Search
- Page Path
- HOME > Search
- Current status of image-enhanced endoscopy in inflammatory bowel disease
- Young Joo Yang
- Clin Endosc 2023;56(5):563-577. Published online September 26, 2023
- DOI: https://doi.org/10.5946/ce.2023.070
-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader ePub
ePub - In inflammatory bowel disease (IBD), chronic inflammation leads to unfavorable clinical outcomes and increases the risk of developing colorectal neoplasm (CRN); thereby highlighting the importance of endoscopically evaluating disease activity as well as detecting and characterizing CRN in patients with IBD. With recent advances in image-enhanced endoscopic (IEE) technologies, especially virtual chromoendoscopy (VCE) platforms, this review discusses state-of-the-art IEE techniques and their applicability in assessing disease activity and surveillance colonoscopy in patients with IBD. Among various IEE, VCE demonstrated the capacity to identify quiescent disease activity. And endoscopic remission defined by the new scoring system using VCE platform better predicted clinical outcomes, which may benefit the tailoring of therapeutic strategies in patients with IBD. High-definition dye-chromoendoscopy (HD-DCE) is numerically superior to high-definition white light endoscopy (HD-WLE) in detecting CRN in IBD; however, discrepancy is observed in the statistical significance. VCE showed comparable performance in detecting dysplasia to HD-WLE or DCE and potential for optical diagnosis to differentiate neoplastic from nonneoplastic lesions during surveillance colonoscopy. Applying these novel advanced IEE technologies would provide opportunities for personalized medicine in IBD and optimal treatment of CRN in patients with IBD.
- 2,037 View
- 107 Download

- Management of complications related to colorectal endoscopic submucosal dissection
- Tae-Geun Gweon, Dong-Hoon Yang
- Clin Endosc 2023;56(4):423-432. Published online July 27, 2023
- DOI: https://doi.org/10.5946/ce.2023.104

-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader ePub
ePub - Compared to endoscopic mucosal resection (EMR), colonoscopic endoscopic submucosal dissection (C-ESD) has the advantages of higher en bloc resection rates and lower recurrence rates of colorectal neoplasms. Therefore, C-ESD is considered an effective treatment method for laterally spread tumors and early colorectal cancer. However, C-ESD is technically more difficult and requires a longer procedure time than EMR. In addition to therapeutic efficacy and procedural difficulty, safety concerns should always be considered when performing C-ESD in clinical practice. Bleeding and perforation are the main adverse events associated with C-ESD and can occur during C-ESD or after the completion of the procedure. Most bleeding associated with C-ESD can be managed endoscopically, even if it occurs during or after the procedure. More recently, most perforations identified during C-ESD can also be managed endoscopically, unless the mural defect is too large to be sutured with endoscopic devices or the patient is hemodynamically unstable. Delayed perforations are quite rare, but they require surgical treatment more frequently than endoscopically identified intraprocedural perforations or radiologically identified immediate postprocedural perforations. Post-ESD coagulation syndrome is a relatively underestimated adverse event, which can mimic localized peritonitis from perforation. Here, we classify and characterize the complications associated with C-ESD and recommend management options for them.
-
Citations
Citations to this article as recorded by- International Digestive Endoscopy Network consensus on the management of antithrombotic agents in patients undergoing gastrointestinal endoscopy
Seung Joo Kang, Chung Hyun Tae, Chang Seok Bang, Cheol Min Shin, Young-Hoon Jeong, Miyoung Choi, Joo Ha Hwang, Yutaka Saito, Philip Wai Yan Chiu, Rungsun Rerknimitr, Christopher Khor, Vu Van Khien, Kee Don Choi, Ki-Nam Shim, Geun Am Song, Oh Young Lee
Clinical Endoscopy.2024; 57(2): 141. CrossRef - Is there a best choice of equipment for colorectal endoscopic submucosal dissection?
Francesco Cocomazzi, Sonia Carparelli, Nunzia Labarile, Antonio Capogreco, Marco Gentile, Roberta Maselli, Jahnvi Dhar, Jayanta Samanta, Alessandro Repici, Cesare Hassan, Francesco Perri, Antonio Facciorusso
Expert Review of Medical Devices.2024; : 1. CrossRef
- International Digestive Endoscopy Network consensus on the management of antithrombotic agents in patients undergoing gastrointestinal endoscopy
- 1,726 View
- 130 Download
- 4 Web of Science
- 2 Crossref

-
Feasibility and safety of endoscopic submucosal dissection for lesions in proximity to a colonic diverticulum

- Nobuaki Ikezawa, Takashi Toyonaga, Shinwa Tanaka, Tetsuya Yoshizaki, Toshitatsu Takao, Hirofumi Abe, Hiroya Sakaguchi, Kazunori Tsuda, Satoshi Urakami, Tatsuya Nakai, Taku Harada, Kou Miura, Takahisa Yamasaki, Stuart Kostalas, Yoshinori Morita, Yuzo Kodama
- Clin Endosc 2022;55(3):417-425. Published online May 12, 2022
- DOI: https://doi.org/10.5946/ce.2021.245
-
 Abstract
Abstract
 PDF
PDF Supplementary Material
Supplementary Material PubReader
PubReader ePub
ePub - Background
/Aims: Endoscopic submucosal dissection (ESD) for diverticulum-associated colorectal lesions is generally contraindicated because of the high risk of perforation. Several studies on patients with such lesions treated with ESD have been reported recently. However, the feasibility and safety of ESD for lesions in proximity to a colonic diverticulum (D-ESD) have not been fully clarified. The aim of this study was to evaluate the feasibility and safety of D-ESD.
Methods
D-ESD was defined as ESD for lesions within approximately 3 mm of a diverticulum. Twenty-six consecutive patients who underwent D-ESD were included. Two strategic approaches were used depending on whether submucosal dissection of the diverticulum-related part was required (strategy B) or not (strategy A). Treatment outcomes and adverse events associated with each strategy were analyzed.
Results
The en bloc resection rate was 96.2%. The rates of R0 and curative resection in strategies A and B were 80.8%, 73.1%, 84.6%, and 70.6%, respectively. Two cases of intraoperative perforation and one case of delayed perforation occurred. The delayed perforation case required emergency surgery, but the other cases were managed conservatively.
Conclusions
D-ESD may be a feasible treatment option. However, it should be performed in a high-volume center by expert hands because it requires highly skilled endoscopic techniques. -
Citations
Citations to this article as recorded by- Endoscopic submucosal dissection for diverticulum using combination of countertraction and circumferential-inversion method
Hiroshi Takayama, Yoshinori Morita, Toshitatsu Takao, Douglas Motomura, Madoka Takao, Takashi Toyonaga, Yuzo Kodama
Endoscopy.2024; 56(S 01): E91. CrossRef - Traction-assisted endoscopic submucosal dissection for resection of ileocecal valve neoplasia: a French retrospective multicenter case series
Clara Yzet, Timothée Wallenhorst, Jérémie Jacques, Mariana Figueiredo Ferreira, Jérôme Rivory, Florian Rostain, Louis-Jean Masgnaux, Jean Grimaldi, Romain Legros, Pierre Lafeuille, Jérémie Albouys, Fabien Subtil, Marion Schaefer, Mathieu Pioche
Endoscopy.2024;[Epub] CrossRef - The role of cap-assisted endoscopy and its future implications
Sol Kim, Bo-In Lee
Clinical Endoscopy.2024; 57(3): 293. CrossRef - Successful planned piecemeal endoscopic resection using gel immersion and an over-the-scope clip for a lesion extensively extended into the colonic diverticulum
Tomoaki Tashima, Takahiro Muramatsu, Tomonori Kawasaki, Tsubasa Ishikawa, Shomei Ryozawa
VideoGIE.2023; 8(4): 167. CrossRef - Future therapeutic implications of new molecular mechanism of colorectal cancer
Sen Lu, Cheng-You Jia, Jian-She Yang
World Journal of Gastroenterology.2023; 29(16): 2359. CrossRef - Iatrogenic colorectal perforation caused by a clip
Hirotaka Oura, Yasuki Hatayama, Erika Nomura, Harutoshi Sugiyama, Daisuke Murakami, Makoto Arai, Takayoshi Nishino
Endoscopy.2023; 55(S 01): E1091. CrossRef
- Endoscopic submucosal dissection for diverticulum using combination of countertraction and circumferential-inversion method
- 3,725 View
- 171 Download
- 4 Web of Science
- 6 Crossref

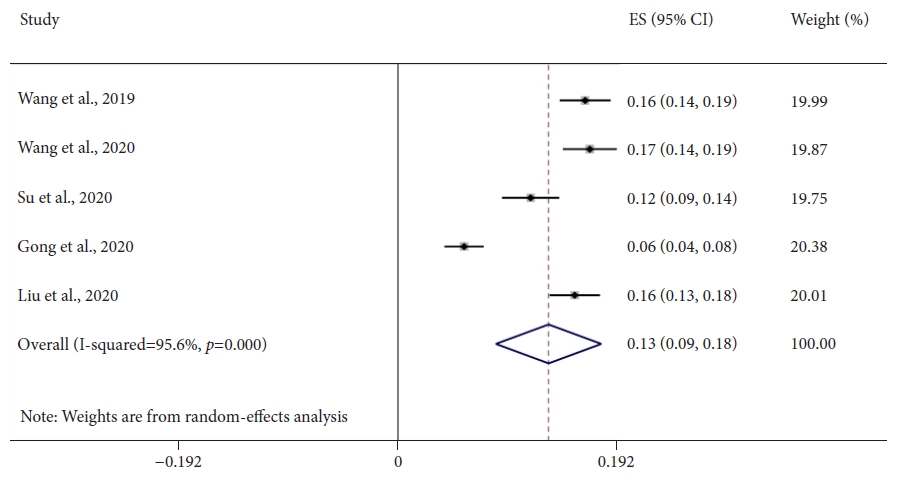
- Does computer-aided diagnostic endoscopy improve the detection of commonly missed polyps? A meta-analysis
- Arun Sivananthan, Scarlet Nazarian, Lakshmana Ayaru, Kinesh Patel, Hutan Ashrafian, Ara Darzi, Nisha Patel
- Clin Endosc 2022;55(3):355-364. Published online May 12, 2022
- DOI: https://doi.org/10.5946/ce.2021.228
-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader ePub
ePub - Background
/Aims: Colonoscopy is the gold standard diagnostic method for colorectal neoplasia, allowing detection and resection of adenomatous polyps; however, significant proportions of adenomas are missed. Computer-aided detection (CADe) systems in endoscopy are currently available to help identify lesions. Diminutive (≤5 mm) and nonpedunculated polyps are most commonly missed. This meta-analysis aimed to assess whether CADe systems can improve the real-time detection of these commonly missed lesions.
Methods
A comprehensive literature search was performed. Randomized controlled trials evaluating CADe systems categorized by morphology and lesion size were included. The mean number of polyps and adenomas per patient was derived. Independent proportions and their differences were calculated using DerSimonian and Laird random-effects modeling.
Results
Seven studies, including 2,595 CADe-assisted colonoscopies and 2,622 conventional colonoscopies, were analyzed. CADe-assisted colonoscopy demonstrated an 80% increase in the mean number of diminutive adenomas detected per patient compared with conventional colonoscopy (0.31 vs. 0.17; effect size, 0.13; 95% confidence interval [CI], 0.09–0.18); it also demonstrated a 91.7% increase in the mean number of nonpedunculated adenomas detected per patient (0.32 vs. 0.19; effect size, 0.05; 95% CI, 0.02–0.07).
Conclusions
CADe-assisted endoscopy significantly improved the detection of most commonly missed adenomas. Although this method is a potentially exciting technology, limitations still apply to current data, prompting the need for further real-time studies. -
Citations
Citations to this article as recorded by- Use of artificial intelligence in the management of T1 colorectal cancer: a new tool in the arsenal or is deep learning out of its depth?
James Weiquan Li, Lai Mun Wang, Katsuro Ichimasa, Kenneth Weicong Lin, James Chi-Yong Ngu, Tiing Leong Ang
Clinical Endoscopy.2024; 57(1): 24. CrossRef - As how artificial intelligence is revolutionizing endoscopy
Jean-Francois Rey
Clinical Endoscopy.2024; 57(3): 302. CrossRef - Eye tracking technology in endoscopy: Looking to the future
Arun Sivananthan, Jabed Ahmed, Alexandros Kogkas, George Mylonas, Ara Darzi, Nisha Patel
Digestive Endoscopy.2023; 35(3): 314. CrossRef - Artificial intelligence and the push for small adenomas: all we need?
Katharina Zimmermann-Fraedrich, Thomas Rösch
Endoscopy.2023; 55(04): 320. CrossRef - Recent advances in devices and technologies that might prove revolutionary for colonoscopy procedures
Jonathan S. Galati, Kevin Lin, Seth A. Gross
Expert Review of Medical Devices.2023; 20(12): 1087. CrossRef - Detecting colorectal lesions with image-enhanced endoscopy: an updated review from clinical trials
Mizuki Nagai, Sho Suzuki, Yohei Minato, Fumiaki Ishibashi, Kentaro Mochida, Ken Ohata, Tetsuo Morishita
Clinical Endoscopy.2023; 56(5): 553. CrossRef - KI-Werkzeuge als smarte Helfer in Klinik und Forschung
Zeitschrift für Gastroenterologie.2023; 61(11): 1544. CrossRef - AI-powered medical devices for practical clinicians including the diagnosis of colorectal polyps
Donghwan Kim, Eunsun Kim
Journal of the Korean Medical Association.2023; 66(11): 658. CrossRef - The Role of Artificial Intelligence in Colorectal Cancer Screening: Lesion Detection and Lesion Characterization
Edward Young, Louisa Edwards, Rajvinder Singh
Cancers.2023; 15(21): 5126. CrossRef - Artificial intelligence for colorectal neoplasia detection during colonoscopy: a systematic review and meta-analysis of randomized clinical trials
Shenghan Lou, Fenqi Du, Wenjie Song, Yixiu Xia, Xinyu Yue, Da Yang, Binbin Cui, Yanlong Liu, Peng Han
eClinicalMedicine.2023; 66: 102341. CrossRef - Pouring some water into the wine—Poor performance of endoscopists in artificial intelligence studies
Jochen Weigt
United European Gastroenterology Journal.2022; 10(8): 793. CrossRef
- Use of artificial intelligence in the management of T1 colorectal cancer: a new tool in the arsenal or is deep learning out of its depth?
- 3,031 View
- 155 Download
- 11 Web of Science
- 11 Crossref

- Radiation Proctitis and Management Strategies
- Dushyant Singh Dahiya, Asim Kichloo, Faiz Tuma, Michael Albosta, Farah Wani
- Clin Endosc 2022;55(1):22-32. Published online November 18, 2021
- DOI: https://doi.org/10.5946/ce.2020.288

