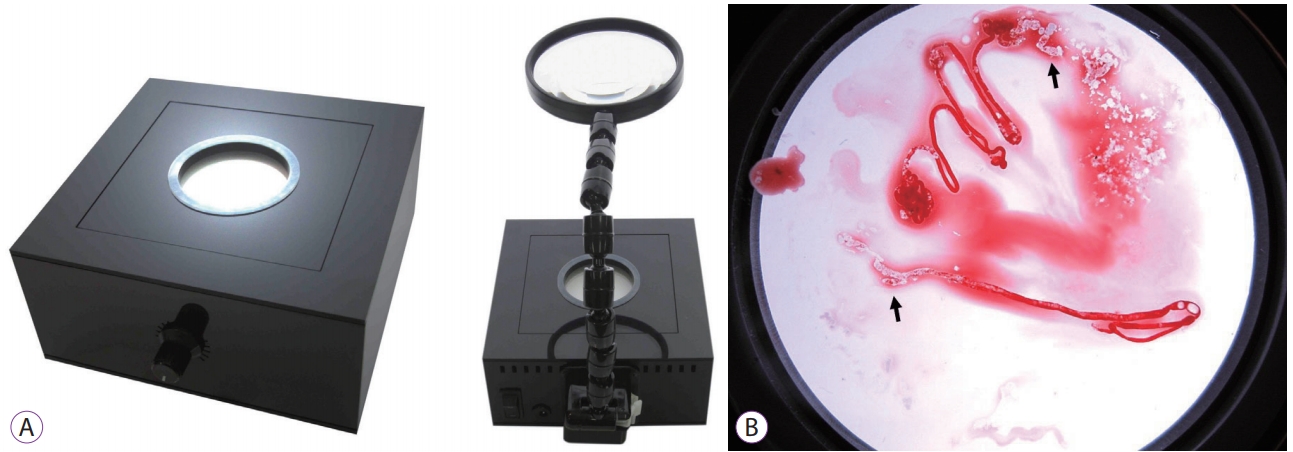
- A “Back Light System” for Identification of Sites for Endoscopic Ultrasound-Guided Fine-Needle Aspiration in Solid Pancreatic Masses: A Prospective, Randomized Study with a Crossover Design
- Ryo Harada, Hironari Kato, Soichiro Fushimi, Hirofumi Inoue, Daisuke Uchida, Yutaka Akimoto, Takeshi Tomoda, Kazuyuki Matsumoto, Yasuhiro Noma, Naoki Yamamoto, Shigeru Horiguchi, Koichiro Tsutsumi, Hiroyuki Okada
- Clin Endosc 2019;52(4):334-339. Published online May 16, 2019
- DOI: https://doi.org/10.5946/ce.2019.004
-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader ePub
ePub - Background
/Aims: We applied a back light system (BLS) with a magnifying glass to improve the ability to assess the adequacy of specimen sampling using endosonography. We conducted this study to evaluate the efficacy of the BLS in sampling of specimens by endoscopic ultrasound-guided fine needle aspiration of solid pancreatic masses.
Methods
This was a prospective, randomized, crossover, single-center clinical trial. An endosonographer evaluated adequacy on gross visual inspection and identified whitish specimen sampling sites with and without the BLS according to a randomization sequence in the first and second passes with a 25-G needle. On cytological evaluation, the presence of well-defined pancreatic ductal epithelium was evaluated by a cytopathologist who was blinded to any clinical information.
Results
A total of 80 consecutive patients were eligible during the study period. Adequacy was observed for 52 specimens (65%) with the BLS and 54 (68%) without the BLS (p=0.88). In assessment of specimen adequacy on gross examination, only fair agreement was observed both with and without BLS (kappa score 0.40 and 0.29, respectively).
Conclusions
The BLS did not influence the ability to identify specimen sampling sites or reliable assessment of specimen site adequacy using gross visual inspection. -
Citations
Citations to this article as recorded by- Tissue processing of endoscopic ultrasound-guided fine-needle aspiration specimens from solid pancreatic lesions
Kenji Notohara, Kaori Nakamura
Journal of Medical Ultrasonics.2024; 51(2): 261. CrossRef - Macroscopic qualitative evaluation of solid pancreatic lesion specimens from endoscopic ultrasound-guided fine needle aspiration/biopsies
Kaori Nakamura, Kenji Notohara, Ryoji Nishizaki, Etsuji Ishida, Midori Sato, Akemi Kodera, Junya Itakura, Motowo Mizuno
Pancreatology.2023; 23(8): 1028. CrossRef - Unfortunately, a “Back Light System” As a Global Positioning System Failed to Guide the Route in 25-G Fine-Needle Aspiration
Rungsun Rerknimitr, Phonthep Angsuwatcharakon
Clinical Endoscopy.2019; 52(4): 295. CrossRef
- Tissue processing of endoscopic ultrasound-guided fine-needle aspiration specimens from solid pancreatic lesions
- 4,297 View
- 81 Download
- 5 Web of Science
- 3 Crossref