-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader ePub
ePub - Radiotherapy (RT) is a treatment modality that uses high-energy rays or radioactive agents to generate ionizing radiation against rapidly dividing cells. The main objective of using radiation in cancer therapy is to impair or halt the division of the tumor cells. Over the past few decades, advancements in technology, the introduction of newer methods of RT, and a better understanding of the pathophysiology of cancers have enabled physicians to deliver doses of radiation that match the exact dimensions of the tumor for greater efficacy, with minimal exposure of the surrounding tissues. However, RT has numerous complications, the most common being radiation proctitis (RP). It is characterized by damage to the rectal epithelium by secondary ionizing radiation. Based on the onset of signs and symptoms, post-radiotherapy RP can be classified as acute or chronic, each with varying levels of severity and complication rates. The treatment options available for RP are limited, with most of the data on treatment available from case reports or small studies. Here, we describe the types of RT used in modern-day medicine and radiation-mediated tissue injury. We have primarily focused on the classification, epidemiology, pathogenesis, clinical features, treatment strategies, complications, and prognosis of RP.
-
Citations
Citations to this article as recorded by- Concurrent rectal perforation and obstruction following neoadjuvant chemoradiation for locally advanced rectal cancer: A case report
Tahmineh Tahouri, Sahand Hedayati Omami, Maryam Hosseini, Ehsanollah Rahimi-Movaghar
International Journal of Surgery Case Reports.2024; 116: 109337. CrossRef - Endoscopic resection of residual rectal neoplasia after definitive chemoradiotherapy for rectal cancer
Robert Klimkowski, Jakub Krzyzkowiak, Nastazja Dagny Pilonis, Krzysztof Bujko, Michal F. Kaminski
Best Practice & Research Clinical Gastroenterology.2024; 68: 101896. CrossRef - Radiation injuries of organs and tissues: mechanisms of occurrence, methods of prevention and treatment: A review
Daiana A. Balaeva, Denis S. Romanov, Oxana P. Trofimova, Zarina Z. Gadzhibabaeva, Yury Yu. Gorchak, Garia A. Gariaev
Journal of Modern Oncology.2024; 25(4): 504. CrossRef - Interventions for Managing Late Gastrointestinal Symptoms Following Pelvic Radiotherapy: a Systematic Review and Meta-analysis
H. Berntsson, A. Thien, D. Hind, L. Stewart, M. Mahzabin, W.S. Tung, M. Bradburn, M. Kurien
Clinical Oncology.2024; 36(5): 318. CrossRef - Intestinal microecological transplantation for a patient with chronic radiation enteritis: A case report
Lin Wang, Yan Li, Yu-Jing Zhang, Li-Hua Peng
World Journal of Gastroenterology.2024; 30(19): 2603. CrossRef - Intrarectal formalin treatment for haemorrhagic radiation‐induced proctopathy: efficacy and safety
Darina Kohoutova, Ana Wilson, Caroline Gee, Ramy Elhusseiny, Linda Wanders, David Cunningham
Colorectal Disease.2024; 26(5): 932. CrossRef - Emodin ameliorates acute radiation proctitis in mice by regulating AKT/MAPK/NF-κB/VEGF pathways
Jinsheng Gao, Yousong Li, Jiaohua Chen, Wen Feng, Jianchen Bu, Zixuan Lu, Jiandong Wang
International Immunopharmacology.2024; 132: 111945. CrossRef - Protocolo diagnóstico de la rectitis (proctitis)
C. Iniesta Cavero, L. Menchén-Viso
Medicine - Programa de Formación Médica Continuada Acreditado.2024; 14(8): 468. CrossRef - Administration of modified Gegen Qinlian decoction for hemorrhagic chronic radiation proctitis: A case report and review of literature
Shao-Yong Liu, Liu-Ling Hu, Shi-Jun Wang, Zhong-Li Liao
World Journal of Clinical Cases.2023; 11(5): 1129. CrossRef - A Retrospective Single-Arm Cohort Study in a Single Center of Radiofrequency Ablation in Treatment of Chronic Radiation Proctitis
Chien-En Tang, Kung-Chuan Cheng, Kuen-Lin Wu, Hong-Hwa Chen, Ko-Chao Lee
Life.2023; 13(2): 566. CrossRef - Survivorship in Early-Stage Rectal Cancer Patients Who Have Received Combined Modality Therapy
Saboor E. Randhawa, Laura Tenner
Clinical Colorectal Cancer.2023; 22(4): 375. CrossRef - A Systematic Review of Population-Based Studies of Chronic Bowel Symptoms in Cancer Survivors following Pelvic Radiotherapy
Adam Biran, Iakov Bolnykh, Ben Rimmer, Anthony Cunliffe, Lisa Durrant, John Hancock, Helen Ludlow, Ian Pedley, Colin Rees, Linda Sharp
Cancers.2023; 15(16): 4037. CrossRef - The effectiveness of hyperbaric oxygen therapy for managing radiation-induced proctitis – results of a 10-year retrospective cohort study
António Moreira Monteiro, Diogo Alpuim Costa, Virgínia Mareco, Carla Espiney Amaro
Frontiers in Oncology.2023;[Epub] CrossRef - Chinese clinical practice guidelines for the prevention and treatment of radiation‐induced rectal injury
Hui Zhang, Zhen Zhang, Shuanghu Yuan
Precision Radiation Oncology.2023; 7(4): 237. CrossRef - Progress in multidisciplinary treatment of hemorrhagic radiation proctitis
Qiulian Li, Guangjie Liao
Annals of Oncology Research and Therapy.2022; 2(1): 10. CrossRef
- Concurrent rectal perforation and obstruction following neoadjuvant chemoradiation for locally advanced rectal cancer: A case report
- 6,844 View
- 548 Download
- 13 Web of Science
- 15 Crossref

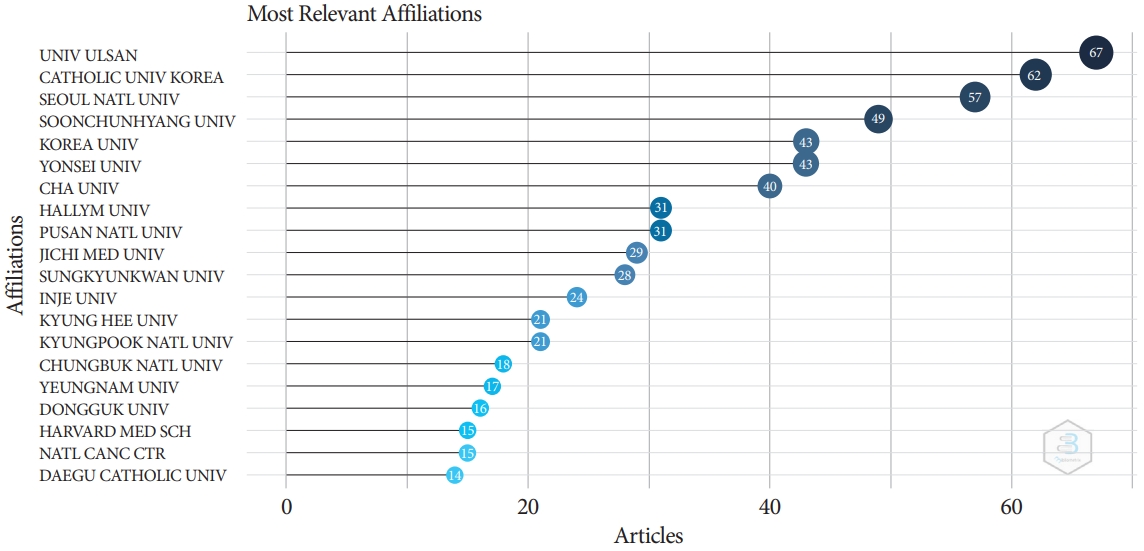
- Document Network and Conceptual and Social Structures of Clinical Endoscopy from 2015 to July 2021 Based on the Web of Science Core Collection: A Bibliometric Study
- Sun Huh
- Clin Endosc 2021;54(5):641-650. Published online September 30, 2021
- DOI: https://doi.org/10.5946/ce.2021.207
-
 Abstract
Abstract
 PDF
PDF Supplementary Material
Supplementary Material PubReader
PubReader ePub
ePub - Background
/Aims: The present study investigated the relevance and network of institutions, keywords, and authors’ countries of the articles in Clinical Endoscopy published from 2015 to May 2021 based on the Web of Science Core Collection.
Methods
The Web of Science Core Collection was searched with the term Clinical Endoscopy as the publication title on July 12, 2021. All 776 citations published from 2015 to May 2021 and 2,964 articles citing those 776 articles were analyzed using Biblioshiny.
Results
The corresponding authors were from 73 countries. Document coupling showed that the colorectal cancer-colonoscopyrandomized controlled trial cluster had the most significant impact and highest centrality. There were 442 articles with corresponding authors from Korea (57.0%). The number of collaborative works by Korean authors with the authors of other countries was 33 (7.5%). The articles were cited 2,964 times by corresponding authors from 37 countries.
Conclusions
The above results show that Clinical Endoscopy has published several studies on gastrointestinal endoscopy. A large proportion of citations (84.7 %) were from outside Korea, indicating that the journal content is useful for global physicians. Collaborative work between authors from Korea and other countries should be encouraged to promote the journal. -
Citations
Citations to this article as recorded by- Research Progress in Land Consolidation and Rural Revitalization: Current Status, Characteristics, Regional Differences, and Evolution Laws
Shuchang Li, Wei Song
Land.2023; 12(1): 210. CrossRef - Journal metrics, document network, and conceptual and social structures of the Korean Journal of Anesthesiology from 2017 to July 2022: a bibliometric study
Sun Huh
Korean Journal of Anesthesiology.2023; 76(1): 3. CrossRef - Promotion to Top-Tier Journal and Development Strategy of the Annals of Laboratory Medicine for Strengthening its Leadership in the Medical Laboratory Technology Category: A Bibliometric Study
Sun Huh
Annals of Laboratory Medicine.2022; 42(3): 321. CrossRef - Research trends on endoscopic mucosal resection: A bibliometric analysis from 1991 to 2021
Yihan Yang, Xuan Xu, Menghui Wang, Yang Zhang, Pinglang Zhou, Sifan Yang, Xu Shu, Chuan Xie
Frontiers in Surgery.2022;[Epub] CrossRef - Riesgo de sangrado gastrointestinal por uso de anticoagulantes directos orales: ¿cuál es más seguro?
Ivan David Lozada Martinez, Luis Carlos Solano Díaz, Marcela Barbosa Pérez, Víctor Andrés Rueda Oviedo, Brainerd Lenin Caicedo Moncada, Gustavo Andrés Diaz Cruz, Adriana cristina Ceballos Espitia, David Esteban Diaz Gómez, Daiana Andrea Rojas Ramí
Revista Cuarzo.2022; 28(2): 31. CrossRef
- Research Progress in Land Consolidation and Rural Revitalization: Current Status, Characteristics, Regional Differences, and Evolution Laws
- 3,432 View
- 61 Download
- 4 Web of Science
- 5 Crossref

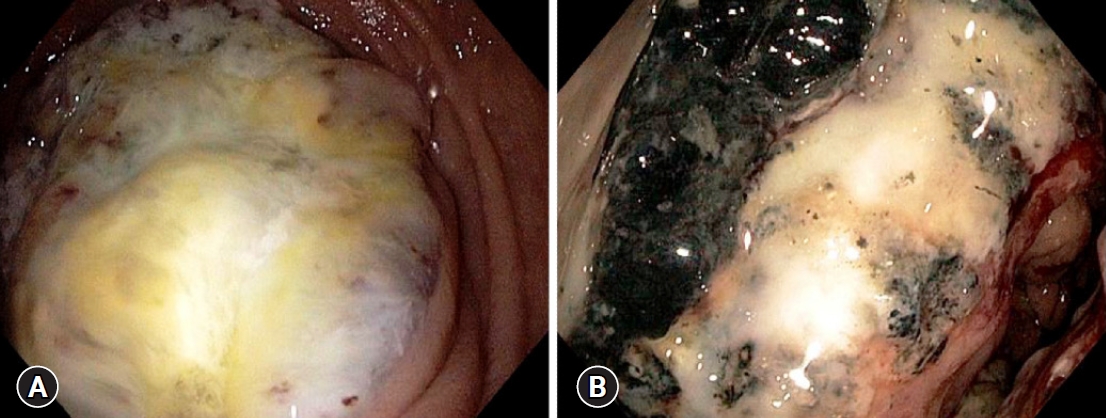
- Colorectal carcinoma and chronic inflammatory demyelinating polyneuropathy: is there a possible paraneoplastic association?
- Adnan Malik, Faisal Inayat, Muhammad Hassan Naeem Goraya, Gul Nawaz, Ahmad Mehran, Atif Aziz, Saad Saleem
- Clin Endosc 2023;56(2):245-251. Published online July 28, 2021
- DOI: https://doi.org/10.5946/ce.2021.076
-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader ePub
ePub - A plethora of paraneoplastic syndromes have been reported as remote effects of colorectal carcinoma (CRC). However, there is a dearth of data pertaining to the association of this cancer with demyelinating neuropathies. Herein, we describe the case of a young woman diagnosed with chronic inflammatory demyelinating polyneuropathy (CIDP). Treatment with intravenous immunoglobulins and prednisone did not improve her condition, and her neurological symptoms worsened. Subsequently, she was readmitted with exertional dyspnea, lightheadedness, malaise, and black stools. Colonoscopy revealed a necrotic mass in the ascending colon, which directly invaded the second part of the duodenum. Pathologic results confirmed the diagnosis of locally advanced CRC. Upon surgical resection of the cancer, her CIDP showed dramatic resolution without any additional therapy. Patients with CRC may develop CIDP as a type of paraneoplastic syndrome. Clinicians should remain cognizant of this potential association, as it is of paramount importance for the necessary holistic clinical management.
- 4,094 View
- 323 Download

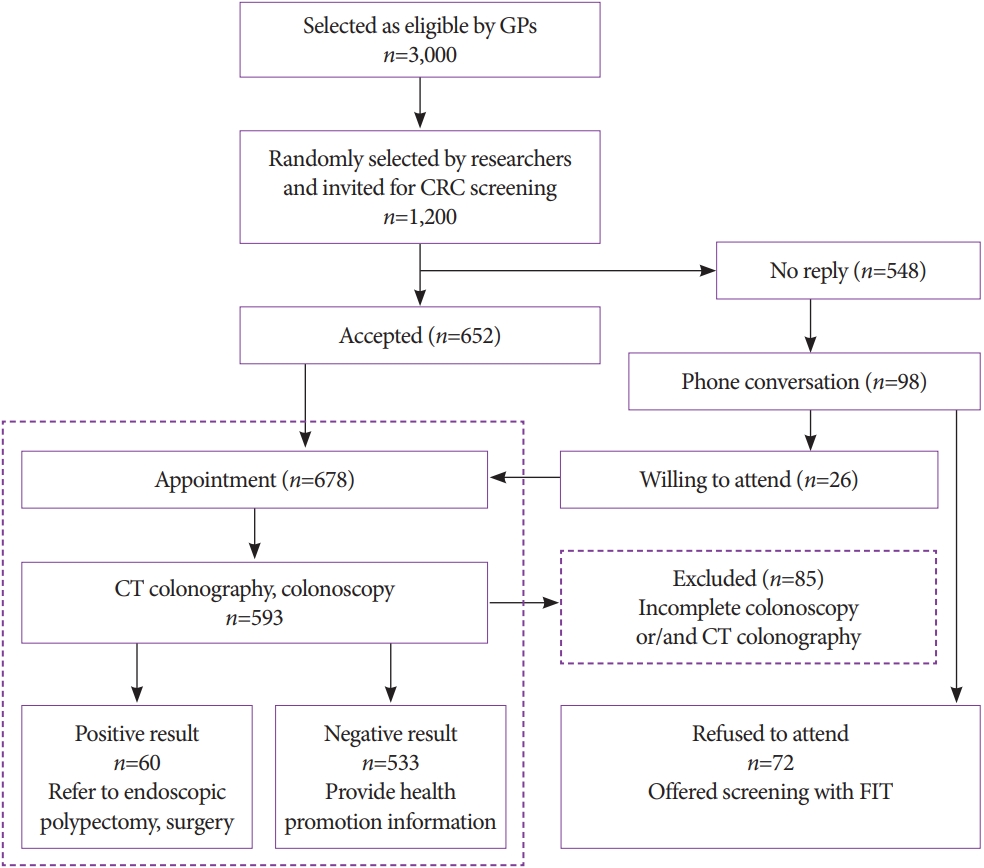
- Colorectal Cancer Screening with Computed Tomography Colonography: Single Region Experience in Kazakhstan
- Jandos Amankulov, Dilyara Kaidarova, Zhamilya Zholdybay, Marianna Zagurovskaya, Nurlan Baltabekov, Madina Gabdullina, Akmaral Ainakulova, Dias Toleshbayev, Alexandra Panina, Elvira Satbayeva, Zhansaya Kalieva
- Clin Endosc 2022;55(1):101-112. Published online July 15, 2021
- DOI: https://doi.org/10.5946/ce.2021.066
-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader ePub
ePub - Background
/Aims: The aim of our study was to determine the efficacy of computed tomography colonography (CTC) in screening for colorectal cancer (CRC).
Methods
A total of 612 females and 588 males aged 45 to 75 years were enrolled in CTC screening. CTC was performed following standard bowel preparation and colonic insufflation with carbon dioxide. The main outcomes were the detection rate of CRC and advanced adenoma (AA), prevalence of colorectal lesions in relation to socio-demographic and health factors, and overall diagnostic performance of CTC.
Results
Overall, 56.5% of the 1,200 invited subjects underwent CTC screening. The sensitivity for CRC and AA was 0.89 and 0.97, respectively, while the specificity was 0.71 and 0.99, respectively. The prevalence of CRC and AA was 3.0% (18/593) and 7.1% (42/593), respectively, with the highest CRC prevalence in the 66-75 age group (≥12 times; odds ratio [OR], 12.11; 95% confidence interval [CI], 4.45-32.92). CRC and AA prevalence were inversely correlated with Asian descent, physical activity, and negative fecal immunochemical test results (OR=0.43; 95% CI, 0.22-0.83; OR=0.16; 95% CI, 0.04-0.68; OR=0.5; 95% CI, 0.07-3.85, respectively).
Conclusions
Our study revealed high accuracy of CTC in diagnosing colonic neoplasms, good compliance with CTC screening, and high detection rate of CRC.
- 5,700 View
- 205 Download
- 1 Web of Science

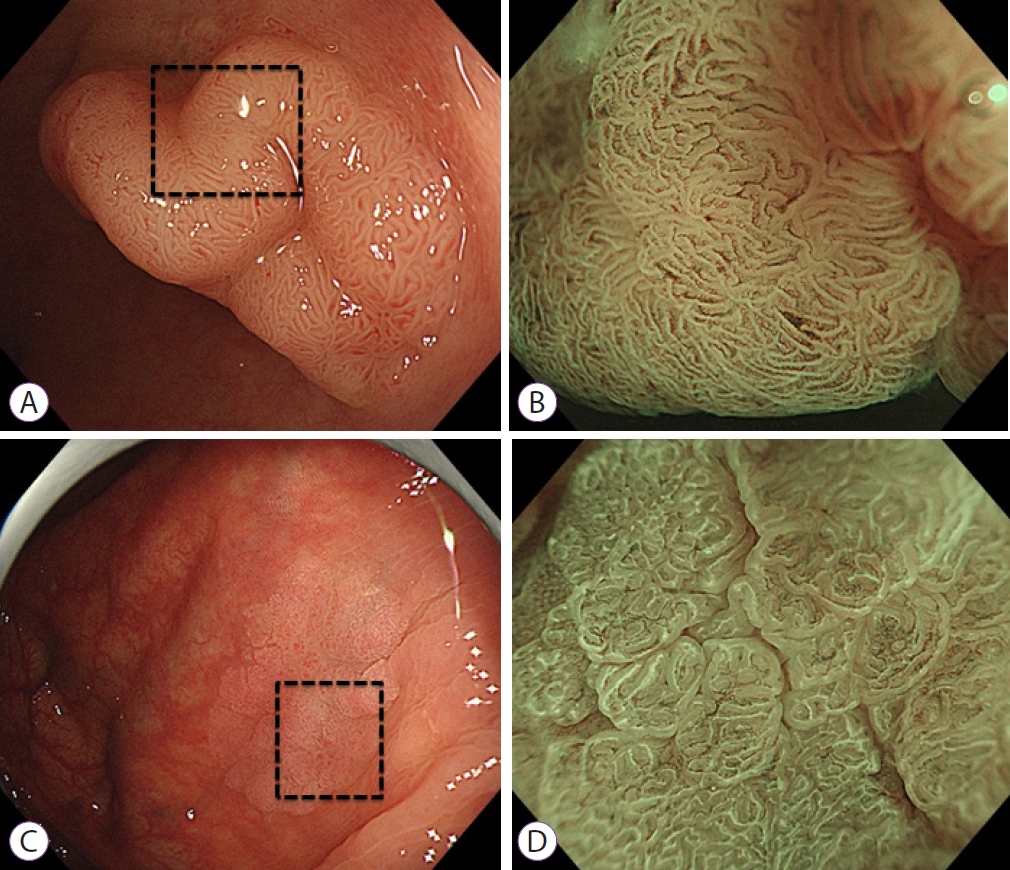
- White Opaque Substance, a New Optical Marker on Magnifying Endoscopy: Usefulness in Diagnosing Colorectal Epithelial Neoplasms
- Kazutomo Yamasaki, Takashi Hisabe, Kenshi Yao, Hiroshi Ishihara, Kentaro Imamura, Tatsuhisa Yasaka, Hiroshi Tanabe, Akinori Iwashita, Toshiharu Ueki
- Clin Endosc 2021;54(4):570-577. Published online January 13, 2021
- DOI: https://doi.org/10.5946/ce.2020.205
-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader ePub
ePub - Background
/Aims: A white substance that is opaque to endoscopic light is sometimes observed in the epithelium during narrowband imaging with magnifying endoscopy of gastric or colorectal epithelial neoplasms. This prospective observational study aimed to determine whether the morphology of the white opaque substance (WOS) allows differential diagnosis between colorectal adenoma and carcinoma.
Methods
A consecutive series of patients with colorectal adenomas or early carcinomas who underwent endoscopic resection or surgical excision were studied. The morphology of the WOS was determined based on endoscopic images before the histopathological diagnosis was performed. The primary outcome was the diagnostic performance of an irregular WOS as a marker of colorectal carcinoma.
Results
The study analyzed 125 lesions. A total of 33 lesions showed an irregular WOS, and 92 lesions showed a regular WOS. Among the 33 lesions found to show an irregular WOS, 30 were carcinomas. Among the 92 lesions showing a regular WOS, 79 were adenomas. With irregular WOS as a marker of carcinoma, the diagnostic accuracy was 87%, sensitivity was 91%, and specificity was 86%.
Conclusions
This study demonstrated the potential usefulness of the morphology of the WOS as a marker for the differential diagnosis between adenoma and carcinoma in cases of colorectal epithelial neoplasms. -
Citations
Citations to this article as recorded by- Emergence of a New Optical Marker for Colorectal Neoplasms: To What Extent Should We Accept It?
Han Hee Lee
Clinical Endoscopy.2022; 55(2): 315. CrossRef
- Emergence of a New Optical Marker for Colorectal Neoplasms: To What Extent Should We Accept It?
- 4,090 View
- 106 Download
- 1 Web of Science
- 1 Crossref

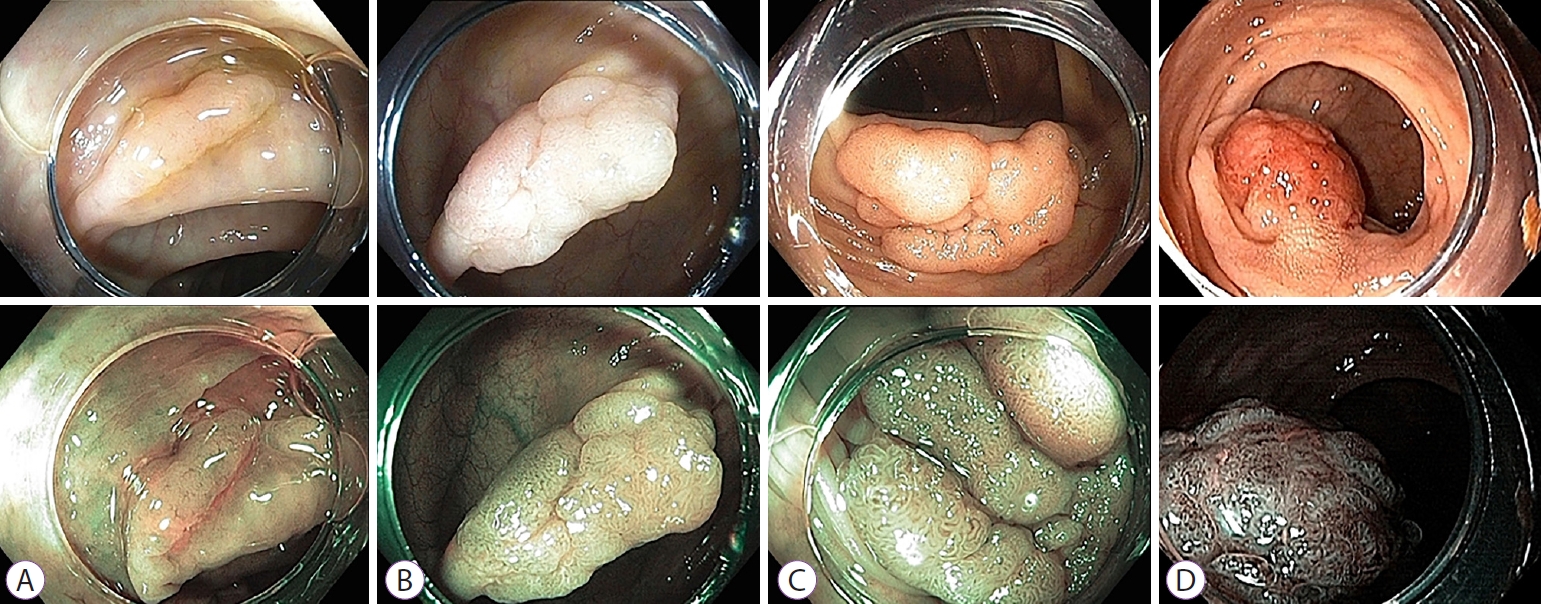
- Comparative Study of Narrow-Band Imaging and i-scan for Predicting the Histology of Intermediate-to-Large Colorectal Polyps: A Prospective, Randomized Pilot Study
- Joon Seop Lee, Seong Woo Jeon, Yong Hwan Kwon
- Clin Endosc 2021;54(6):881-887. Published online January 6, 2021
- DOI: https://doi.org/10.5946/ce.2020.257
-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader ePub
ePub - Background
/Aims: To date, no reports have compared the diagnostic efficacy of narrow-band imaging (NBI) and i-scan for the histologic prediction of intermediate-to-large colorectal polyps. We aimed to compare the diagnostic accuracy of NBI and i-scan in predicting histology, and their inter-/intra-observer agreement.
Methods
We performed a prospective, randomized study that included 66 patients (NBI, n=33 vs. i-scan, n=33) with colorectal polyps (size >10 mm but <50 mm) who underwent colonoscopic resection. During the procedure, three endoscopists documented their prediction using the Japan NBI Expert Team (JNET) classification. Two months after study completion, the endoscopists reviewed still images and video clips for analysis.
Results
The overall diagnostic accuracies in the NBI and i-scan groups were 73.7% (73/99) and 75.8% (75/99), respectively, and there was no statistical significance between the two groups (p=0.744). The JNET classification as applied to NBI and i-scan showed substantial inter-observer agreement (NBI κ-value 0.612, p=0.001 vs. i-scan κ-value 0.662, p=0.002). Additionally, the κ-values of intra-observer agreement were in the range of 0.385–0.660 with NBI and 0.364–0.741 with i-scan.
Conclusions
NBI and i-scan have similar diagnostic accuracies for the histologic prediction of intermediate-to-large colorectal polyps. Furthermore, the inter-/intra-observer agreement was acceptable for both modalities when the JNET classification was applied. -
Citations
Citations to this article as recorded by- Ultra-minimally invasive endoscopic techniques and colorectal diseases: Current status and its future
Nalini Kanta Ghosh, Ashok Kumar
Artificial Intelligence in Gastrointestinal Endoscopy.2024;[Epub] CrossRef - The Utility of Narrow-Band Imaging International Colorectal Endoscopic Classification in Predicting the Histologies of Diminutive Colorectal Polyps Using I-Scan Optical Enhancement: A Prospective Study
Yeo Wool Kang, Jong Hoon Lee, Jong Yoon Lee
Diagnostics.2023; 13(16): 2720. CrossRef - Detecting colorectal lesions with image-enhanced endoscopy: an updated review from clinical trials
Mizuki Nagai, Sho Suzuki, Yohei Minato, Fumiaki Ishibashi, Kentaro Mochida, Ken Ohata, Tetsuo Morishita
Clinical Endoscopy.2023; 56(5): 553. CrossRef - Classification and endoscopic diagnosis of colorectal polyps
Ji Hyun Kim, Sung Chul Park
Journal of the Korean Medical Association.2023; 66(11): 633. CrossRef - Usefulness of optical enhancement endoscopy combined with magnification to improve detection of intestinal metaplasia in the stomach
Sergio Sobrino-Cossío, Oscar Teramoto-Matsubara, Fabian Emura, Raúl Araya, Vítor Arantes, Elymir S. Galvis-García, Marisi Meza-Caballero, Blanca Sinahi García-Aguilar, Arturo Reding-Bernal, Noriya Uedo
Endoscopy International Open.2022; 10(04): E441. CrossRef - Interventions to improve adenoma detection rates for colonoscopy
Aasma Shaukat, Anne Tuskey, Vijaya L. Rao, Jason A. Dominitz, M. Hassan Murad, Rajesh N. Keswani, Fateh Bazerbachi, Lukejohn W. Day
Gastrointestinal Endoscopy.2022; 96(2): 171. CrossRef - A modified fujinon intelligent color enhancement (FICE) in the diagnostics of superficial epithelial neoplasms of the colon
V. A. Duvanskiy, A. V. Belkov
Experimental and Clinical Gastroenterology.2022; (5): 154. CrossRef - Mucosal imaging in colon polyps: New advances and what the future may hold
Edward John Young, Arvinf Rajandran, Hamish Lachlan Philpott, Dharshan Sathananthan, Sophie Fenella Hoile, Rajvinder Singh
World Journal of Gastroenterology.2022; 28(47): 6632. CrossRef - Commentary on “Comparative Study of Narrow-Band Imaging and i-scan for Predicting the Histology of Intermediate-to-Large Colorectal Polyps: A Prospective, Randomized Pilot Study”
Yunho Jung, Masayuki Kato
Clinical Endoscopy.2021; 54(6): 781. CrossRef
- Ultra-minimally invasive endoscopic techniques and colorectal diseases: Current status and its future
- 4,427 View
- 145 Download
- 8 Web of Science
- 9 Crossref