- Endoscopic Ultrasound-Guided Liver Biopsy Using a Core Needle for Hepatic Solid Mass
- Hyung Ku Chon, Hee Chan Yang, Keum Ha Choi, Tae Hyeon Kim
- Clin Endosc 2019;52(4):340-346. Published online July 15, 2019
- DOI: https://doi.org/10.5946/ce.2018.175
-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader ePub
ePub - Background
/Aims: This study aimed to evaluate the feasibility and efficacy of endoscopic ultrasound-guided fine needle biopsy (EUSFNB) using a core needle for hepatic solid masses (HSMs). Additionally, the study aimed to assess factors that influence the diagnostic accuracy of EUS-FNB for HSMs.
Methods
A retrospective analysis of patients who underwent EUS-FNB for the pathological diagnosis of HSMs was conducted between January 2013 and July 2017. The procedure had been performed using core needles of different calibers. The assessed variables were mass size, puncture route, needle type, and the number of needle passes.
Results
Fifty-eight patients underwent EUS-FNB for the pathologic evaluation of HSMs with a mean mass size of 21.4±9.2 mm. EUSFNB was performed with either a 20-G (n=14), 22-G (n=29) or a 25-G core needle (n=15). The diagnostic accuracy for this procedure was 89.7%, but both specimen adequacy for histology and available immunohistochemistry stain were 91.4%. The sensitivity and specificity of EUS-FNB were 89.7% and 100%, respectively. There was one case involving bleeding as a complication, which was controlled with endoscopic hemostasis. According to the multivariate analysis, no variable was independently associated with a correct final diagnosis.
Conclusions
EUS-FNB with core biopsy needle is a safe and highly accurate diagnostic option for assessing HSMs. There were no variable factors associated with diagnostic accuracy. -
Citations
Citations to this article as recorded by- Endoscopic ultrasound‐guided tissue acquisition for focal liver lesions in patients with a history of multiple primary malignant neoplasms
Yuichi Takano, Naoki Tamai, Masataka Yamawaki, Jun Noda, Tetsushi Azami, Fumitaka Niiya, Fumiya Nishimoto, Naotaka Maruoka, Tatsuya Yamagami, Masatsugu Nagahama
DEN Open.2025;[Epub] CrossRef - Diagnostic and therapeutic role of endoscopic ultrasound in liver diseases: A systematic review and meta-analysis
Eyad Gadour, Abeer Awad, Zeinab Hassan, Khalid Jebril Shrwani, Bogdan Miutescu, Hussein Hassan Okasha
World Journal of Gastroenterology.2024; 30(7): 742. CrossRef - Endoscopic Ultrasound-Guided Tissue Sampling for the Cytohistological Diagnosis of Focal Liver Lesions
Jose Lariño-Noia, Andrea Jardi-Cuadrado, Juan Enrique Dominguez-Muñoz, Yessica Domínguez-Novoa, Marco Galego, Alberto Rama, Daniel de la Iglesia-Garcia, Xurxo Martinez-Seara, Ihab Abdulkader-Nallib, Julio Iglesias-Garcia
Diagnostics.2024; 14(11): 1155. CrossRef - Role of endoscopic ultrasound and endoscopic ultrasound-guided tissue acquisition in diagnosing hepatic focal lesions
Hussein Hassan Okasha, Hanane Delsa, Abdelmoneim Alsawaf, Ahmed Morad Hashim, Hani M Khattab, Dalia Abdelfatah, Abeer Abdellatef, Amr Albitar
World Journal of Methodology.2023; 13(4): 287. CrossRef - Endo-Hepatology: The Buzz Goes Much beyond Liver Biopsy—A Narrative Review
Rajesh Puri, Zubin Sharma, Swapnil Dhampalwar, Abhishek Kathuria, Bimal Sahu
Journal of Digestive Endoscopy.2023; 14(04): 227. CrossRef - A comparison of the antegrade core trap and reverse bevel needles for EUS-guided fine-needle biopsy sampling of liver mass: a prospective randomized cross over study
Pradermchai Kongkam, Nutbordee Nalinthassanai, Piyapan Prueksapanich, Anapat Sanpavat, Arlyn R. Cañones, Thanawat Luangsukrerk, Phonthep Angsuwatcharakon, Wiriyaporn Ridtitid, Pinit Kullavanijaya, Sombat Treeprasertsuk, Rungsun Rerknimitr
HPB.2022; 24(6): 797. CrossRef - Alternativen histologischer Materialgewinnung – Wann und wie ist die histologische Sicherung mittels Ultraschall (US), Computertomografie (CT) oder Endosonografie (EUS) sinnvoll?
Kathleen Möller, Christoph F. Dietrich, Siegbert Faiss, Sven Mutze, Leonie Goelz
Zeitschrift für Gastroenterologie.2022; 60(06): 937. CrossRef -
Ranunculus ternatus
Thunb extract attenuates renal fibrosis of diabetic nephropathy via inhibiting SMYD2
Weiwei Xu, Rui Peng, Siyu Chen, Congcong Wu, Xiaoxiao Wang, Ting Yu, Jiuying Jian, Ni Zhang, Siyang Zuo, Min Chen, Bing Guo, Lirong Liu
Pharmaceutical Biology.2022; 60(1): 300. CrossRef - Update on endoscopic ultrasound-guided liver biopsy
Shiva Rangwani, Devarshi R Ardeshna, Khalid Mumtaz, Sean G Kelly, Samuel Y Han, Somashekar G Krishna
World Journal of Gastroenterology.2022; 28(28): 3586. CrossRef - Endoscopic Ultrasound-Guided Fine-Needle Biopsy versus Fine-Needle Aspiration in the Diagnosis of Focal Liver Lesions: Prospective Head-to-Head Comparison
Marcel Gheorghiu, Andrada Seicean, Sorana D. Bolboacă, Ioana Rusu, Radu Seicean, Cristina Pojoga, Ofelia Moșteanu, Zeno Sparchez
Diagnostics.2022; 12(9): 2214. CrossRef - Diagnostic and interventional EUS in hepatology: An updated review
Vaneet Jearth, Sridhar Sundaram, SurinderSingh Rana
Endoscopic Ultrasound.2022; 11(5): 355. CrossRef - Endoscopic ultrasound guided hepatic interventions
Rintaro Hashimoto, Kenneth J. Chang
Digestive Endoscopy.2021; 33(1): 54. CrossRef - Biopsy or cytology for diagnosing hepatic focal lesions?
Haeryoung Kim
Clinical and Molecular Hepatology.2021; 27(2): 278. CrossRef - Endoscopic Ultrasound-Guided Fine Needle Aspiration Using a 22-G Needle for Hepatic Lesions: Single-Center Experience
Ebru Akay, Deniz Atasoy, Engin Altınkaya, Ali Koç, Tamer Ertan, Hatice Karaman, Erkan Caglar
Clinical Endoscopy.2021; 54(3): 404. CrossRef - Role of Endoscopic Ultrasound in Liver Disease: Where Do We Stand?
Tajana Pavic, Ivana Mikolasevic, Dominik Kralj, Nina Blazevic, Anita Skrtic, Ivan Budimir, Ivan Lerotic, Davor Hrabar
Diagnostics.2021; 11(11): 2021. CrossRef - Role of endoscopic ultrasound in the field of hepatology: Recent advances and future trends
Jahnvi Dhar, Jayanta Samanta
World Journal of Hepatology.2021; 13(11): 1459. CrossRef - The utility of liver biopsy in 2020
Ali Khalifa, Don C. Rockey
Current Opinion in Gastroenterology.2020; 36(3): 184. CrossRef - A State-of-the-Art Review on the Evolving Utility of Endoscopic Ultrasound in Liver Diseases Diagnosis
Wisam Sbeit, Anas Kadah, Mahmud Mahamid, Rinaldo Pellicano, Amir Mari, Tawfik Khoury
Diagnostics.2020; 10(8): 512. CrossRef - A Comprehensive Narrative Review on the Evolving Role of Endoscopic Ultrasound in Focal Solid Liver Lesions Diagnosis and Management
Wisam Sbeit, Anas Kadah, Amir Mari, Mahmud Mahamid, Tawfik Khoury
Diagnostics.2020; 10(9): 688. CrossRef - Endoscopic Ultrasound-Guided Liver Biopsies: Is the Future Here Yet?
Ihab I. El Hajj, Mohammad Al-Haddad
Clinical Endoscopy.2019; 52(4): 297. CrossRef
- Endoscopic ultrasound‐guided tissue acquisition for focal liver lesions in patients with a history of multiple primary malignant neoplasms
- 5,156 View
- 125 Download
- 20 Web of Science
- 20 Crossref

- Comparison of Endoscopic Ultrasound Biopsy Needles for Endoscopic Ultrasound-Guided Liver Biopsy
- Armen Eskandari, Patrick Koo, Heejung Bang, Dorina Gui, Shiro Urayama
- Clin Endosc 2019;52(4):347-352. Published online July 10, 2019
- DOI: https://doi.org/10.5946/ce.2019.005

-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader ePub
ePub - Background
/Aims: To compare the performance of latest commercially available endoscopic ultrasound biopsy needles.
Methods
Six latest commercially available needles were tested on a freshly harvested bovine liver; the tested needles included three 19 G, one 20 G, and two 22 G needles. Five biopsies were performed per needle with 10 mL of wet suction. The primary outcome was the number of complete portal tracts (CPTs) per needle aspirate. The secondary outcomes were the mean specimen length and mean fragment length. Analysis of variance and Tukey’s test were applied.
Results
All 19 G needles and the 20 G needle yielded similar mean CPTs and were superior to the SharkCore 22 G needle (p<0.001 adjusted for multiplicity). There was no statistically significant difference in total specimen length among the three 19 G needles and the 20 G needle tested. The two 22 G needles performed similarly with respect to the number of CPTs, mean fragment length, and mean specimen length (adjusted p=0.07, p=0.59, and p=0.10, respectively).
Conclusions
The specimen adequacy was similar among the 3 latest commercially available 19 G needles. The endoscopist may choose a larger-bore needle based on availability without concerns of specimen adequacy. Further studies are needed to assess the ease of needle use in various anatomical locations and to confirm the optimal needle design. -
Citations
Citations to this article as recorded by- EUS-guided versus percutaneous liver biopsy: A comprehensive review and meta-analysis of outcomes
Saurabh Chandan, Smit Deliwala, ShahabR Khan, BabuP Mohan, BanreetS Dhindsa, Jay Bapaye, Hemant Goyal, LenaL Kassab, Faisal Kamal, HarlanR Sayles, GursimranS Kochhar, DouglasG Adler
Endoscopic Ultrasound.2023; 12(2): 171. CrossRef - The Role of Endoscopic Ultrasound in Hepatology
Saleh A. Alqahtani, Floriane Ausloos, Ji Seok Park, Sunguk Jang
Gut and Liver.2023; 17(2): 204. CrossRef - EUS Guided Liver Biopsy
Itegbemie Obaitan, Romil Saxena, Mohammad A Al-Haddad
Techniques and Innovations in Gastrointestinal Endoscopy.2022; 24(1): 66. CrossRef - Endoscopic Ultrasound-Guided Liver Biopsy
Ishaan K. Madhok, Nasim Parsa, Jose M. Nieto
Clinics in Liver Disease.2022; 26(1): 127. CrossRef - Role of endoscopic ultrasound-guided liver biopsy: a meta-analysis
Keyu Zeng, Zhenpeng Jiang, Jie Yang, Kefei Chen, Qiang Lu
Scandinavian Journal of Gastroenterology.2022; 57(5): 545. CrossRef - Update on endoscopic ultrasound-guided liver biopsy
Shiva Rangwani, Devarshi R Ardeshna, Khalid Mumtaz, Sean G Kelly, Samuel Y Han, Somashekar G Krishna
World Journal of Gastroenterology.2022; 28(28): 3586. CrossRef - Diagnostic and interventional EUS in hepatology: An updated review
Vaneet Jearth, Sridhar Sundaram, SurinderSingh Rana
Endoscopic Ultrasound.2022; 11(5): 355. CrossRef - Endoscopic ultrasound guided hepatic interventions
Rintaro Hashimoto, Kenneth J. Chang
Digestive Endoscopy.2021; 33(1): 54. CrossRef - Comparison of Two Specialized Histology Needles for Endoscopic Ultrasound (EUS)-Guided Liver Biopsy: A Pilot Study
Rintaro Hashimoto, David P. Lee, Jason B. Samarasena, Vishal S. Chandan, Wenchang Guo, John G. Lee, Kenneth J. Chang
Digestive Diseases and Sciences.2021; 66(5): 1700. CrossRef - Emerging role of endoscopic ultrasound-guided liver biopsy
John David Chetwood, Sanjivan Mudaliar, Dominic Staudenmann, Joo-Shik Shin, Ken Liu, Avik Majumdar, Arthur Kaffes, Simone Strasser, Geoffrey W McCaughan, Payal Saxena
Gut.2021; 70(8): 1600. CrossRef - A prospective, head-to-head comparison of 2 EUS-guided liver biopsy needles in vivo
Soorya N. Aggarwal, Travis Magdaleno, Farina Klocksieben, Jennifer E. MacFarlan, Shanth Goonewardene, Zachary Zator, Shashin Shah, Hiral N. Shah
Gastrointestinal Endoscopy.2021; 93(5): 1133. CrossRef - Endoscopic Ultrasound-Guided Fine Needle Aspiration Using a 22-G Needle for Hepatic Lesions: Single-Center Experience
Ebru Akay, Deniz Atasoy, Engin Altınkaya, Ali Koç, Tamer Ertan, Hatice Karaman, Erkan Caglar
Clinical Endoscopy.2021; 54(3): 404. CrossRef - How Can We Optimize Tools and Techniques for Endoscopic Ultrasound–Guided Liver Biopsy?
Itegbemie Obaitan, Mohammad A. Al-Haddad
Clinical Gastroenterology and Hepatology.2020; 18(5): 1025. CrossRef - Guidelines on the use of liver biopsy in clinical practice from the British Society of Gastroenterology, the Royal College of Radiologists and the Royal College of Pathology
James Neuberger, Jai Patel, Helen Caldwell, Susan Davies, Vanessa Hebditch, Coral Hollywood, Stefan Hubscher, Salil Karkhanis, Will Lester, Nicholas Roslund, Rebecca West, Judith I Wyatt, Mathis Heydtmann
Gut.2020; 69(8): 1382. CrossRef - Endoscopic ultrasound in chronic liver disease
Brian M Fung, Alexander P Abadir, Armen Eskandari, Michael J Levy, James H Tabibian
World Journal of Hepatology.2020; 12(6): 262. CrossRef - Endoscopic Ultrasound-Guided Liver Biopsies: Is the Future Here Yet?
Ihab I. El Hajj, Mohammad Al-Haddad
Clinical Endoscopy.2019; 52(4): 297. CrossRef
- EUS-guided versus percutaneous liver biopsy: A comprehensive review and meta-analysis of outcomes
- 6,445 View
- 123 Download
- 19 Web of Science
- 16 Crossref