- Management of Complications of Colorectal Submucosal Dissection
- Eun Ran Kim, Dong Kyung Chang
- Clin Endosc 2019;52(2):114-119. Published online March 29, 2019
- DOI: https://doi.org/10.5946/ce.2019.063
-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader ePub
ePub - Endoscopic submucosal dissection (ESD) is a useful procedure for the treatment of superficial gastrointestinal neoplasm. Compared with endoscopic mucosal resection (EMR), ESD has several benefits, which include resectability of various difficult lesion, accurate histologic assessment of specimen, and lower recurrence rate. However, the risk of procedure- related complications is higher with ESD than with EMR. Moreover, because the colon has a thin wall and limited endoscopic maneuverability, ESD is considered a more challenging and risky procedure when performed in the colon than in the stomach. ESD-related complications are more likely to occur. The significant complications associated with ESD are bleeding, perforation, coagulation syndrome and stenosis, most of which can be treated and prevented by endoscopic intervention and preparation. Therefore, it is important to know how to occur and manage the ESD related complication.
-
Citations
Citations to this article as recorded by- Feasibility and safety of 0.6% sodium alginate in endoscopic submucosal dissection for colorectal neoplastic lesion: A pilot study
Hajime Nakamura, Rie Morita, Ryo Ito, Akira Sakurada, Natsumi Tomita, Yuya Hirata, Yusuke Kanari, Yuya Komatsu, Kunihiro Takanashi, Tomonori Anbo, Shinichi Katsuki
DEN Open.2024;[Epub] CrossRef - Endoscopic Submucosal Dissection for Resections Larger than 10 cm: Outcomes from a Portuguese Center
Raquel R. Mendes, Pedro Barreiro, André Mascarenhas, Ana Rita Franco, Liliana Carvalho, Cristina Chagas
GE - Portuguese Journal of Gastroenterology.2024; 31(1): 33. CrossRef - A Phase II Clinical Trial to Study the Safety of Triamcinolone after Endoscopic Radial Incision and Cutting Dilatation for Benign Stenosis of the Lower Gastrointestinal Tract: A Study Protocol
RINTARO MOROI, HISASHI SHIGA, KOTARO NOCHIOKA, HIROFUMI CHIBA, YUSUKE SHIMOYAMA, MOTOYUKI ONODERA, TAKEO NAITO, MASAKI TOSA, YOICHI KAKUTA, YUICHIRO SATO, SHOICHI KAYABA, SEICHI TAKAHASHI, SATOSHI MIYATA, YOSHITAKA KINOUCHI, ATSUSHI MASAMUNE
The Kurume Medical Journal.2024;[Epub] CrossRef - Meta-Analysis of Endoscopic Full-Thickness Resection Versus Endoscopic Submucosal Dissection for Complex Colorectal Lesions
Sahib Singh, Babu P. Mohan, Rakesh Vinayek, Sudhir Dutta, Dushyant S. Dahiya, Manesh K. Gangwani, Vishnu C. Suresh Kumar, Ganesh Aswath, Ishfaq Bhat, Sumant Inamdar, Neil Sharma, Douglas G. Adler
Journal of Clinical Gastroenterology.2024;[Epub] CrossRef - Management of giant colorectal polyps (≥3 cm) by endoscopic submucosal dissection (ESD) versus surgery: a propensity score–based analysis
Lo Hau Ching Michelle, Poon Chi Ming Michael
Surgical Practice.2024;[Epub] CrossRef - The role of endoluminal surgery in a colorectal surgical practice. A global view
Ilker Ozgur, Fevzi Cengiz
Seminars in Colon and Rectal Surgery.2024; 35(2): 101023. CrossRef - Endoscopic submucosal dissection for colorectal polyps: outcome determining factors
Chi Woo Samuel Chow, Tak Lit Derek Fung, Pak Tat Chan, Kam Hung Kwok
Surgical Endoscopy.2023; 37(2): 1293. CrossRef - A novel strategy to perform endoscopic full-thickness resection at the ileocecal valve and securing the orifice with a double-pigtail catheter
Moritz Meiborg, Nicolae-Catalin Mechie, Tobias Blasberg, Marie Weber, Edris Wedi
Endoscopy.2023; 55(S 01): E375. CrossRef - A novel strategy to perform endoscopic full-thickness resection at the ileocecal valve and securing the orifice with a double-pigtail catheter
Moritz Meiborg, Nicolae-Catalin Mechie, Tobias Blasberg, Marie Weber, Edris Wedi
Endoscopy.2023; 55(06): 583. CrossRef - Experience of surgical treatment in a granular cell tumor in the qscending colon: a case report
In-Kyeong Kim, Young-Tae Ju, Han-Gil Kim, Jin-Kwon Lee, Dong-Chul Kim, Jae-Myung Kim, Jin Kyu Cho, Ji-Ho Park, Ju-Yeon Kim, Chi-Young Jeong, Soon-Chan Hong, Seung-Jin Kwag
Annals of Coloproctology.2023; 39(3): 275. CrossRef - Management of complications related to colorectal endoscopic submucosal dissection
Tae-Geun Gweon, Dong-Hoon Yang
Clinical Endoscopy.2023; 56(4): 423. CrossRef - Endoscopic Mucosal Resection and Endoscopic Submucosal Dissection
Sumeyye Yilmaz, Emre Gorgun
Clinics in Colon and Rectal Surgery.2023;[Epub] CrossRef - Colorectal Endoscopic Submucosal Dissection: Performance of a Novel Hybrid-Technology Knife in an Animal Trial
Jérémie Jacques, Horst Neuhaus, Markus D. Enderle, Ulrich Biber, Walter Linzenbold, Martin Schenk, Kareem Khalaf, Alessandro Repici
Diagnostics.2023; 13(21): 3347. CrossRef - Delayed Perforation of Colorectal Endoscopic Submucosal Dissection Treated by Endoscopic Ultrasound-Guided Drainage
Koichi Hamada, Yoshiki Shiwa, Akira Kurita, Yukitoshi Todate, Yoshinori Horikawa, Kae Techigawara, Masafumi Ishikawa, Takayuki Nagahashi, Yuki Takeda, Daizo Fukushima, Noriyuki Nishino, Hideo Sakuma, Michitaka Honda
Case Reports in Gastroenterology.2023; 17(1): 155. CrossRef - Colonoscopic‐assisted laparoscopic wedge resection versus segmental colon resection for benign colonic polyps: a comparative cost analysis
Julia Hanevelt, Laura W. Leicher, Leon M. G. Moons, Frank P. Vleggaar, Jelle F. Huisman, Henderik L. van Westreenen, Wouter H. de Vos tot Nederveen Cappel
Colorectal Disease.2023; 25(11): 2147. CrossRef - Temperature profile and residual heat of monopolar laparoscopic and endoscopic dissection instruments
Franz Brinkmann, Ronny Hüttner, Philipp J. Mehner, Konrad Henkel, Georgi Paschew, Moritz Herzog, Nora Martens, Andreas Richter, Sebastian Hinz, Justus Groß, Clemens Schafmayer, Jochen Hampe, Alexander Hendricks, Frank Schwandner
Surgical Endoscopy.2022; 36(6): 4507. CrossRef - A pilot study investigating the safety and feasibility of endoscopic dilation using a radial incision and cutting technique for benign strictures of the small intestine: a study protocol
Rintaro Moroi, Hisashi Shiga, Kotaro Nochioka, Yusuke Shimoyama, Masatake Kuroha, Yoichi Kakuta, Yoshitaka Kinouchi, Atsushi Masamune
Pilot and Feasibility Studies.2022;[Epub] CrossRef - Applicability of endoscopic submucosal dissection after unsuccessful endoscopic mucosal resection in colorectal laterally spreading tumors: a single center experience
Abdullah Murat BUYRUK, Ayten LİVAOĞLU, Aydın AKTAŞ
Ege Tıp Dergisi.2022; 61(2): 151. CrossRef - One thousand endoscopic submucosal dissections. Experience of the national center
S.I. Achkasov, Yu.A. Shelygin, A.A. Likutov, D.A. Mtvralashvili, V.V. Veselov, O.A. Mainovskaya, M.A. Nagudov, S.V. Chernyshov
Khirurgiya. Zhurnal im. N.I. Pirogova.2022; (8): 5. CrossRef - Post-polypectomy syndrome—a rare complication in colonoscopy procedures: a case report
Julián A Romo, Jorge David Peña, Laura A López, Carlos Figueroa, Horacio Garzon, Andrea Recamán
Journal of Surgical Case Reports.2022;[Epub] CrossRef - Clinical outcomes of endoscopic submucosal dissection for colorectal neoplasms: A single-center experience in Southern Taiwan
Chen-Yu Ko, Chih-Chien Yao, Yu-Chi Li, Lung-Sheng Lu, Yeh-Pin Chou, Ming-Luen Hu, Yi-Chun Chiu, Seng-Kee Chuah, Wei-Chen Tai, Hsu-Heng Yen
PLOS ONE.2022; 17(10): e0275723. CrossRef - Safety and feasibility of same-day discharge after esophageal endoscopic submucosal dissection
Yuri Hanada, Kenneth K. Wang
Gastrointestinal Endoscopy.2021; 93(4): 853. CrossRef - Evaluations on laser ablation of ex vivo porcine stomach tissue for development of Ho:YAG-assisted endoscopic submucosal dissection (ESD)
Hanjae Pyo, Hyeonsoo Kim, Hyun Wook Kang
Lasers in Medical Science.2021; 36(7): 1437. CrossRef - Evaluation of improved bi-manual endoscopic resection using a customizable 3D-printed manipulator system designed for use with standard endoscopes: a feasibility study using a porcine ex-vivo model
Benjamin Walter, Yannick S. Krieger, Dirk Wilhelm, Hubertus Feussner, Tim C. Lueth, Alexander Meining
Endoscopy International Open.2021; 09(06): E881. CrossRef - A patient-like swine model of gastrointestinal fibrotic strictures for advancing therapeutics
Ling Li, Mohamad I. Itani, Kevan J. Salimian, Yue Li, Olaya Brewer Gutierrez, Haijie Hu, George Fayad, Jean A. Donet, Min Kyung Joo, Laura M. Ensign, Vivek Kumbhari, Florin M. Selaru
Scientific Reports.2021;[Epub] CrossRef - Review on colorectal endoscopic submucosal dissection focusing on the technical aspect
Tak Lit Derek Fung, Chi Woo Samuel Chow, Pak Tat Chan, Kam Hung Kwok
Surgical Endoscopy.2020; 34(9): 3766. CrossRef - Endovascular hemostasis for endoscopic procedure-related gastrointestinal bleeding
Minho Park, Jong Woo Kim, Ji Hoon Shin
International Journal of Gastrointestinal Intervention.2019; 8(3): 134. CrossRef
- Feasibility and safety of 0.6% sodium alginate in endoscopic submucosal dissection for colorectal neoplastic lesion: A pilot study
- 8,803 View
- 339 Download
- 27 Web of Science
- 27 Crossref

- Estimation of Invasion Depth: The First Key to Successful Colorectal ESD
- Bo-In Lee, Takahisa Matsuda
- Clin Endosc 2019;52(2):100-106. Published online March 27, 2019
- DOI: https://doi.org/10.5946/ce.2019.012

-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader ePub
ePub - Colorectal tumors with superficial submucosal invasion, which cannot be removed by snaring, are one of the most optimal indications for colorectal endoscopic submucosal dissection (ESD). Therefore, estimation of the invasion depth is the first key to successful colorectal ESD. Although estimation of the invasion depth based on the gross morphology may be useful in selected cases, its diagnostic accuracy could not reach the clinical requirement. The Japan Narrow-band Imaging (NBI) Expert Team (JNET) classification of NBI magnifying endoscopy findings is a useful method for histologic prediction and invasion depth estimation. However, magnifying chromoendoscopy is still necessary for JNET type 2B lesions to reach a satisfactory diagnostic accuracy. Endocytoscopy with artificial intelligence is a promising technology in invasion depth estimation; however, more data are needed for its clinical application.
-
Citations
Citations to this article as recorded by- Comparison of two pathological processing methods for large endoscopic submucosal dissection (ESD) specimens
Zixiang Yu, Dongxian Jiang, Wen Huang, Rongkui Luo, Haixing Wang, Jieakesu Su, Jia Liu, Chen Xu, Yingyong Hou
Journal of Clinical Pathology.2023; 76(11): 757. CrossRef - Endoscopic submucosal dissection for colorectal polyps: outcome determining factors
Chi Woo Samuel Chow, Tak Lit Derek Fung, Pak Tat Chan, Kam Hung Kwok
Surgical Endoscopy.2023; 37(2): 1293. CrossRef - Endoscopic management of patients with high-risk colorectal colitis–associated neoplasia: a Delphi study
Michiel T.J. Bak, Eduardo Albéniz, James E. East, Nayantara Coelho-Prabhu, Noriko Suzuki, Yutaka Saito, Takayuki Matsumoto, Rupa Banerjee, Michal F. Kaminski, Ralf Kiesslich, Emmanuel Coron, Annemarie C. de Vries, C. Janneke van der Woude, Raf Bisschops,
Gastrointestinal Endoscopy.2023; 97(4): 767. CrossRef - Detecting colorectal lesions with image-enhanced endoscopy: an updated review from clinical trials
Mizuki Nagai, Sho Suzuki, Yohei Minato, Fumiaki Ishibashi, Kentaro Mochida, Ken Ohata, Tetsuo Morishita
Clinical Endoscopy.2023; 56(5): 553. CrossRef - Development of artificial intelligence technology in diagnosis, treatment, and prognosis of colorectal cancer
Feng Liang, Shu Wang, Kai Zhang, Tong-Jun Liu, Jian-Nan Li
World Journal of Gastrointestinal Oncology.2022; 14(1): 124. CrossRef - Linear-array EUS improves the accuracy of predicting deep submucosal invasion in non-pedunculated rectal polyps compared with radial EUS: a prospective observational study
Zhixian Lan, Kangyue Sun, Yuchen Luo, Haiyan Hu, Wei Zhu, Wen Guo, Jing Wen, Wenting Mi, Junsheng Chen, Xiang Chen, Venkata Akshintala, Ying Huang, Side Liu, Yue Li
Surgical Endoscopy.2021; 35(4): 1734. CrossRef - Deep learning analysis for the detection of pancreatic cancer on endosonographic images: a pilot study
Ryosuke Tonozuka, Takao Itoi, Naoyoshi Nagata, Hiroyuki Kojima, Atsushi Sofuni, Takayoshi Tsuchiya, Kentaro Ishii, Reina Tanaka, Yuichi Nagakawa, Shuntaro Mukai
Journal of Hepato-Biliary-Pancreatic Sciences.2021; 28(1): 95. CrossRef - Comparison of long-term recurrence-free survival between primary surgery and endoscopic resection followed by secondary surgery in T1 colorectal cancer
Eun Hye Oh, Nayoung Kim, Sung Wook Hwang, Sang Hyoung Park, Dong-Hoon Yang, Byong Duk Ye, Seung-Jae Myung, Suk-Kyun Yang, Chang Sik Yu, Jin Cheon Kim, Jeong-Sik Byeon
Gastrointestinal Endoscopy.2021; 94(2): 394. CrossRef - The impact of transanal local excision of early rectal cancer on completion rectal resection without neoadjuvant chemoradiotherapy: a systematic review
R. Zinicola, R. Nascimbeni, R. Cirocchi, G. Gagliardi, N. Cracco, M. Giuffrida, G. Pedrazzi, G. A. Binda
Techniques in Coloproctology.2021; 25(9): 997. CrossRef - Role of Artificial Intelligence in Video Capsule Endoscopy
Ioannis Tziortziotis, Faidon-Marios Laskaratos, Sergio Coda
Diagnostics.2021; 11(7): 1192. CrossRef - Controversies in EUS: Do we need miniprobes?
Hans Seifert, Pietro Fusaroli, PaoloGiorgio Arcidiacono, Barbara Braden, Felix Herth, Michael Hocke, Alberto Larghi, Bertrand Napoleon, Mihai Rimbas, BogdanSilvio Ungureanu, Adrian Sãftoiu, AnandV Sahai, ChristophF Dietrich
Endoscopic Ultrasound.2021; 10(4): 246. CrossRef - RNA-sequencing identification and validation of genes differentially expressed in high-risk adenoma, advanced colorectal cancer, and normal controls
Namjoo Kim, Jeong-An Gim, Beom Jae Lee, Byung il Choi, Seung Bin Park, Hee Sook Yoon, Sang Hee Kang, Seung Han Kim, Moon Kyung Joo, Jong-Jae Park, Chungyeul Kim, Han-Kyeom Kim
Functional & Integrative Genomics.2021; 21(3-4): 513. CrossRef - Editors' Choice of Noteworthy Clinical Endoscopy Publications in the First Decade
Gwang Ha Kim, Kwang An Kwon, Do Hyun Park, Jimin Han
Clinical Endoscopy.2021; 54(5): 633. CrossRef - Artificial intelligence in gastrointestinal endoscopy: general overview
Ahmad El Hajjar, Jean-François Rey
Chinese Medical Journal.2020; 133(3): 326. CrossRef - Curriculum for optical diagnosis training in Europe: European Society of Gastrointestinal Endoscopy (ESGE) Position Statement
Evelien Dekker, Britt B. S. L. Houwen, Ignasi Puig, Marco Bustamante-Balén, Emmanuel Coron, Daniela E. Dobru, Roman Kuvaev, Helmut Neumann, Gavin Johnson, Pedro Pimentel-Nunes, David S. Sanders, Mario Dinis-Ribeiro, Marianna Arvanitakis, Thierry Ponchon,
Endoscopy.2020;[Epub] CrossRef - Use of artificial intelligence in improving adenoma detection rate during colonoscopy: Might both endoscopists and pathologists be further helped
Emanuele Sinagra, Matteo Badalamenti, Marcello Maida, Marco Spadaccini, Roberta Maselli, Francesca Rossi, Giuseppe Conoscenti, Dario Raimondo, Socrate Pallio, Alessandro Repici, Andrea Anderloni
World Journal of Gastroenterology.2020; 26(39): 5911. CrossRef - The Role of Artificial Intelligence in Endoscopic Ultrasound for Pancreatic Disorders
Ryosuke Tonozuka, Shuntaro Mukai, Takao Itoi
Diagnostics.2020; 11(1): 18. CrossRef - Endoscopic imaging techniques for detecting early colorectal cancer
Ignasi Puig, Carlos Mármol, Marco Bustamante
Current Opinion in Gastroenterology.2019; 35(5): 432. CrossRef
- Comparison of two pathological processing methods for large endoscopic submucosal dissection (ESD) specimens
- 7,485 View
- 335 Download
- 18 Web of Science
- 18 Crossref

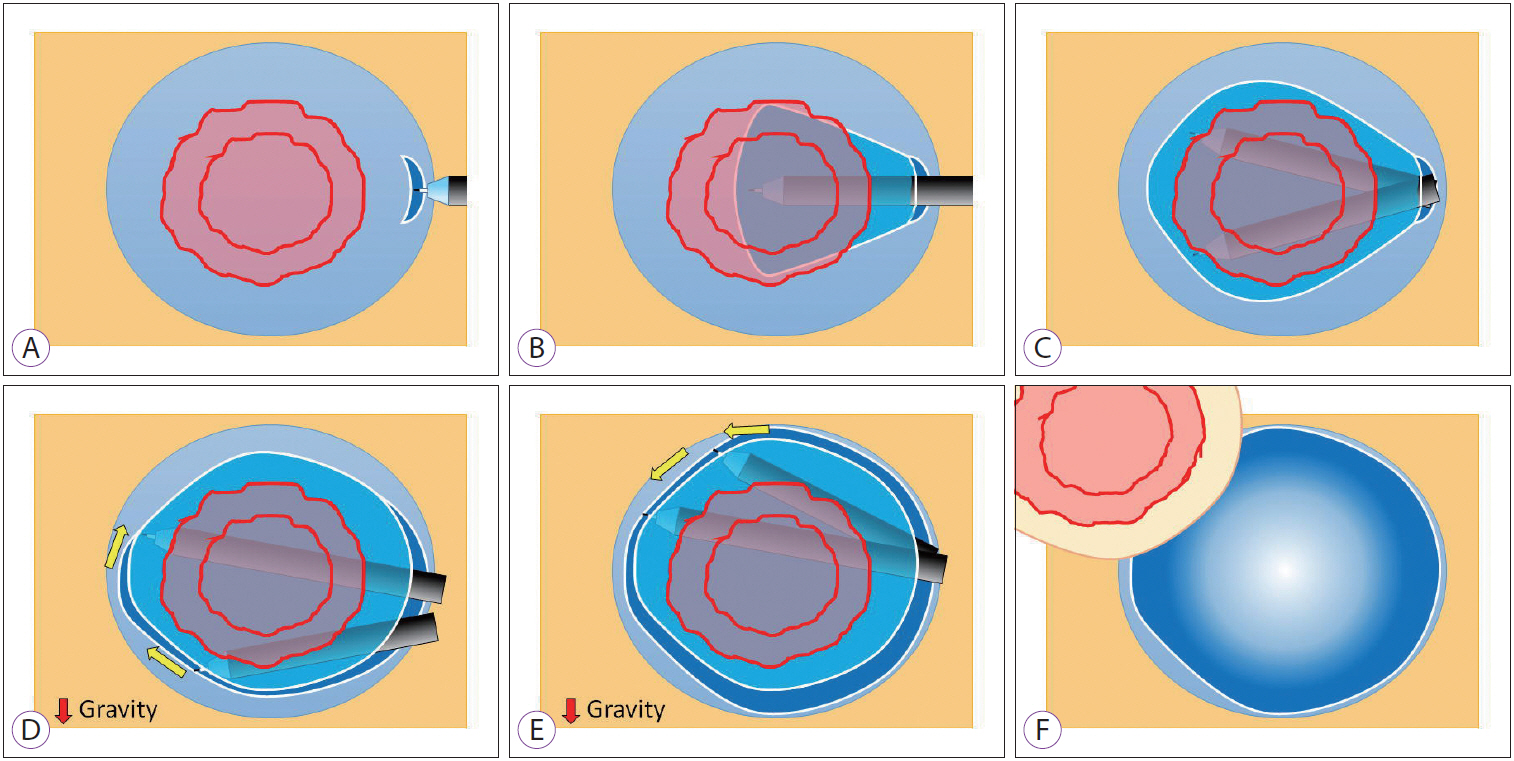
- Advanced Treatment and Imaging in Colonoscopy: The Pocket-Creation Method for Complete Resection and Linked Color Imaging for Better Detection of Early Neoplastic Lesions by Colonoscopy
- Hironori Yamamoto, Satoshi Shinozaki, Yoshikazu Hayashi, Yoshimasa Miura, Tsevelnorov Khurelbaatar, Hiroyuki Osawa, Alan Kawarai Lefor
- Clin Endosc 2019;52(2):107-113. Published online January 10, 2019
- DOI: https://doi.org/10.5946/ce.2018.189
-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader ePub
ePub - Early detection and resection of neoplastic lesions are key objectives to diminish colorectal cancer mortality. Resection of superficial colorectal neoplasms, cold snare polypectomy, endoscopic mucosal resection, and endoscopic submucosal dissection have all been developed and used worldwide. The pocket-creation method facilitates the resection of tumors in difficult and routine locations. Early detection is the most important first step to maximize the benefits of recent advancements in endoscopic techniques. However, the detection of small, flat-shaped, or faded color lesions remains difficult. Linked color imaging, a novel multi-light technology, facilitates the recognition of minor differences in tissue by enhancing the color contrast between early colorectal neoplasms and surrounding normal mucosa in a bright field of view. The most striking feature of linked color imaging is its ability to display the color of early neoplastic lesions as distinct from inflammatory changes, both of which have similar “redness” when viewed using white light imaging. To increase the detection rate of neoplasms, linked color imaging should be used from the outset for endoscopic observation. Early detection of superficial colorectal tumors can result in decreased mortality from colorectal cancer and maintain a good quality of life for patients.
-
Citations
Citations to this article as recorded by- Comparison of blue laser imaging and light‐emitting diode‐blue light imaging for the characterization of colorectal polyps using the Japan narrow‐band imaging expert team classification: The LASEREO and ELUXEO COLonoscopic study
Masahiro Okada, Naohisa Yoshida, Hiroshi Kashida, Yoshikazu Hayashi, Satoshi Shinozaki, Shiori Yoshimoto, Toshihiro Fujinuma, Hirotsugu Sakamoto, Keijiro Sunada, Yuri Tomita, Osamu Dohi, Ken Inoue, Ryohei Hirose, Yoshito Itoh, Yoriaki Komeda, Ikue Sekai,
DEN Open.2024;[Epub] CrossRef - Linked color imaging versus white light imaging in the diagnosis of colorectal lesions: a meta-analysis of randomized controlled trials
Yining Sun, Xiu-He Lv, Xian Zhang, Jin Wang, Huimin Wang, Jin-Lin Yang
Therapeutic Advances in Gastroenterology.2023;[Epub] CrossRef - The Pocket-Creation Method Facilitates Endoscopic Submucosal Dissection of Gastric Neoplasms Along the Lesser Curvature at the Gastric Angle
Masafumi Kitamura, Yoshimasa Miura, Satoshi Shinozaki, Alan Kawarai Lefor, Hironori Yamamoto
Frontiers in Medicine.2022;[Epub] CrossRef - Development and validation of the linked color imaging classification for endoscopic prediction of colorectal polyp histology
Min Min, Shou Bin Ning, Duan Min Hu, Yoshikazu Hayashi, Yan Liu
Journal of Digestive Diseases.2022; 23(5-6): 310. CrossRef - Combination of endoscopic submucosal dissection techniques, a practical solution for difficult cases
Dong-Hoon Yang
Clinical Endoscopy.2022; 55(5): 626. CrossRef - Medical needs related to the endoscopic technology and colonoscopy for colorectal cancer diagnosis
Juan Francisco Ortega-Morán, Águeda Azpeitia, Luisa F. Sánchez-Peralta, Luis Bote-Curiel, Blas Pagador, Virginia Cabezón, Cristina L. Saratxaga, Francisco M. Sánchez-Margallo
BMC Cancer.2021;[Epub] CrossRef - Comparison of long-term recurrence-free survival between primary surgery and endoscopic resection followed by secondary surgery in T1 colorectal cancer
Eun Hye Oh, Nayoung Kim, Sung Wook Hwang, Sang Hyoung Park, Dong-Hoon Yang, Byong Duk Ye, Seung-Jae Myung, Suk-Kyun Yang, Chang Sik Yu, Jin Cheon Kim, Jeong-Sik Byeon
Gastrointestinal Endoscopy.2021; 94(2): 394. CrossRef - The pocket-creation method facilitates gastric endoscopic submucosal dissection and overcomes challenging situations
Masafumi Kitamura, Yoshimasa Miura, Satoshi Shinozaki, Hironori Yamamoto
VideoGIE.2021; 6(9): 390. CrossRef - Application of linked color imaging in the diagnosis of early gastrointestinal neoplasms and precancerous lesions: a review
Shanshan Wang, Lei Shen, Hesheng Luo
Therapeutic Advances in Gastroenterology.2021; 14: 175628482110259. CrossRef - Colon polyp detection using linked color imaging compared to white light imaging: Systematic review and meta‐analysis
Satoshi Shinozaki, Yasutoshi Kobayashi, Yoshikazu Hayashi, Hirotsugu Sakamoto, Keijiro Sunada, Alan Kawarai Lefor, Hironori Yamamoto
Digestive Endoscopy.2020; 32(6): 874. CrossRef - The Role of Cholangioscopy in the Management of Primary Sclerosing Cholangitis
Aldo J. Montano-Loza, Maryam Ebadi, Gurpal Sandha
Current Hepatology Reports.2020; 19(2): 78. CrossRef - Linked color imaging for the detection of early gastrointestinal neoplasms
Satoshi Shinozaki, Hiroyuki Osawa, Yoshikazu Hayashi, Alan Kawarai Lefor, Hironori Yamamoto
Therapeutic Advances in Gastroenterology.2019; 12: 175628481988524. CrossRef
- Comparison of blue laser imaging and light‐emitting diode‐blue light imaging for the characterization of colorectal polyps using the Japan narrow‐band imaging expert team classification: The LASEREO and ELUXEO COLonoscopic study
- 6,479 View
- 214 Download
- 11 Web of Science
- 12 Crossref

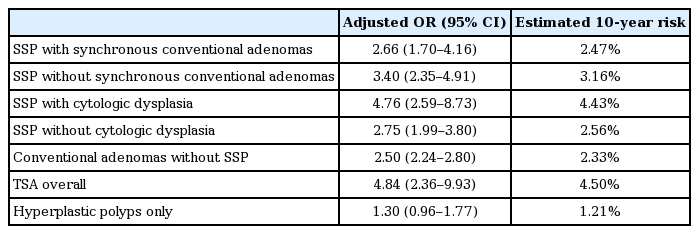
- Screening Relevance of Sessile Serrated Polyps
- Charles J. Kahi
- Clin Endosc 2019;52(3):235-238. Published online January 8, 2019
- DOI: https://doi.org/10.5946/ce.2018.112
-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader ePub
ePub - Conventional adenomas have historically been considered to be the only screening-relevant colorectal cancer (CRC) precursor lesion. The prevailing paradigm was that most CRCs arise along the chromosomal instability pathway, where adenomas accumulate incremental genetic alterations over time, leading eventually to malignancy. However, it is now recognized that this “conventional” pathway accounts for only about two-thirds of CRCs. The serrated pathway is responsible for most of the remainder, and is a disproportionate contributor to postcolonoscopy CRC. Hallmarks of the serrated pathway are mutations in the BRAF gene, high levels of methylation of promoter CpG islands, and the sessile serrated polyp (SSP). Accumulating evidence shows that SSPs can be considered adenoma-equivalent from the standpoint of CRC screening. SSPs have a higher prevalence than previously thought, and appear to have a relatively long dwell time similar to that of conventional adenomas. In addition, SSPs, whether sporadic or as part of the serrated polyposis syndrome, are associated with increased risk of synchronous and metachronous neoplasia. These features collectively support that SSPs are highly relevant to CRC prevention.
-
Citations
Citations to this article as recorded by- Sessile serrated lesion detection rates continue to increase: 2008–2020
Nicholas Edwardson, Prajakta Adsul, Zorisadday Gonzalez, V. Shane Pankratz, Gulshan Parasher, Kevin English, Shiraz Mishra
Endoscopy International Open.2023; 11(01): E107. CrossRef - Long-term Use of Hormone Replacement Therapy is Associated With a Lower Risk of Developing High-risk Serrated Polyps in Women
Dylan E. O’Sullivan, Yibing Ruan, Nauzer Forbes, Steven J. Heitman, Robert J. Hilsden, Joy Pader, Darren R. Brenner
Journal of Clinical Gastroenterology.2022; 56(8): 697. CrossRef - Distinct colon mucosa microbiomes associated with tubular adenomas and serrated polyps
Julio Avelar-Barragan, Lauren DeDecker, Zachary N. Lu, Bretton Coppedge, William E. Karnes, Katrine L. Whiteson
npj Biofilms and Microbiomes.2022;[Epub] CrossRef - Impact of looping on premalignant polyp detection during colonoscopy
Osamu Toyoshima, Toshihiro Nishizawa, Shuntaro Yoshida, Tatsuya Matsuno, Toru Arano, Ryo Kondo, Kazunori Kinoshita, Yuki Yasumi, Yosuke Tsuji, Mitsuhiro Fujishiro
World Journal of Gastrointestinal Endoscopy.2022; 14(11): 694. CrossRef - Cost-Effectiveness of Colorectal Cancer Surveillance in Hodgkin Lymphoma Survivors Treated with Procarbazine and/or Infradiaphragmatic Radiotherapy
Berbel L.M. Ykema, Andrea Gini, Lisanne S. Rigter, Manon C.W. Spaander, Leon M.G. Moons, Tanya M. Bisseling, Jan Paul de Boer, Wieke H.M. Verbeek, Pieternella J. Lugtenburg, Cecile P.M. Janus, Eefke J. Petersen, Judith M. Roesink, Richard W.M. van der Maa
Cancer Epidemiology, Biomarkers & Prevention.2022; 31(12): 2157. CrossRef - Serrated Lesions in Inflammatory Bowel Disease: Genotype-Phenotype Correlation
Iva Brcic, Heather Dawson, Hans Peter Gröchenig, Christoph Högenauer, Karl Kashofer
International Journal of Surgical Pathology.2021; 29(1): 46. CrossRef - Increased Sessile Serrated Adenoma Detection Rate With Mechanical New Technology Devices
Elijah Verheyen, Daniel Castaneda, Seth A. Gross, Violeta Popov
Journal of Clinical Gastroenterology.2021; 55(4): 335. CrossRef - The prevalence of sessile serrated lesion in the colorectum and its relationship to synchronous colorectal advanced neoplasia: a systemic review and meta-analysis
Sz-Iuan Shiu, Hiroshi Kashida, Yoriaki Komeda
European Journal of Gastroenterology & Hepatology.2021; 33(12): 1495. CrossRef - Detection of Microsatellite Instability in Colorectal Cancer Patients With a Plasma-Based Real-Time PCR Analysis
Namjoo Kim, Sung Min Kim, Beom Jae Lee, Byung il Choi, Hee Sook Yoon, Sang Hee Kang, Seung Han Kim, Moon Kyung Joo, Jong-Jae Park, Chungyeul Kim
Frontiers in Pharmacology.2021;[Epub] CrossRef - Biology and Therapeutic Targets of Colorectal Serrated Adenocarcinoma; Clues for a Histologically Based Treatment against an Aggressive Tumor
Begoña Alburquerque-González, Fernando F. López-Calderón, María Dolores López-Abellán, Ángel Esteban-Gil, José García-Solano, Pablo Conesa-Zamora
International Journal of Molecular Sciences.2020; 21(6): 1991. CrossRef - Computer aided detection for laterally spreading tumors and sessile serrated adenomas during colonoscopy
Guanyu Zhou, Xun Xiao, Mengtian Tu, Peixi Liu, Dan Yang, Xiaogang Liu, Renyi Zhang, Liangping Li, Shan Lei, Han Wang, Yan Song, Pu Wang, Wajid Mumtaz
PLOS ONE.2020; 15(4): e0231880. CrossRef - The Molecular Hallmarks of the Serrated Pathway in Colorectal Cancer
Fatima De Palma, Valeria D’Argenio, Jonathan Pol, Guido Kroemer, Maria Maiuri, Francesco Salvatore
Cancers.2019; 11(7): 1017. CrossRef
- Sessile serrated lesion detection rates continue to increase: 2008–2020
- 6,870 View
- 275 Download
- 12 Web of Science
- 12 Crossref

- Endoscopic Features of Mucous Cap Polyps: A Way to Predict Serrated Polyps
- Brian T. Moy, Faripour Forouhar, Chia-Ling Kuo, Thomas J. Devers
- Clin Endosc 2018;51(4):368-374. Published online April 27, 2018
- DOI: https://doi.org/10.5946/ce.2017.155