- Comparison of Clinical Outcomes between Plastic Stent and Novel Lumen-apposing Metal Stent for Endoscopic Ultrasound-Guided Drainage of Peripancreatic Fluid Collections
- Ho Cheol Shin, Chang Min Cho, Min Kyu Jung, Seong Jae Yeo
- Clin Endosc 2019;52(4):353-359. Published online March 13, 2019
- DOI: https://doi.org/10.5946/ce.2018.154
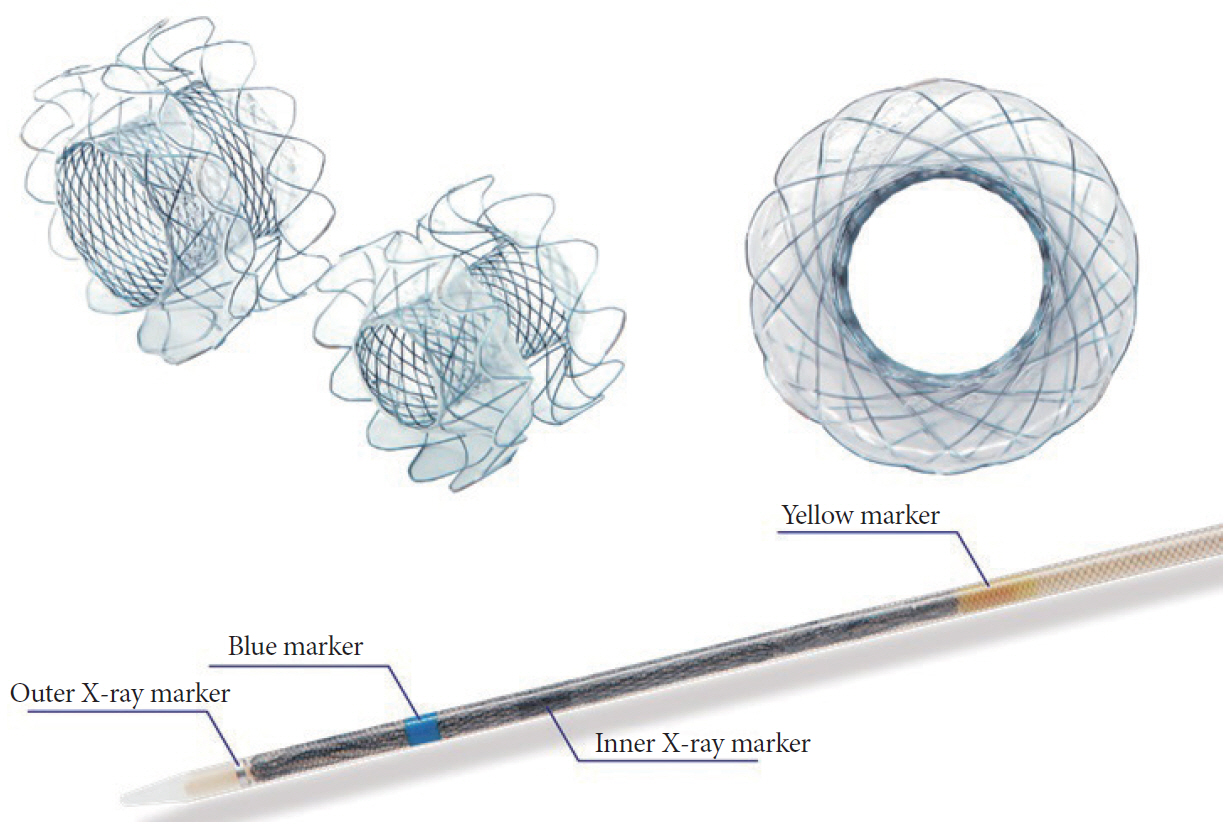
-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader ePub
ePub - Background
/Aims: Endoscopic ultrasound (EUS)-guided transmural drainage for peripancreatic fluid collections (PFCs) has gained wide acceptance as a nonsurgical intervention. Although a lumen-apposing metal stent (LAMS) was recently introduced, there are few data comparing the clinical outcomes between LAMS and plastic stent (PS) drainage.
Methods
Endoscopy databases of all patients who had undergone EUS-guided drainage for PFCs were searched and the clinical outcomes of EUS-guided drainage according to stent-type used were compared.
Results
A total of 27 patients (median age, 56 years) with PFCs underwent EUS-guided transmural drainage between January 2011 and December 2017. Of these, 17 underwent PS placement and 10 underwent LAMS placement. There was no significant difference in the technical success rate between the 2 groups (94.1% vs. 100%, p=1.0). Procedure time was shorter in the LAMS group compared to that in the PS group (10.6±2.5 min vs. 21.4±9.5 min, p=0.002). Among subjects with clinical success, recurrence of PFC after stent removal occurred in 5 of 12 patients with PS and 4 of 10 with LAMS, without statistical difference (41.7% vs. 40.0%, p=1.0).
Conclusions
Although our study showed similar clinical outcomes for LAMS and PS, further prospective trials are required to validate the superiority of LAMS. -
Citations
Citations to this article as recorded by- Efficacy and safety of long-term indwelling plastic stents after resolution of pancreatic fluid collections with endoscopic transmural drainage: a systematic review and meta-analysis
Fadi Hawa, Jean M. Chalhoub, Ana Vilela, Elit Quingalahua, Carol Shannon, George M. Philips, Richard S. Kwon, Erik-Jan Wamsteker, Allison R. Schulman, Matthew J. DiMagno, Jorge D. Machicado
Surgical Endoscopy.2024; 38(5): 2350. CrossRef - Multicenter study of the efficacy and safety of electrocautery-enhanced lumen-apposing metal stents for the internal drainage of pancreatic fluid collections
Chen-Shuan Chung, Yu-Ting Kuo, Yi-Chun Chiu, Yang-Chao Lin, Chi-Ying Yang, Kuan-Chih Chen, Szu-Chia Liao, Cheuk-Kay Sun, Yen-Chih Lin, Hsiu-Po Wang
Scientific Reports.2024;[Epub] CrossRef - Endoscopic management of pancreatic collections. Endoscopic Ultrasound Group from the Spanish Society of Digestive Endoscopy (GSEED-USE) Clinical Guidelines
Mariano González-Haba Ruiz, María Teresa Betés Ibáñez, Belén Martínez Moreno , Alejandro Repiso Ortega, Carlos de la Serna Higuera, Julio Iglesias García, Oriol Sendino García, María Moris Felgueroso, Belén Agudo Castillo, José Miguel Esteban Lóp
Revista Española de Enfermedades Digestivas.2024;[Epub] CrossRef - Head‐to‐head comparison between endoscopic ultrasound guided lumen apposing metal stent and plastic stents for the treatment of pancreatic fluid collections: A systematic review and meta‐analysis
Edson Guzmán‐Calderón, Alfonso Chacaltana, Ramiro Díaz, Bruno Li, Belen Martinez‐Moreno, José Ramón Aparicio
Journal of Hepato-Biliary-Pancreatic Sciences.2022; 29(2): 198. CrossRef - JPN clinical practice guidelines 2021 with easy‐to‐understand explanations for the management of acute pancreatitis
Tadahiro Takada, Shuji Isaji, Toshihiko Mayumi, Masahiro Yoshida, Yoshifumi Takeyama, Takao Itoi, Keiji Sano, Yusuke Iizawa, Atsushi Masamune, Morihisa Hirota, Kohji Okamoto, Dai Inoue, Nobuya Kitamura, Yasuhisa Mori, Shuntaro Mukai, Seiki Kiriyama, Kunih
Journal of Hepato-Biliary-Pancreatic Sciences.2022; 29(10): 1057. CrossRef - Comparison Between Lumen-Apposing Metal Stents and Plastic Stents in Endoscopic Ultrasound–Guided Drainage of Pancreatic Fluid Collection
Yunxiao Lyu, Ting Li, Bin Wang, Yunxiao Cheng, Liang Chen, Sicong Zhao
Pancreas.2021; 50(4): 571. CrossRef - Current status of treatments of pancreatic and peripancreatic collections of acute pancreatitis
Nian-Jun Xiao, Ting-Ting Cui, Fang Liu, Wen Li
World Journal of Gastrointestinal Surgery.2021; 13(7): 633. CrossRef - Current treatment of pancreatic pseudocysts: a systematic review
V. M. Durleshter, S. R. Genrikh, A. V. Makarenko, D. S. Kirakosyan
Kuban Scientific Medical Bulletin.2021; 28(4): 85. CrossRef - Metal Versus Plastic Stents for Pancreatic Fluid Collection Drainage
Xianzhu Zhou, Han Lin, Xiaoju Su, Pingping Zhang, Chunting Fu, Xiangyu Kong, Zhendong Jin, Zhaoshen Li, Yiqi Du, Huiyun Zhu
Journal of Clinical Gastroenterology.2021; 55(8): 652. CrossRef - Editors' Choice of Noteworthy Clinical Endoscopy Publications in the First Decade
Gwang Ha Kim, Kwang An Kwon, Do Hyun Park, Jimin Han
Clinical Endoscopy.2021; 54(5): 633. CrossRef - Are Lumen-Apposing Metal Stents More Effective Than Plastic Stents for the Management of Pancreatic Fluid Collections: An Updated Systematic Review and Meta-analysis
Shali Tan, Chunyu Zhong, Yutang Ren, Xujuan Luo, Jin Xu, Yan Peng, Xiangsheng Fu, Xiaowei Tang
Gastroenterology Research and Practice.2020; 2020: 1. CrossRef - A Comparison of Endoscopic Versus Surgical Creation of a Cystogastrostomy to Drain Pancreatic Pseudocysts and Walled-Off Pancreatic Necrosis in 5500 Patients
Patrick Suggs, Timothy NeCamp, John Alfred Carr
Annals of Surgery Open.2020; 1(2): e024. CrossRef - Endoscopic Ultrasound-Guided Drainage of Peripancreatic Fluid Collections
Eun Young Kim, Robert H. Hawes
Clinical Endoscopy.2019; 52(4): 299. CrossRef
- Efficacy and safety of long-term indwelling plastic stents after resolution of pancreatic fluid collections with endoscopic transmural drainage: a systematic review and meta-analysis
- 5,530 View
- 202 Download
- 11 Web of Science
- 13 Crossref