-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader ePub
ePub - Background
/Aims: The aims of the study were to identify whether a mucous-cap predicts the presence of serrated polyps, and to determine whether additional endoscopic findings predict the presence of a sessile serrated adenomas/polyp (SSA/P).
Methods
We analyzed 147 mucous-capped polyps with corresponding histology, during 2011–2014. Eight endoscopic features (presence of borders, elevation, rim of debris, location in the colon, size ≥10 mm, varicose vessels, nodularity, and alteration in mucosal folds) of mucous-capped polyps were examined to see if they can predict SSA/Ps.
Results
A total of 86% (n=126) of mucous-capped polyps were from the right sided serrated pathway (right-sided hyperplastic [n=83], SSA/Ps [n=43], traditional serrated adenoma [n=1]), 10% (n=15) were left-sided hyperplastic polyps, and 3% (n=5) were from the adenoma-carcinoma sequence. The presence of a mucous cap combined with varicose vessels was the only significant predictor for SSA/Ps. The other seven characteristics were not found to be statistically significant for SSA/Ps, although location in the colon and the presence of nodularity trended towards significance.
Conclusions
Our study suggests that mucous-capped polyps have high predictability for being a part of the serrated pathway. Gastroenterologists should be alert for a mucous-capped polyp with varicose veins, as these lesions have a higher risk of SSA/P. -
Citations
Citations to this article as recorded by- Clinicopathological features and expression of regulatory mechanism of the Wnt signaling pathway in colorectal sessile serrated adenomas/polyps with different syndrome types
Dan Qiao, Xiao-Yan Liu, Lie Zheng, Ya-Li Zhang, Ren-Ye Que, Bing-Jing Ge, Hong-Yan Cao, Yan-Cheng Dai
World Journal of Clinical Cases.2023; 11(9): 1963. CrossRef - Coagulase-negative staphylococci (CoNS) as a significant etiological factor of laryngological infections: a review
Michał Michalik, Alfred Samet, Adrianna Podbielska-Kubera, Vincenzo Savini, Jacek Międzobrodzki, Maja Kosecka-Strojek
Annals of Clinical Microbiology and Antimicrobials.2020;[Epub] CrossRef - Endoscopic features of sessile serrated adenoma/polyps under narrowband imaging: A retrospective study
Xin Tian Zhang, Qing Wei Zhang, Fei Liu, Xiao Lu Lin, Jin Nan Chen, Xiao Bo Li
Journal of Digestive Diseases.2019; 20(3): 135. CrossRef - How to Detect Sessile Serrated Adenoma/Polyps
Eun Ran Kim, Dong Kyung Chang
Clinical Endoscopy.2018; 51(4): 313. CrossRef
- Clinicopathological features and expression of regulatory mechanism of the Wnt signaling pathway in colorectal sessile serrated adenomas/polyps with different syndrome types
- 7,064 View
- 149 Download
- 4 Web of Science
- 4 Crossref

- Colorectal Cancer Screening—Who, How, and When?
- Roisin Bevan, Matthew D Rutter
- Clin Endosc 2018;51(1):37-49. Published online January 31, 2018
- DOI: https://doi.org/10.5946/ce.2017.141
-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader ePub
ePub - Colorectal cancer (CRC) is the third most common cancer worldwide. It is amenable to screening as it occurs in premalignant, latent, early, and curable stages. PubMed, Cochrane Database of Systematic Reviews, and national and international CRC screening guidelines were searched for CRC screening methods, populations, and timing. CRC screening can use direct or indirect tests, delivered opportunistically or via organized programs. Most CRCs are diagnosed after 60 years of age; most screening programs apply to individuals 50–75 years of age. Screening may reduce disease-specific mortality by detecting CRC in earlier stages, and CRC incidence by detecting premalignant polyps, which can subsequently be removed. In randomized controlled trials (RCTs) guaiac fecal occult blood testing (gFOBt) was found to reduce CRC mortality by 13%–33%. Fecal immunochemical testing (FIT) has no RCT data comparing it to no screening, but is superior to gFOBt. Flexible sigmoidoscopy (FS) trials demonstrated an 18% reduction in CRC incidence and a 28% reduction in CRC mortality. Currently, RCT evidence for colonoscopy screening is scarce. Although not yet corroborated by RCTs, it is likely that colonoscopy is the best screening modality for an individual. From a population perspective, organized programs are superior to opportunistic screening. However, no nation can offer organized population-wide colonoscopy screening. Thus, organized programs using cheaper modalities, such as FS/FIT, can be tailored to budget and capacity.
-
Citations
Citations to this article as recorded by- Evaluating the potential impact of lifestyle-based behavior change interventions delivered at the time of colorectal cancer screening
Veeraj Shah, Greta Geller, Diane Xu, Lily Taylor, Simon Griffin, Juliet A. Usher-Smith
Cancer Causes & Control.2024; 35(3): 561. CrossRef - Evaluation of tumor-educated platelet long non-coding RNAs (lncRNAs) as potential diagnostic biomarkers for colorectal cancer
Seidamir Pasha Tabaeian, Zahra Shokati Eshkiki, Fatemeh Dana, Farimah Fayyaz, Mansoureh Baniasadi, Shahram Agah, Mohsen Masoodi, Elahe Safari, Meghdad Sedaghat, Paria Abedini, Abolfazl Akbari
Journal of Cancer Research and Therapeutics.2024;[Epub] CrossRef - Restrictive diets are unnecessary for colonoscopy: Non-inferiority randomized trial
Salvador Machlab, Eva Martínez-Bauer, Pilar López, Pablo Ruiz-Ramirez, Bárbara Gómez, Antonio Z. Gimeno-Garcia, María del Mar Pujals, Sara Tanco, Lluïsa Sargatal, Betty Pérez, Reyes Justicia, Mónica Enguita, Nùria Piqué, Oliver Valero, Xavier Calvet, Rafe
Endoscopy International Open.2024; 12(03): E352. CrossRef - Managing Colorectal Cancer from Ethology to Interdisciplinary Treatment: The Gains and Challenges of Modern Medicine
Monika Berbecka, Maciej Berbecki, Anna Maria Gliwa, Monika Szewc, Robert Sitarz
International Journal of Molecular Sciences.2024; 25(4): 2032. CrossRef - Incidental Diagnosis of Metastatic Colorectal Cancer in a Habitual Hookah Smoker: A Case Report Emphasizing Early Detection and Lifestyle Risks
Omar B Banamah, Renad A Sagim, Abdullah A Al Qurashi
Cureus.2024;[Epub] CrossRef -
Armamentarium in Drug Delivery for Colorectal Cancer
Asad Ali, Juber Akhtar, Usama Ahmad, Abdul Samad Basheer, Neha Jaiswal, Afroz Jahan
Critical Reviews™ in Therapeutic Drug Carrier Systems.2023; 40(1): 1. CrossRef - Older Age at First Screening Colonoscopy is Associated With an Increased Risk of Colorectal Adenomas and Cancer
David Obadina, Haider Haider, Dejan Micic, Atsushi Sakuraba
Journal of Clinical Gastroenterology.2023; 57(8): 804. CrossRef - A Pilot Colorectal Cancer Study Using Fecal Occult Blood Tests and Colonoscopy to Identify the Weaknesses of the Romanian Public Healthcare System before Implementing National Screening
Linda-Nicoleta Bărbulescu, Stelian-Ștefăniță Mogoantă, Lucian-Florentin Bărbulescu, Constantin Kamal, Didi-Liliana Popa, Radu-Teodoru Popa
International Journal of Environmental Research and Public Health.2023; 20(3): 2531. CrossRef - Effectiveness of Colorectal Cancer (CRC) Screening on All-Cause and CRC-Specific Mortality Reduction: A Systematic Review and Meta-Analysis
Senshuang Zheng, Jelle J. A. Schrijvers, Marcel J. W. Greuter, Gürsah Kats-Ugurlu, Wenli Lu, Geertruida H. de Bock
Cancers.2023; 15(7): 1948. CrossRef - A new modality of colorectal cancer screening based on chronic disease management
Mo Liu, Shi-Jun Liu, Ming-Jun Chen, Tingting Ning
BMC Gastroenterology.2023;[Epub] CrossRef - Does Health Literacy Affect Colorectal Cancer Screening Rates?
Melike Yalçın Gürsoy, Canan Bulut Ayaz
Journal of Community Health Nursing.2023; 40(2): 147. CrossRef - Clinical utility of colon capsule endoscopy: a moving target?
Gohar Jalayeri Nia, Ramesh P. Arasaradnam, Anastasios Koulaouzidis
Therapeutic Advances in Gastroenterology.2023;[Epub] CrossRef - External validation of the Moroccan Arabic version of the European Organization for Research and Treatment of Cancer colorectal (CR29) module: Monocentric study
Houda Bachri, Hajar Essangri, Nezha El Bahaoui, Amine Benkabbou, Raouf Mohsine, Anass Mohammed Majbar, Amine Souadka
World Journal of Methodology.2023; 13(4): 259. CrossRef -
Association of
HLA-G
3′ Untranslated Region Indel Polymorphism and Its Serum Expression with Susceptibility to Colorectal Cancer
Garrach Behaeddin, Ben Othmen Abdelwaheb, Khamlaoui Wided, Yatouji Sonia, Toumi Iheb, Zaied Sonia, Zouari Khadija, Hammami Mohamed, Hammami Sonia
Biomarkers in Medicine.2023; 17(12): 541. CrossRef - Swiss cost-effectiveness analysis of universal screening for Lynch syndrome of patients with colorectal cancer followed by cascade genetic testing of relatives
Islam Salikhanov, Karl Heinimann, Pierre Chappuis, Nicole Buerki, Rossella Graffeo, Viola Heinzelmann, Manuela Rabaglio, Monica Taborelli, Simon Wieser, Maria C. Katapodi
Journal of Medical Genetics.2022; 59(9): 924. CrossRef - A Noninvasive Risk Stratification Tool Build Using an Artificial Intelligence Approach for Colorectal Polyps Based on Annual Checkup Data
Chieh Lee, Tsung-Hsing Lin, Chen-Ju Lin, Chang-Fu Kuo, Betty Chien-Jung Pai, Hao-Tsai Cheng, Cheng-Chou Lai, Tsung-Hsing Chen
Healthcare.2022; 10(1): 169. CrossRef - Association between colorectal cancer and the degree of ITGA4 promoter methylation in peripheral blood mononuclear cells
Sima Jafarpour, Farideh Saberi, Maryam Yazdi, Reza Nedaeinia, Guilda Amini, Gordon A. Ferns, Rasoul Salehi
Gene Reports.2022; 27: 101580. CrossRef - Geographic variation and association of risk factors with incidence of colorectal cancer at small-area level
Getachew A. Dagne
Cancer Causes & Control.2022; 33(9): 1155. CrossRef - Intervention during wait time: identification and referral of individuals non-adherent for colorectal cancer screening
Beau Abar, Chanjun Syd Park, Preeti Dalawari, Howard Klausner, Chinwe Ogedegbe, Steven Valassis, Haran Koneswaran, David Adler, Keith Bradley
Emergency Cancer Care.2022;[Epub] CrossRef - Impact of an Abdominal Compression Bandage on the Completion of Colonoscopy for Obese Adults: A Prospective Randomized Controlled Trial
Ting-Ting Liu, Yi-Teng Meng, Feng Xiong, Cheng Wei, Su Luo, Sheng-Gang Zhan, Yang Song, Ying-Xue Li, Rui-Yue Shi, Jun Yao, Li-Sheng Wang, De-Feng Li, Xingshun Qi
Canadian Journal of Gastroenterology and Hepatology.2022; 2022: 1. CrossRef - Impact of looping on premalignant polyp detection during colonoscopy
Osamu Toyoshima, Toshihiro Nishizawa, Shuntaro Yoshida, Tatsuya Matsuno, Toru Arano, Ryo Kondo, Kazunori Kinoshita, Yuki Yasumi, Yosuke Tsuji, Mitsuhiro Fujishiro
World Journal of Gastrointestinal Endoscopy.2022; 14(11): 694. CrossRef - How Can the EU Beating Cancer Plan Help in Tackling Lung Cancer, Colorectal Cancer, Breast Cancer and Melanoma?
Denis Horgan, Anne-Marie Baird, Mark Middleton, Zhasmina Mihaylova, Jan P. Van Meerbeeck, Jens Vogel-Claussen, Paul E. Van Schil, Josep Malvehy, Paolo Antonio Ascierto, France Dube, Michael Zaiac, Jonathan A. Lal, Grażyna Kamińska-Winciorek, Marco Donia,
Healthcare.2022; 10(9): 1618. CrossRef - Culturally-adapted behavioral intervention to improve colorectal cancer screening uptake among foreign-born South Asians in New Jersey: the Desi Sehat trial
Sharon L. Manne, Nadia Islam, Sara Frederick, Usman Khan, Sunanda Gaur, Anam Khan
Ethnicity & Health.2021; 26(4): 554. CrossRef - Guaiac Fecal Occult Blood Tests and Mortality: A 30-Year Follow-up of Two Pooled Trials
Emma C. Robbins, Amanda J. Cross
Clinical Gastroenterology and Hepatology.2021; 19(5): 892. CrossRef - Comparable quality of bowel preparation with single‐day versus three‐day low‐residue diet: Randomized controlled trial
Salvador Machlab, Eva Martínez‐Bauer, Pilar López, Núria Piqué, Valentí Puig‐Diví, Félix Junquera, Alba Lira, Enric Brullet, Anna Selva, Pilar García‐Iglesias, Xavier Calvet, Rafel Campo
Digestive Endoscopy.2021; 33(5): 797. CrossRef - Race/ethnicity, sex and insurance disparities in colorectal cancer screening among individuals with and without cardiovascular disease
Swati Sakhuja, Mackenzie E. Fowler, Akinyemi I. Ojesina
Preventive Medicine Reports.2021; 21: 101263. CrossRef - Computed tomography colonography and radiation risk: How low can we go?
Jelena Popic, Sanda Tipuric, Ivan Balen, Anna Mrzljak
World Journal of Gastrointestinal Endoscopy.2021; 13(3): 72. CrossRef - A Sigmoid-Colon-Straightening Soft Actuator With Peristaltic Motion for Colonoscopy Insertion Assistance: Easycolon
Hansoul Kim, Joonhwan Kim, Jae Min You, Seung Woo Lee, Ki-Uk Kyung, Dong-Soo Kwon
IEEE Robotics and Automation Letters.2021; 6(2): 3577. CrossRef - A Disintegrin and Metalloprotease 12 Promotes Tumor Progression by Inhibiting Apoptosis in Human Colorectal Cancer
Young-Lan Park, Sun-Young Park, Hyung-Hoon Oh, Min-Woo Chung, Ji-Yun Hong, Ki-Hyun Kim, Dae-Seong Myung, Sung-Bum Cho, Wan-Sik Lee, Hyun-Soo Kim, Young-Eun Joo
Cancers.2021; 13(8): 1927. CrossRef - Current Landscape in Organic Nanosized Materials Advances for Improved Management of Colorectal Cancer Patients
Octav Ginghină, Ariana Hudiță, Cătălin Zaharia, Aristidis Tsatsakis, Yaroslav Mezhuev, Marieta Costache, Bianca Gălățeanu
Materials.2021; 14(9): 2440. CrossRef - The immunomodulatory effects of low molecular weight garlic protein in crosstalk between peripheral blood mononuclear cells and colon cancer cells
Mona Amani, Elham Shokati, Kobra Entezami, Samaneh Khorrami, Mir Hadi Jazayeri, Elahe Safari
Process Biochemistry.2021; 108: 161. CrossRef - Predictors of clinical outcomes of self-expandable metal stent treatment for malignant colorectal obstruction
Bora Han, Ji-Yun Hong, Eun Myung, Hyung-Hoon Oh, Hee-Chan Yang, Sang-Wook Kim, Jun Lee, Seong-Jung Kim, Yeom-Dong Han, Geom-Seok Seo, Gun-Young Hong, Ho-Dong Kim, Hyun-Soo Kim, Young-Eun Joo
Medicine.2021; 100(27): e26616. CrossRef - RNA-sequencing identification and validation of genes differentially expressed in high-risk adenoma, advanced colorectal cancer, and normal controls
Namjoo Kim, Jeong-An Gim, Beom Jae Lee, Byung il Choi, Seung Bin Park, Hee Sook Yoon, Sang Hee Kang, Seung Han Kim, Moon Kyung Joo, Jong-Jae Park, Chungyeul Kim, Han-Kyeom Kim
Functional & Integrative Genomics.2021; 21(3-4): 513. CrossRef - Evaluation of colonoscopic findings in patients undergoing colonoscopy due to positive fecal occult blood test: a single center experience
Tolga DÜZENLİ, Mevlut KİYAK
Journal of Health Sciences and Medicine.2021; 4(5): 646. CrossRef - Editors' Choice of Noteworthy Clinical Endoscopy Publications in the First Decade
Gwang Ha Kim, Kwang An Kwon, Do Hyun Park, Jimin Han
Clinical Endoscopy.2021; 54(5): 633. CrossRef - Nanoparticle-Mediated Delivery Systems in Photodynamic Therapy of Colorectal Cancer
Nokuphila Winifred Nompumelelo Simelane, Heidi Abrahamse
International Journal of Molecular Sciences.2021; 22(22): 12405. CrossRef - Undertaking general practice quality improvement to improve cancer screening - a thematic analysis of provider experiences
Steven A. Trankle, Christine Metusela, Jennifer Reath
BMC Family Practice.2021;[Epub] CrossRef - Colorectal cancer in adolescents and young adults with Lynch syndrome: a Danish register-based study
Jon Ambæk Durhuus, Christina Therkildsen, Thomas Kallemose, Mef Nilbert
BMJ Open.2021; 11(12): e053538. CrossRef - Gut Microbiota Profiles in Early- and Late-Onset Colorectal Cancer: A Potential Diagnostic Biomarker in the Future
Murdani Abdullah, Ninik Sukartini, Saskia Aziza Nursyirwan, Rabbinu Rangga Pribadi, Hasan Maulahela, Amanda Pitarini Utari, Virly Nanda Muzellina, Agustinus Wiraatmadja, Kaka Renaldi
Digestion.2021; 102(6): 823. CrossRef - A Critical Review of Second-Generation Anti-EGFR Monoclonal Antibodies in Metastatic Colorectal Cancer
Daniel Sur, Andrei Havasi, Alecsandra Gorzo, Claudia Burz
Current Drug Targets.2021; 22(9): 1034. CrossRef - Association Between Carotid Ultrasonography Findings and Colorectal Adenoma in Asymptomatic Adults
Jeongseok Kim, Ji Young Lee, Nam Seok Ham, Eun Hye Oh, Hye-Sook Chang, Hyewon Park, Yoon Suh Do, Sung Wook Hwang, Dong-Hoon Yang, Jae Won Choe, Jeong-Sik Byeon
Digestive Diseases and Sciences.2020; 65(6): 1816. CrossRef - DNA methylation and gene expression profiles characterize epigenetic regulation of lncRNAs in colon adenocarcinoma
Zhijin Li, Hua Tan, Hai Yu, Zhong Deng, Xiaobo Zhou, Maode Wang
Journal of Cellular Biochemistry.2020; 121(3): 2406. CrossRef - Colonoscopy using back brace support belt: A randomized, prospective trial
Osamu Toyoshima, Toshihiro Nishizawa, Kosuke Sakitani, Tadahiro Yamakawa, Shuntaro Yoshida, Kazushi Fukagawa, Keisuke Hata, Soichiro Ishihara, Hidekazu Suzuki
JGH Open.2020; 4(3): 441. CrossRef - Clinical outcomes of submucosal colorectal cancer diagnosed after endoscopic resection: a focus on the need for surgery
Yun Sik Choi, Wan Soo Kim, Sung Wook Hwang, Sang Hyoung Park, Dong-Hoon Yang, Byong Duk Ye, Seung-Jae Myung, Suk-Kyun Yang, Jeong-Sik Byeon
Intestinal Research.2020; 18(1): 96. CrossRef - Association of low skeletal muscle mass with the presence of advanced colorectal neoplasm: integrative analysis using three skeletal muscle mass indices
H. J. Lee, J. Y. Lee, M. J. Lee, H.‐K. Kim, N. Kim, G.‐U. Kim, J.‐S. Lee, H. W. Park, H.‐S. Chang, D.‐H. Yang, J. Choe, J.‐S. Byeon
Colorectal Disease.2020; 22(10): 1293. CrossRef - Nanoparticles in Colorectal Cancer Therapy: Latest In Vivo Assays, Clinical Trials, and Patents
Laura Cabeza, Gloria Perazzoli, Cristina Mesas, Cristina Jiménez-Luna, José Prados, Ana Rosa Rama, Consolación Melguizo
AAPS PharmSciTech.2020;[Epub] CrossRef - Acceptance on colorectal cancer screening upper age limit in South Korea
Xuan Quy Luu, Kyeongmin Lee, Yun Yoeng Lee, Mina Suh, Yeol Kim, Kui Son Choi
World Journal of Gastroenterology.2020; 26(27): 3963. CrossRef - Acceptance on colorectal cancer screening upper age limit in South Korea
Xuan Quy Luu, Kyeongmin Lee, Yun Yoeng Lee, Mina Suh, Yeol Kim, Kui Son Choi
World Journal of Gastroenterology.2020; 26(27): 3959. CrossRef - Effects of Alternative Offers of Screening Sigmoidoscopy and Colonoscopy on Utilization and Yield of Endoscopic Screening for Colorectal Neoplasms: Protocol of the DARIO Randomized Trial
Petra Schrotz-King, Michael Hoffmeister, Peter Sauer, Anja Schaible, Hermann Brenner
JMIR Research Protocols.2020; 9(8): e17516. CrossRef - Colonoscopy and flexible sigmoidoscopy for follow-up of patients with left-sided diverticulitis
Z Abdulazeez, N Kukreja, N Qureshi, S Lascelles
The Annals of The Royal College of Surgeons of England.2020; 102(9): 744. CrossRef - Rapid review of factors associated with flexible sigmoidoscopy screening use
Robert S. Kerrison, Christian von Wagner, Trish Green, Monica Gibbins, Una Macleod, Mark Hughes, Colin J. Rees, Stephen Duffy, Lesley M. McGregor
Preventive Medicine.2019; 120: 8. CrossRef - Facilitating colorectal cancer cell metastasis by targeted binding of long non-coding RNA ENSG00000231881 with miR-133b via VEGFC signaling pathway
Jianyu Zhou, Yueyi Zou, Gui Hu, Changwei Lin, Yihang Guo, Kai Gao, Mayrong Wu
Biochemical and Biophysical Research Communications.2019; 509(1): 1. CrossRef - Is it Possible to Successfully Treat Locally Advanced Colon Cancer Using Pre-Operative Chemoradiotherapy?
Ji Hun Choi, Jae Hyun Kim, Won Moon, Seung Hun Lee, Sung Uhn Baek, Byung Kwon Ahn, Jung Gu Park, Seun Ja Park
Clinical Endoscopy.2019; 52(2): 191. CrossRef - Colorectal Cancer Screening in People With and Without HIV in an Integrated Health Care Setting
Jennifer O. Lam, Leo B. Hurley, Natalia Udaltsova, Stacey E. Alexeeff, Daniel B. Klein, Douglas A. Corley, Michael J. Silverberg
JAIDS Journal of Acquired Immune Deficiency Syndromes.2019; 81(3): 284. CrossRef - A Soft Pneumatic Inchworm Double balloon (SPID) for colonoscopy
Luigi Manfredi, Elisabetta Capoccia, Gastone Ciuti, Alfred Cuschieri
Scientific Reports.2019;[Epub] CrossRef - Colorectal cancer screening guidelines for Nigeria in 2019
OlusegunIsaac Alatise, Olalekan Olasehinde, AbdulfataiBamidele Olokoba, BabatundeM Duduyemi, OlusolaC Famurewa, OludareF Adeyemi, ElugwaraonuA Agbakwuru, AW Asombang
NIGERIAN JOURNAL OF GASTROENTEROLOGY AND HEPATOLOGY.2019; 11(2): 42. CrossRef - Microbial markers in colorectal cancer detection and/or prognosis
Romain Villéger, Amélie Lopès, Julie Veziant, Johan Gagnière, Nicolas Barnich, Elisabeth Billard, Delphine Boucher, Mathilde Bonnet
World Journal of Gastroenterology.2018; 24(22): 2327. CrossRef
- Evaluating the potential impact of lifestyle-based behavior change interventions delivered at the time of colorectal cancer screening
- 14,471 View
- 396 Download
- 55 Web of Science
- 57 Crossref

- Full-Thickness Resection Device for Complex Colorectal Lesions in High-Risk Patients as a Last-Resort Endoscopic Treatment: Initial Clinical Experience and Review of the Current Literature
- Edris Wedi, Beatrice Orlandini, Mark Gromski, Carlo Felix Maria Jung, Irina Tchoumak, Stephanie Boucher, Volker Ellenrieder, Jürgen Hochberger
- Clin Endosc 2018;51(1):103-108. Published online January 31, 2018
- DOI: https://doi.org/10.5946/ce.2017.093
-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader ePub
ePub - The full-thickness resection device (FTRD) is a novel endoscopic device approved for the resection of colorectal lesions. This case-series describes the device and its use in high-risk patients with colorectal lesions and provides an overview of the potential indications in recently published data.
Between December 2014 and September 2015, 3 patients underwent endoscopic full thickness resection using the FTRD for colorectal lesions: 1 case for a T1 adenocarcinoma in the region of a surgical anastomosis after recto-sigmoidectomy, 1 case for a non-lifting colonic adenoma with low-grade dysplasia in an 89-year old patient and 1 for a recurrent adenoma with high-grade dysplasia in a young patient with ulcerative rectocolitis who was under immunosuppression after renal transplantation. Both technical and clinical success rates were achieved in all cases. The size of removed lesions ranged from 9 to 30 mm.
Overall, the most frequent indication in the literature has been for lifting or non-lifting adenoma, submucosal tumors, neuroendocrin tumors, incomplete endoscopic resection (R1) or T1 carcinoma.
Colorectal FTRD is a feasible technique for the treatment of colorectal lesions and represents a minimally invasive alternative for either surgical or conventional endoscopic resection strategies. -
Citations
Citations to this article as recorded by- Ensayo clínico multicéntrico para la resección de pólipos rectales mediante un nuevo dispositivo de acceso transanal híbrido laparoendoscópico
José Francisco Noguera Aguilar, Alba Gómez Dovigo, Javier Aguirrezabalaga González, Benito González Conde, Pedro Alonso Aguirre, David Martínez Ares, Javier Sánchez González, María Pilar Díez Redondo, Olga Maseda Díaz, María Ignacia Torres García, Andres
Cirugía Española.2023; 101(6): 435. CrossRef - Multicenter clinical trial for the resection of rectal polyps using a new laparoendoscopic hybrid transanal access device
José Francisco Noguera Aguilar, Alba Gómez Dovigo, Javier Aguirrezabalaga González, Benito González Conde, Pedro Alonso Aguirre, David Martínez Ares, Javier Sánchez González, María Pilar Díez Redondo, Olga Maseda Díaz, Maria Ignacia Torres García, Andrés
Cirugía Española (English Edition).2023; 101(6): 435. CrossRef - A novel strategy to perform endoscopic full-thickness resection at the ileocecal valve and securing the orifice with a double-pigtail catheter
Moritz Meiborg, Nicolae-Catalin Mechie, Tobias Blasberg, Marie Weber, Edris Wedi
Endoscopy.2023; 55(S 01): E375. CrossRef - Novel Sequence of Endoscopic Therapy for the Management of Colonic Adenocarcinoma and Surrounding Adenoma
Adam W. Scott, Khalid Amin, Justin R. Howard, Stuart K. Amateau
ACG Case Reports Journal.2023; 10(2): e01008. CrossRef - Distal Cap-assisted Endoscopic Mucosal Resection for Non-lifting Colorectal Polyps: An International, Multicenter Study
Scott R. Douglas, Douglas K. Rex, Alessandro Repici, Melissa Kelly, J. Wes Heinle, Marco Spadaccini, Matthew T. Moyer
Techniques and Innovations in Gastrointestinal Endoscopy.2023; 25(3): 236. CrossRef - A novel strategy to perform endoscopic full-thickness resection at the ileocecal valve and securing the orifice with a double-pigtail catheter
Moritz Meiborg, Nicolae-Catalin Mechie, Tobias Blasberg, Marie Weber, Edris Wedi
Endoscopy.2023; 55(06): 583. CrossRef - Endoscopic mucosal resection and endoscopic submucosal dissection with an external additional working channel (EMR+ and ESD+) are equivalent to using a double-channel endoscope: a systematic evaluation in a porcine ex vivo model
Richard F. Knoop, Ahmad Amanzada, Golo Petzold, Volker Ellenrieder, Michael Engelhardt, Albrecht Neesse, Sebastian C. B. Bremer, Steffen Kunsch
Surgical Endoscopy.2023; 37(10): 7749. CrossRef - EMR + with the AWC improves endoscopic resection speed compared to ESD: a porcine ex-vivo pilot study
Edris Wedi, Richard Knoop, Carlo Jung, Mark Gromski, Chi Nghia Ho, Gabor Conrad, Juergen Maiss, Sinisa Milenovic, David Klemme, Ulrich Baulain, Ali Seif Amir Hosseini, Volker Ellenrieder, Peter Koehler
Minimally Invasive Therapy & Allied Technologies.2021; 30(1): 47. CrossRef - Endoscopic submucosal dissection with an additional working channel (ESD+): a novel technique to improve procedure time and safety of ESD
Richard F. Knoop, Edris Wedi, Golo Petzold, Sebastian C. B. Bremer, Ahmad Amanzada, Volker Ellenrieder, Albrecht Neesse, Steffen Kunsch
Surgical Endoscopy.2021; 35(7): 3506. CrossRef - Novel modified endoscopic mucosal resection of large GI lesions (> 20 mm) using an external additional working channel (AWC) may improve R0 resection rate: initial clinical experience
A. Sportes, Jung CFM, M. A. Gromski, P. Koehler, A. Seif Amir Hosseini, P. Kauffmann, V. Ellenrieder, E. Wedi
BMC Gastroenterology.2020;[Epub] CrossRef - Devices and techniques for endoscopic treatment of residual and fibrotic colorectal polyps (with videos)
Arvind J. Trindade, Nikhil A. Kumta, Manoop S. Bhutani, Vinay Chandrasekhara, Pichamol Jirapinyo, Kumar Krishnan, Joshua Melson, Rahul Pannala, Mansour A. Parsi, Allison R. Schulman, Guru Trikudanathan, Rabindra R. Watson, John T. Maple, David R. Lichtens
Gastrointestinal Endoscopy.2020; 92(3): 474. CrossRef - Endoskopisches Management von kolorektalen Adenomen, HGIEN und Frühkarzinomen
Michael Hünerbein, Frank Kolligs
Onkologie up2date.2020; 2(04): 311. CrossRef - Comparison of Endoscopic Submucosal Dissection for Primary and Recurrent Colorectal Lesions: A Single-Center European Study
Michał Spychalski, Aleksander Skulimowski, Makoto Nishimura, Adam Dziki
Journal of Laparoendoscopic & Advanced Surgical Techniques.2019; 29(3): 366. CrossRef - Optimal Management of Malignant Polyps, From Endoscopic Assessment and Resection to Decisions About Surgery
Douglas K. Rex, Aasma Shaukat, Michael B. Wallace
Clinical Gastroenterology and Hepatology.2019; 17(8): 1428. CrossRef - Endoscopic full-thickness resection of early mucosal neoplasms
Andreas Wannhoff, Karel Caca
Techniques in Gastrointestinal Endoscopy.2019; 21(1): 13. CrossRef - Endoscopic full-thickness resection for early colorectal cancer
Armin Kuellmer, Julius Mueller, Karel Caca, Patrick Aepli, David Albers, Brigitte Schumacher, Anne Glitsch, Claus Schäfer, Ingo Wallstabe, Christopher Hofmann, Andreas Erhardt, Benjamin Meier, Dominik Bettinger, Robert Thimme, Arthur Schmidt
Gastrointestinal Endoscopy.2019; 89(6): 1180. CrossRef - Future of full thickness resection – Devices, indications, robotics, what is missing
Philip WY Chiu
Techniques in Gastrointestinal Endoscopy.2019; 21(1): 48. CrossRef - Clips for managing perforation and bleeding after colorectal endoscopic mucosal resection
A. S. Turan, G. Ultee, E. J. M. Van Geenen, P. D. Siersema
Expert Review of Medical Devices.2019; 16(6): 493. CrossRef - Technical Feasibility of a Guidetube for Various Endoscopic Procedures in Human Gastrointestinal Simulators
Dong Seok Lee, Byeong Gwan Kim, Kook Lae Lee, Yong Jin Jung, Ji Won Kim
Clinical Endoscopy.2019; 52(3): 247. CrossRef - Now, More than Ever Before, Colonoscopy Is a Therapeutic Procedure
Ana Catarina Ribeiro Gomes, Rolando Pinho
GE - Portuguese Journal of Gastroenterology.2019; 26(4): 229. CrossRef
- Ensayo clínico multicéntrico para la resección de pólipos rectales mediante un nuevo dispositivo de acceso transanal híbrido laparoendoscópico
- 9,306 View
- 188 Download
- 25 Web of Science
- 20 Crossref