- Diagnostic Efficacy of Endoscopic Ultrasound Elastography in Differentiating Solid Pancreatic Lesions: A Single-Center Experience
- Ahmed Youssef Altonbary, Hazem Hakim, Ahmed Mohamed El-Shamy
- Clin Endosc 2019;52(4):360-364. Published online January 8, 2019
- DOI: https://doi.org/10.5946/ce.2018.160

-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader ePub
ePub - Background
/Aims: Endoscopic ultrasound (EUS) has a limited ability to determine the nature of solid pancreatic lesions (SPLs). Most recent ultrasound processors are provided with elastography software, which allows quantification of the tissue hardness. The aim of this study is to evaluate the effectiveness of the elasticity score (ES) and strain ratio (SR) in the differentiation of benign pancreatic lesions from malignant pancreatic lesions.
Methods
The study had a retrospective design; it included 97 patients with SPLs and 19 patients with inflammatory lesions. The ES and SR were determined during the examination; finally, EUS-guided fine needle aspiration was performed.
Results
In this 2-year study, 116 patients were enrolled (97 with malignant lesions and 19 with benign lesions). There were 69 men and 47 women. Their median age was 55.9 years. A cut-off point was detected at SR of 7.75 with a specificity of 99.9%, sensitivity of 90.7%, positive predictive value (PPV) of 99.9%, negative predictive value (NPV) of 67.9%, and accuracy of 92.2%. After adding the ES to the SR, the cut-off point at 7.75 resulted in a specificity of 94.6%, sensitivity of 99%, PPV of 98%, NPV of 98.5%, and accuracy of 97%.
Conclusions
The use of the ES combined with the SR increases the accuracy of differentiation between benign and malignant SPLs and is an effective method for the evaluation of pancreatic masses. -
Citations
Citations to this article as recorded by- Diagnostic performance of endoscopic ultrasound elastography for differential diagnosis of solid pancreatic lesions: A propensity score-matched analysis
In Rae Cho, Seok-Hoo Jeong, Huapyong Kang, Eui Joo Kim, Yeon Suk Kim, Soyoung Jeon, Jae Hee Cho
Pancreatology.2023; 23(1): 105. CrossRef - The expanding role of endoscopic ultrasound elastography
Jahnvi Dhar, Jayanta Samanta
Clinical Journal of Gastroenterology.2022; 15(5): 841. CrossRef - Endoscopic ultrasound elastography for malignant pancreatic masses and associated lymph nodes: Critical evaluation of strain ratio cutoff value
Miguel Puga-Tejada, Raquel Del Valle, Roberto Oleas, Maria Egas-Izquierdo, Martha Arevalo-Mora, Jorge Baquerizo-Burgos, Jesenia Ospina, Miguel Soria-Alcivar, Hannah Pitanga-Lukashok, Carlos Robles-Medranda
World Journal of Gastrointestinal Endoscopy.2022; 14(9): 524. CrossRef - Role of Endoscopic Ultrasonography and Endoscopic Retrograde Cholangiopancreatography in the Diagnosis of Pancreatic Cancer
Yasutaka Ishii, Masahiro Serikawa, Tomofumi Tsuboi, Ryota Kawamura, Ken Tsushima, Shinya Nakamura, Tetsuro Hirano, Ayami Fukiage, Takeshi Mori, Juri Ikemoto, Yusuke Kiyoshita, Sho Saeki, Yosuke Tamura, Sayaka Miyamoto, Kazuaki Chayama
Diagnostics.2021; 11(2): 238. CrossRef - Impact of endoscopic ultrasound elastography in pancreatic lesion evaluation
Cosmas Rinaldi Adithya Lesmana, Maria Satya Paramitha
Artificial Intelligence in Gastrointestinal Endoscopy.2021; 2(4): 168. CrossRef - Utilidad de la elastografía cuantitativa por ultrasonografía endoscópica (USE), para el diagnóstico de las lesiones sólidas del páncreas (LSP).
Martín Alonso Gómez Zuleta, Oscar Fernando Ruíz Morales, Diego Fernando Cano Rosales
Revista colombiana de Gastroenterología.2021; 36(4): 434. CrossRef
- Diagnostic performance of endoscopic ultrasound elastography for differential diagnosis of solid pancreatic lesions: A propensity score-matched analysis
- 4,947 View
- 146 Download
- 4 Web of Science
- 6 Crossref

Case Reports
- Air Embolism during Upper Endoscopy: A Case Report
- Yin Fang, Junbei Wu, Feng Wang, Lihong Cheng, Yunhong Lu, Xiaofei Cao
- Clin Endosc 2019;52(4):365-368. Published online March 13, 2019
- DOI: https://doi.org/10.5946/ce.2018.201

-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader ePub
ePub - Air embolism is a rare complication of upper endoscopy and potentially causes life-threatening events. A 67-year-old man with a history of surgery of cardiac carcinoma and pancreatic neuroendocrine tumor underwent painless upper endoscopy because of tarry stools. During the procedure, air embolism developed, which caused decreased pulse oxygen saturation and delayed sedation recovery. He recovered with some weakness of the left upper limb in the intensive care unit without hyperbaric oxygen therapy. The etiology, clinical manifestations, and treatments of air embolism are discussed based on the literature reports. Although air embolism is uncommon in endoscopic examinations, the patients’ outcomes could be improved if clinicians are alert to this potential complication, and promptly start proper diagnostic and therapeutic measures.
-
Citations
Citations to this article as recorded by- Anesthesia for Advanced Endoscopic Procedures
Basavana Goudra, Monica Saumoy
Clinical Endoscopy.2022; 55(1): 1. CrossRef - Cerebral Air Embolism After Endoscopic Variceal Band Ligation
Maria Azhar, Sunita Upreti, Bruce F. Sabath
ACG Case Reports Journal.2020; 7(8): e00443. CrossRef - Cerebral Air Embolism after Esophagogastroduodenoscopy: Insight on Pathophysiology, Epidemiology, Prevention and Treatment
Malik Ghannam, Azizullah Beran, Dana Ghazaleh, Tanner Ferderer, Brent Berry, Mona Al Banna, Leighton Mohl, Christopher Streib, Tapan Thacker, Ivan Matos
Journal of Stroke and Cerebrovascular Diseases.2019; 28(12): 104403. CrossRef
- Anesthesia for Advanced Endoscopic Procedures
- 4,303 View
- 117 Download
- 3 Web of Science
- 3 Crossref

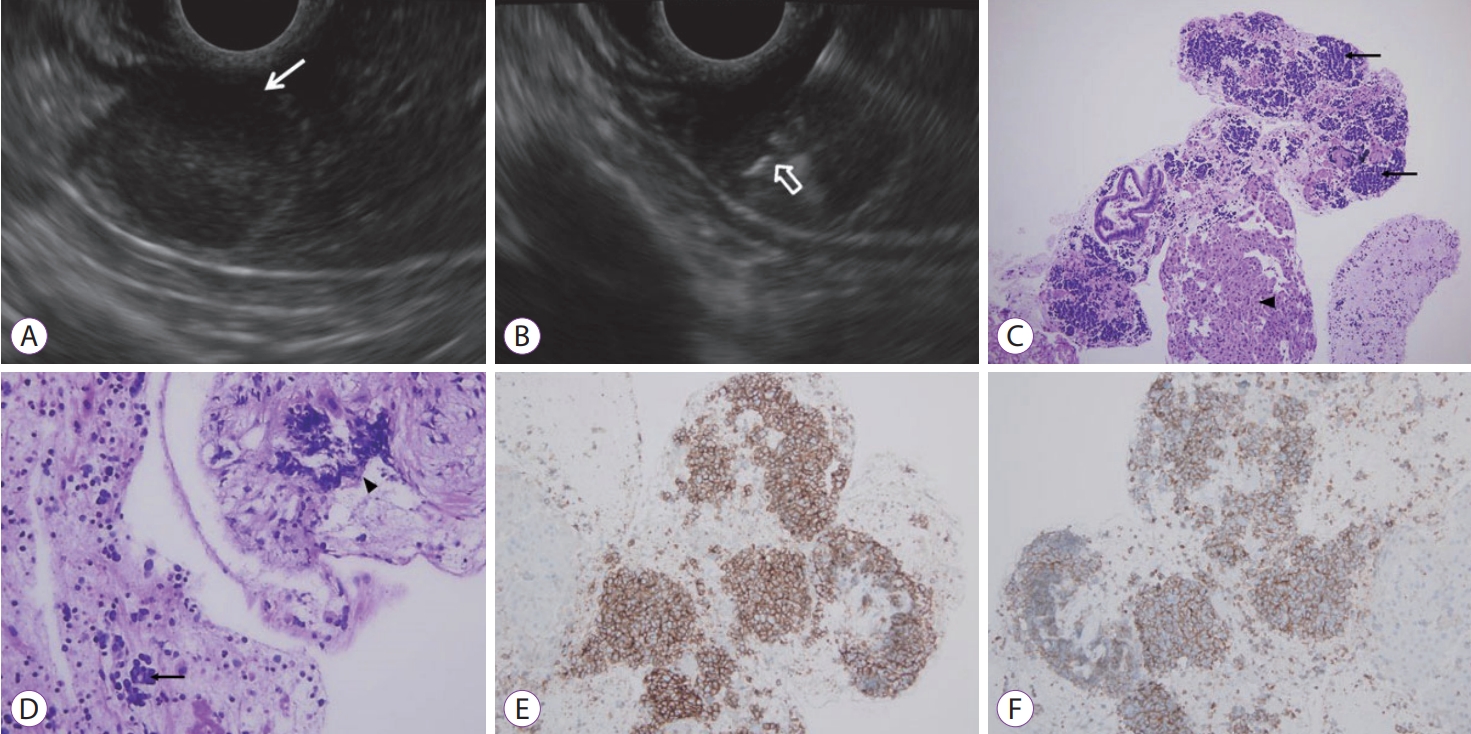
- A Rare Case of Lymph Node Metastasis from Early Gastric Cancer
- Takaaki Yoshikawa, Yoshio Kadokawa, Masaya Ohana, Akihisa Fukuda, Hiroshi Seno
- Clin Endosc 2019;52(4):369-372. Published online October 5, 2018
- DOI: https://doi.org/10.5946/ce.2018.130
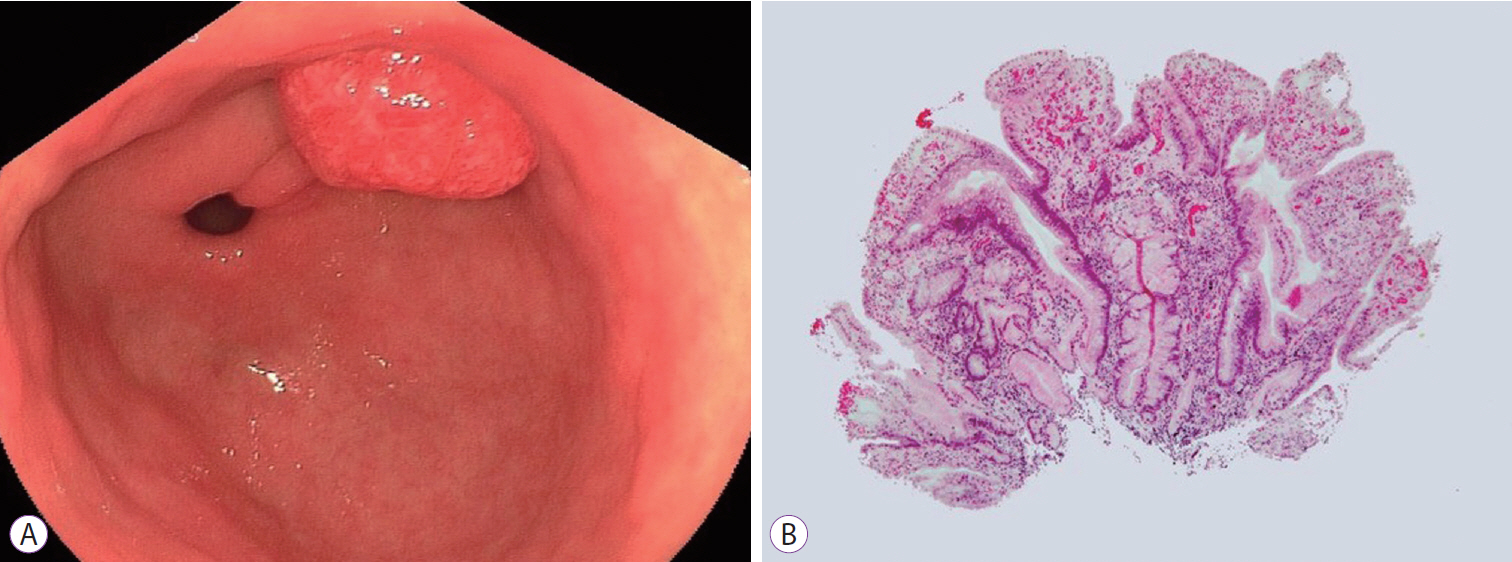
-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader ePub
ePub - Gastric cancers that fulfill the Japanese criteria for curative endoscopic resection show a low risk of lymph node (LN) metastasis. Here, we report a case of LN metastasis from early gastric cancer that fulfilled the curative criteria. A 74-year-old Japanese woman was referred to our hospital for treatment of early gastric cancer identified at the site of a hyperplastic polyp that had been diagnosed 10 years prior to presentation. Contrast-enhanced computed tomography did not show any lymphadenopathy and laparoscopy-assisted distal gastrectomy was performed. Histopathological examination revealed a predominantly moderately differentiated adenocarcinoma that measured 15 mm in size and was confined to the mucosa. However, a single metastatic regional LN was observed. A few cancer cells showed positive staining for alpha-fetoprotein. It should be noted that early gastric cancer can be accompanied by LN metastasis even if it fulfills the criteria for curative endoscopic resection.
- 4,689 View
- 125 Download

- Endoscopic Ultrasound-Guided Transgastric Drainage of an IntraAbdominal Abscess following Gastrectomy
- Satoru Kikuchi, Tetsushi Kubota, Shinji Kuroda, Masahiko Nishizaki, Shunsuke Kagawa, Hironari Kato, Hiroyuki Okada, Toshiyoshi Fujiwara
- Clin Endosc 2019;52(4):373-376. Published online February 15, 2019
- DOI: https://doi.org/10.5946/ce.2018.134
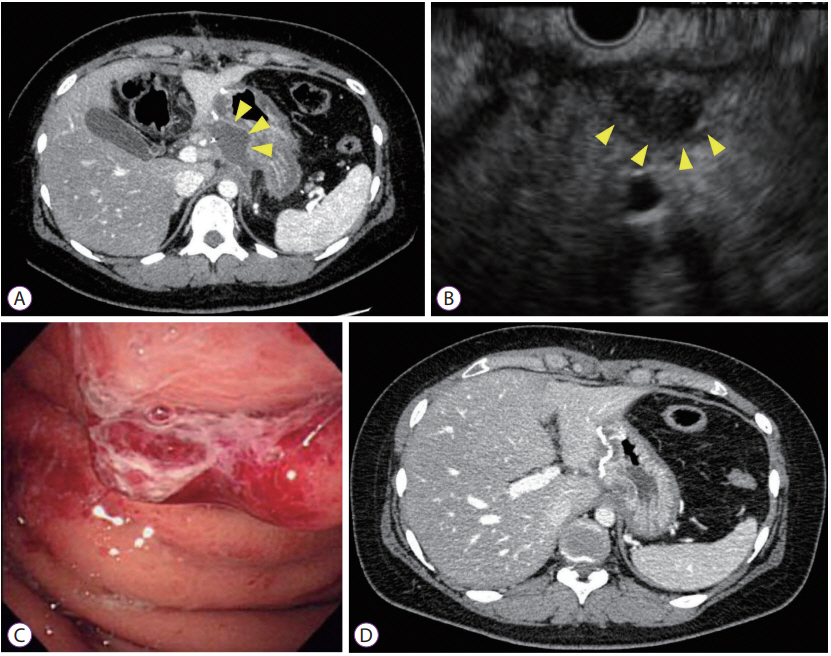
-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader ePub
ePub - Endoscopic ultrasound (EUS)-guided transgastric drainage has been performed as a less invasive procedure for pancreatic fistulas and intra-abdominal abscesses occurring after surgery in recent years. However, there are no reports of EUS-guided transgastric drainage of intra-abdominal abscesses following gastrectomy. This case report describes 2 patients who developed an intra-abdominal abscess following gastrectomy and underwent EUS-guided transgastric drainage. Both patients underwent laparoscopy-assisted distal gastrectomy with Billroth-I reconstruction for gastric cancer. The intra-abdominal abscesses were caused by postoperative pancreatic fistula that developed following gastrectomy. One patient underwent naso-cystic drainage and the other underwent only a needle puncture of the abscess cavity. EUS-guided drainage was performed safely and effectively, although 1 patient developed gastroduodenal anastomotic leakage related to this procedure. In summary, EUS-guided transgastric drainage is safe and technically feasible even in post-gastrectomy patients. However, it is necessary to be careful if this procedure is performed in the early period following gastrectomy.
-
Citations
Citations to this article as recorded by- A Case of Intra-abdominal Abcess following a Pancreatic Fistula after Gastrectomy Treated with Endoscopic Ultrasound-guided Transgastric Drainage
Kenichi ISHIBAYASHI, Toshikatsu TSUJI, Daisuke YAMAMOTO, Hirotaka KITAMURA, Shinichi KADOYA, Hiroyuki BANDO
Nihon Rinsho Geka Gakkai Zasshi (Journal of Japan Surgical Association).2020; 81(6): 1097. CrossRef
- A Case of Intra-abdominal Abcess following a Pancreatic Fistula after Gastrectomy Treated with Endoscopic Ultrasound-guided Transgastric Drainage
- 6,272 View
- 115 Download
- 1 Web of Science
- 1 Crossref

- Colonic Intramucosal Cancer in the Interposed Colon Treated with Endoscopic Mucosal Resection: A Case Report and Review of Literature
- Seung-Ho Baek, Jang-Ho Lee, Dong Ryeol Yoo, Hye Yeong Kim, Meihua Jin, Ah-reum Jang, Dong-Hoon Yang, Jeong-Sik Byeon
- Clin Endosc 2019;52(4):377-381. Published online July 30, 2019
- DOI: https://doi.org/10.5946/ce.2018.129
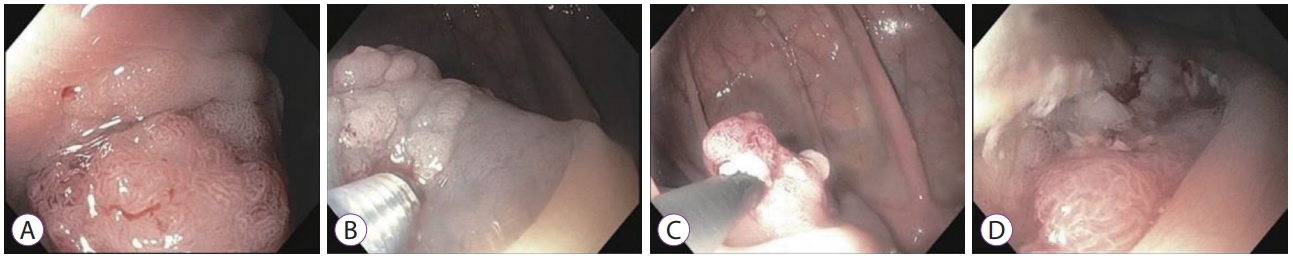
-
 Abstract
Abstract
 PDF
PDF Supplementary Material
Supplementary Material PubReader
PubReader ePub
ePub - Colon interposition is a surgical procedure used for maintenance of luminal conduit after esophagectomy. Although epithelial neoplasia, such as adenoma and adenocarcinoma, may develop in the interposed colon, there are only few case reports on the condition. Due to the rarity of this condition, there is no definite consensus on recommending screening endoscopy for the early detection of neoplasia in the interposed colons. Here, we report a case of intramucosal adenocarcinoma in an interposed colon. Initial endoscopic resection for this tumor failed to accomplish complete resection. A subsequent endoscopic resection was performed 1 month later and complete resection was achieved. Based on our experience and recommendation on screening endoscopy for gastric cancer in Korea, we suggest that regular screening esophagogastroduodenoscopies should be performed following esophagectomy to detect early neoplasia in the stomach and interposed colon and avoid adverse results induced by delayed detection.
-
Citations
Citations to this article as recorded by- The presence of adenocarcinoma of the right colon and polyp in colonic graft in a female patient with colon interposition due to caustic stricture of the esophagus in childhood
Stojan Latincic, Maja Pavlov, Jovica Vasiljevic, Dragan Vasin, Milena Papovic
Srpski arhiv za celokupno lekarstvo.2024; 152(1-2): 71. CrossRef
- The presence of adenocarcinoma of the right colon and polyp in colonic graft in a female patient with colon interposition due to caustic stricture of the esophagus in childhood
- 6,651 View
- 73 Download
- 1 Web of Science
- 1 Crossref

- A Case of Concurrent Ampullary Adenoma and Gangliocytic Paraganglioma at the Minor Papilla Treated with Endoscopic Resection
- Jun Kwon Ko, Do Hyun Park, Hee Sang Hwang
- Clin Endosc 2019;52(4):382-386. Published online April 12, 2019
- DOI: https://doi.org/10.5946/ce.2018.198

-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader ePub
ePub - A gangliocytic paraganglioma is a benign tumor of the digestive system with a very low incidence. The tumor is histopathologically characterized by a triphasic pattern consisting of epithelioid, ganglion, and spindle-shaped Schwann cells. In most cases, it occurs in the second portion of the duodenum near the ampulla of Vater. We report a case of a gangliocytic paraganglioma occurring at the minor duodenal papilla (a rare location) with a concurrent adenoma of the ampulla of Vater. Both lesions were treated simultaneously using endoscopic resection. Additionally, we have presented a literature review.
-
Citations
Citations to this article as recorded by- Estrategia de manejo quirúrgico en tumores de bajo potencial maligno de localización ampular. Presentación de un caso de paraganglioma gangliocítico
Victoria Lucas Guerrero, Anna González Costa, Andreu Romaguera Monzonis, Natalia Bejarano González, Francisco García Borobia
Cirugía Española.2021; 99(8): 621. CrossRef - Endoscopic Mucosal Resection of Adenocarcinoma at the Minor Duodenal Papilla: A Case Report and Suggestions for the Optimal Treatment Strategy
Takao Sato, Ryota Sagami, Hidefumi Nishikiori, Hiroaki Tsuji, Keiji Sato, Tsutomu Daa, Kazunari Murakami
Internal Medicine.2021; 60(16): 2593. CrossRef - Surgical management strategy in ampullary tumors with low malignant potential: Presentation of a patient with a gangliocytic paraganglioma
Victoria Lucas Guerrero, Anna González Costa, Andreu Romaguera Monzonis, Natalia Bejarano González, Francisco García Borobia
Cirugía Española (English Edition).2021; 99(8): 621. CrossRef
- Estrategia de manejo quirúrgico en tumores de bajo potencial maligno de localización ampular. Presentación de un caso de paraganglioma gangliocítico
- 4,899 View
- 100 Download
- 2 Web of Science
- 3 Crossref

Brief Report
- Flower Basket Retrieval: Utilization of a Device with a Unique Design for Endoscopic Rescue in Cases Involving Proximal Migration of Pancreatic Duct Stents
- Vincent Zimmer
- Clin Endosc 2019;52(4):387-389. Published online July 5, 2019
- DOI: https://doi.org/10.5946/ce.2019.057

-
 PDF
PDF PubReader
PubReader ePub
ePub -
Citations
Citations to this article as recorded by- Endoscopic retrieval for a large impacted meat bolus in the oesophagus
Soo In Choi, Jeongmin Choi
BMJ Case Reports.2021; 14(2): e241275. CrossRef
- Endoscopic retrieval for a large impacted meat bolus in the oesophagus
- 4,073 View
- 91 Download
- 1 Web of Science
- 1 Crossref

Letter to the Editor
- Combined Laparoscopic-Endoscopic Techniques for Removal of Small Gastric Tumors: Advantages and Tricks
- Eva Intagliata, Rosario Vecchio
- Clin Endosc 2019;52(4):390-391. Published online July 30, 2019
- DOI: https://doi.org/10.5946/ce.2019.102
- 3,083 View
- 52 Download


 KSGE
KSGE




 First
First Prev
Prev