- External Validation of the Endoscopic Features of Sessile Serrated Adenomas in Expert and Trainee Colonoscopists
- Hyo-Joon Yang, Jeong In Lee, Soo-Kyung Park, Yoon Suk Jung, Jin Hee Sohn, Kyu Yong Choi, Dong Il Park
- Clin Endosc 2017;50(3):279-286. Published online September 13, 2016
- DOI: https://doi.org/10.5946/ce.2016.107
-
 Abstract
Abstract
 PDF
PDF Supplementary Material
Supplementary Material PubReader
PubReader ePub
ePub - Background
/Aims: It is unclear whether the endoscopic features of sessile serrated adenomas (SSAs) would be useful to trainee colonoscopists to predict SSA. Therefore, the present study aimed to identify features that expert and trainee colonoscopists can use to independently and reliably predict SSA by using high-resolution white-light endoscopy.
Methods
Endoscopic images of 81 polyps (39 SSAs, 22 hyperplastic polyps, and 20 tubular adenomas) from 43 patients were retrospectively evaluated by 10 colonoscopists (four experts and six trainees). Eight endoscopic features of SSAs were assessed for each polyp.
Results
According to multivariable analysis, a mucous cap (odds ratio [OR], 10.44; 95% confidence interval [CI], 5.72 to 19.07), indistinctive borders (OR, 4.21; 95% CI, 2.74 to 7.16), dark spots (OR, 3.64; 95% CI, 1.89 to 7.00), and cloud-like surface (OR, 2.43; 95% CI, 1.27 to 4.668) were independent predictors of SSAs. Among these, a mucous cap, indistinctive borders, and cloud-like surface showed moderate interobserver agreement (mean κ >0.40) among experts and trainees. When ≥1 of the three predictors was observed, the sensitivity and specificity for diagnosing SSAs were 79.0% and 81.4%, respectively.
Conclusions
Colonoscopy trainees and experts can use several specific endoscopic features to independently and reliably predict SSAs. -
Citations
Citations to this article as recorded by- Accuracy and Inter-observer Agreement Among Endoscopists for Visual Identification of Colorectal Polyps Using Endoscopy Images
Thi Khuc, Amol Agarwal, Feng Li, Sergey Kantsevoy, Bryan Curtin, Matilda Hagan, Mary Harris, Anurag Maheshwari, Amit Raina, Elinor Zhou, Paul Thuluvath
Digestive Diseases and Sciences.2023; 68(2): 616. CrossRef - Polyp Fact or Polyp Fiction: Endoscopic Identification of Polyps Using Established Criteria to Improve the Quality of Endoscopic Evaluation and Cost Effectiveness
Ihsan Al Bayati, Sarah Al Obaidi, Mohammed Bashashati
Digestive Diseases and Sciences.2023; 68(2): 344. CrossRef - Risk factors for sessile serrated lesions among Chinese patients undergoing colonoscopy
Ru Zhang, Yunbi Ni, Cosmos LT Guo, Rashid NS Lui, William KK Wu, Joseph JY Sung, Vincent WS Wong, Sunny H Wong
Journal of Gastroenterology and Hepatology.2023; 38(9): 1468. CrossRef - Risk factors of traditional serrated adenoma and clinicopathologic characteristics of synchronous conventional adenoma
Jeongseok Kim, Ji Young Lee, Sung Wook Hwang, Sang Hyoung Park, Dong-Hoon Yang, Byong Duk Ye, Seung-Jae Myung, Suk-Kyun Yang, Ja Eun Koo, Hyo Jeong Lee, Jaewon Choe, Jeong-Sik Byeon
Gastrointestinal Endoscopy.2019; 90(4): 636. CrossRef - Identification of risk factors for sessile and traditional serrated adenomas of the colon by using big data analysis
Jeung Hui Pyo, Sang Yun Ha, Sung Noh Hong, Dong Kyung Chang, Hee Jung Son, Kyoung‐Mee Kim, Hyeseung Kim, Kyunga Kim, Jee Eun Kim, Yoon‐Ho Choi, Young‐Ho Kim
Journal of Gastroenterology and Hepatology.2018; 33(5): 1039. CrossRef - Sessile Serrated Adenoma; the Hard-to-Catch Culprit of Interval Cancer
Suk Pyo Shin
Clinical Endoscopy.2017; 50(3): 215. CrossRef - Proximal Sessile Serrated Adenomas Are More Prevalent in Caucasians, and Gastroenterologists Are Better Than Nongastroenterologists at Their Detection
Malav P. Parikh, Sujit Muthukuru, Yash Jobanputra, Kushal Naha, Niyati M. Gupta, Vaibhav Wadhwa, Rocio Lopez, Prashanthi N. Thota, Madhusudhan R. Sanaka
Gastroenterology Research and Practice.2017; 2017: 1. CrossRef
- Accuracy and Inter-observer Agreement Among Endoscopists for Visual Identification of Colorectal Polyps Using Endoscopy Images
- 7,470 View
- 146 Download
- 7 Web of Science
- 7 Crossref

- Endoscopic Mucosal Resection versus Endoscopic Submucosal Dissection for Large Polyps: A Western Colonoscopist’s View
- Ian Holmes, Shai Friedland
- Clin Endosc 2016;49(5):454-456. Published online August 26, 2016
- DOI: https://doi.org/10.5946/ce.2016.077
-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader ePub
ePub - To discuss the rationale for the widespread application of endoscopic mucosal resection (EMR) rather than endoscopic submucosal dissection (ESD) in Western centers. In Western centers, EMR is the treatment of choice for most non-pedunculated colorectal adenomas >2 cm in size. EMR is sufficiently effective and safe to be performed without post-procedure hospitalization. Advances in EMR have led to reduced recurrence rates, and recent studies have demonstrated excellent outcomes with endoscopic treatment of recurrent adenomas. While studies from Asia have demonstrated lower recurrence rates with ESD, concern about the higher perforation risk and lengthy procedure time of ESD are two of the barriers preventing widespread adoption of ESD in the West. EMR is likely to continue as the dominant method for the treatment of large colorectal adenomas in Western centers until the limitations of ESD are overcome.
-
Citations
Citations to this article as recorded by- Identification of Iatrogenic Perforation in Pediatric Gastrointestinal Endoscopy
Oren Ledder, Marek Woynarowski, Diana Kamińska, Izabella Łazowska-Przeorek, Stanislaw Pieczarkowski, Claudio Romano, Raffi Lev-Tzion, Magdalena Holon, Andreia Nita, Anna Rybak, Elżbieta Jarocka-Cyrta, Bartosz Korczowski, Elzbieta Czkwianianc, Iva Hojsak,
Journal of Pediatric Gastroenterology & Nutrition.2023; 77(3): 401. CrossRef - Current status of endoscopic full‐thickness resection with the full‐thickness resection device
Julius Mueller, Armin Kuellmer, Moritz Schiemer, Robert Thimme, Arthur Schmidt
Digestive Endoscopy.2023; 35(2): 232. CrossRef - Endoscopic mucosal resection and endoscopic submucosal dissection with an external additional working channel (EMR+ and ESD+) are equivalent to using a double-channel endoscope: a systematic evaluation in a porcine ex vivo model
Richard F. Knoop, Ahmad Amanzada, Golo Petzold, Volker Ellenrieder, Michael Engelhardt, Albrecht Neesse, Sebastian C. B. Bremer, Steffen Kunsch
Surgical Endoscopy.2023; 37(10): 7749. CrossRef - High-Quality Colonoscopy: A Review of Quality Indicators and Best Practices
Mason Soeder, Alla Turshudzhyan, Lisa Rosenberg, Micheal Tadros
Gastroenterology Insights.2022; 13(2): 162. CrossRef - Risk factors for local recurrence of large gastrointestinal lesions after endoscopic mucosal resection
Yasar Colak, Badar Hasan, Walid Hassaballa, Mamoon Ur Rashid, Victor Strassmann, Giovanna DaSilva, Steven D. Wexner, Tolga Erim
Techniques in Coloproctology.2022; 26(7): 545. CrossRef - From advanced diagnosis to advanced resection in early neoplastic colorectal lesions: Never-ending and trending topics in the 2020s
Francesco Auriemma, Sandro Sferrazza, Mario Bianchetti, Maria Flavia Savarese, Laura Lamonaca, Danilo Paduano, Nicole Piazza, Enrica Giuffrida, Lupe Sanchez Mete, Alessandra Tucci, Sebastian Manuel Milluzzo, Chiara Iannelli, Alessandro Repici, Benedetto M
World Journal of Gastrointestinal Surgery.2022; 14(7): 632. CrossRef - Endoscopic submucosal dissection with an additional working channel (ESD+): a novel technique to improve procedure time and safety of ESD
Richard F. Knoop, Edris Wedi, Golo Petzold, Sebastian C. B. Bremer, Ahmad Amanzada, Volker Ellenrieder, Albrecht Neesse, Steffen Kunsch
Surgical Endoscopy.2021; 35(7): 3506. CrossRef - The Mettle to Use the Petals: Using Over-the-Scope Rings to Optimize Endoscopic Submucosal Dissection
Mike T. Wei, George Triadafilopoulos, Shai Friedland
Digestive Diseases and Sciences.2021; 66(4): 989. CrossRef - Current Endoscopic Resection Techniques for Gastrointestinal Lesions: Endoscopic Mucosal Resection, Submucosal Dissection, and Full-Thickness Resection
Arthur Hoffman, Raja Atreya, Timo Rath, Markus Ferdinand Neurath
Visceral Medicine.2021; 37(5): 358. CrossRef - Japan Gastroenterological Endoscopy Society guidelines for colorectal endoscopic submucosal dissection/endoscopic mucosal resection
Shinji Tanaka, Hiroshi Kashida, Yutaka Saito, Naohisa Yahagi, Hiroo Yamano, Shoichi Saito, Takashi Hisabe, Takashi Yao, Masahiko Watanabe, Masahiro Yoshida, Yusuke Saitoh, Osamu Tsuruta, Ken‐ichi Sugihara, Masahiro Igarashi, Takashi Toyonaga, Yoichi Ajiok
Digestive Endoscopy.2020; 32(2): 219. CrossRef - Features of endoscopic procedure site reaction associated with a recently approved submucosal lifting agent
Carlos A. Castrodad-Rodríguez, Nicole C. Panarelli, Adam J. Gersten, Qiang Liu, Michael Feely, Tony El Jabbour
Modern Pathology.2020; 33(8): 1581. CrossRef - Efficacy and safety of three different endoscopic methods in treatment of 6–20 mm colorectal polyps
Dazhou Li, Wen Wang, Jiao Xie, Gang Liu, Rong Wang, Chuanshen Jiang, Zhou Ye, Binbin Xu, Xiaojian He, Donggui Hong
Scandinavian Journal of Gastroenterology.2020; 55(3): 362. CrossRef - ESD and Pit Pattern Diagnosis: Lessons from a Japanese Endoscopist Working in the United States
Makoto Nishimura
Clinics in Colon and Rectal Surgery.2020; 33(06): 329. CrossRef - Long‐term clinical outcomes of endoscopic submucosal dissection for colorectal neoplasia with or without the hybrid technique
DU Kang, JC Park, SW Hwang, SH Park, DH Yang, KJ Kim, BD Ye, SJ Myung, SK Yang, JS Byeon
Colorectal Disease.2020; 22(12): 2008. CrossRef - Evaluation of recurrence and surgical complementation rates after endoscopic resection of large colorectal non-pedunculated lesions
Alanna Alexandre Silva de Azevedo, Maria Cecilia del Picchia Novaes Ribeiro, Fernando Lander Mota, Paulo Alberto Falco Pires Correa, Jarbas Faraco Maldonado Loureiro
Revista Española de Enfermedades Digestivas.2020;[Epub] CrossRef - How Is Endoscopic Submucosal Dissection for Gastrointestinal Lesions Being Implemented? Results from an International Survey
Miguel Araújo-Martins, Pedro Pimentel-Nunes, Diogo Libânio, Marta Borges-Canha, Mário Dinis-Ribeiro
GE - Portuguese Journal of Gastroenterology.2020; 27(1): 1. CrossRef - Comparison of Endoscopic Submucosal Dissection for Primary and Recurrent Colorectal Lesions: A Single-Center European Study
Michał Spychalski, Aleksander Skulimowski, Makoto Nishimura, Adam Dziki
Journal of Laparoendoscopic & Advanced Surgical Techniques.2019; 29(3): 366. CrossRef - Modular laser-based endoluminal ablation of the gastrointestinal tract: in vivo dose–effect evaluation and predictive numerical model
Giuseppe Quero, Paola Saccomandi, Jung-Myun Kwak, Bernard Dallemagne, Guido Costamagna, Jacques Marescaux, Didier Mutter, Michele Diana
Surgical Endoscopy.2019; 33(10): 3200. CrossRef - Endoscopic resection techniques for colorectal neoplasia: Current developments
Franz Ludwig Dumoulin, Ralf Hildenbrand
World Journal of Gastroenterology.2019; 25(3): 300. CrossRef - Cost Analysis of Endoscopic Submucosal Dissection for the Treatment of Colorectal Lesions in China
Ning Cui, Yu Zhao, Honggang Yu
BioMed Research International.2019; 2019: 1. CrossRef - Endoscopic management of iatrogenic gastrointestinal perforations
Kan Wang, Jihao Shi, Linna Ye
Laparoscopic, Endoscopic and Robotic Surgery.2019; 2(2): 41. CrossRef - Using Endoscopic Submucosal Dissection as a Routine Component of the Standard Treatment Strategy for Large and Complex Colorectal Lesions in a Western Tertiary Referral Unit
Andrew Emmanuel, Shraddha Gulati, Margaret Burt, Bu’Hussain Hayee, Amyn Haji
Diseases of the Colon & Rectum.2018; 61(6): 743. CrossRef - Endoscopic resection of large colorectal adenomas – clinical experience of a tertiary referral centre
L. Mlynarsky, S. Zelber‐Sagi, E. Miller, R. Kariv
Colorectal Disease.2018; 20(5): 391. CrossRef - Implementation of mentor-assisted colorectal endoscopic submucosal dissection in Sweden; learning curve and clinical outcomes
Shunsuke Yamamoto, Tomasz Radomski, Morteza Shafazand
Scandinavian Journal of Gastroenterology.2018; 53(9): 1146. CrossRef
- Identification of Iatrogenic Perforation in Pediatric Gastrointestinal Endoscopy
- 8,770 View
- 283 Download
- 29 Web of Science
- 24 Crossref

- Unusual Local Recurrence with Distant Metastasis after Successful Endoscopic Submucosal Dissection for Colorectal Mucosal Cancer
- Hyo Jeong Lee, Byong Duk Ye, Jeong-Sik Byeon, Jihun Kim, Young Soo Park, Yong Sang Hong, Yong Sik Yoon, Dong-Hoon Yang
- Clin Endosc 2017;50(1):91-95. Published online August 22, 2016
- DOI: https://doi.org/10.5946/ce.2016.054
-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader ePub
ePub - Intramucosal colorectal cancer (CRC) is thought not to metastasize because the colonic lamina propria lacks lymphatics. Only a few recent case reports have suggested lymph node metastasis from intramucosal CRC, but there is no clear evidence supporting the metastatic potential of intramucosal CRC. Hence, endoscopic resection is regarded as curative treatment for intramucosal CRC. This report describes two cases of unusual local recurrence with distant metastasis in patients who had previously undergone successful endoscopic submucosal dissection for intramucosal CRC. The recurrent colorectal lesions developed at the site of the previous endoscopic submucosal dissection scars in a relatively short-term period, and the pathologic findings showed an “undermining” invasion pattern without surrounding mucosal change. Based on the clinical course and pathological findings, we concluded that the second colorectal lesions were recurrences rather than de novo cancers.
-
Citations
Citations to this article as recorded by- Local excision after polypectomy for rectal polyp cancer: when is it worthwhile?
Helen J. S. Jones, Issam al‐Najami, Gunnar Baatrup, Chris Cunningham
Colorectal Disease.2021; 23(4): 868. CrossRef - Presacral lymph node recurrence of rectal intramucosal adenocarcinoma after endoscopic mucosal resection: a case report
Taichi Horino, Yukiharu Hiyoshi, Yuji Miyamoto, Naoya Yoshida, Hideo Baba
Surgical Case Reports.2020;[Epub] CrossRef - Recurrence, death risk, and related factors in patients with stage 0 colorectal cancer
Ming-Hao Hsieh, Pei-Tseng Kung, Wen-Yin Kuo, Tao-Wei Ke, Wen-Chen Tsai
Medicine.2020; 99(36): e21688. CrossRef - Recurrence rate of lateral margin-positive cases after en bloc endoscopic submucosal dissection of colorectal neoplasia
Seohyun Lee, Jihun Kim, Jae Seung Soh, Jungho Bae, Sung Wook Hwang, Sang Hyoung Park, Byong Duk Ye, Jeong-Sik Byeon, Seung-Jae Myung, Suk-Kyun Yang, Dong-Hoon Yang
International Journal of Colorectal Disease.2018; 33(6): 735. CrossRef
- Local excision after polypectomy for rectal polyp cancer: when is it worthwhile?
- 7,211 View
- 199 Download
- 7 Web of Science
- 4 Crossref

- Optimal Colonoscopy Surveillance Interval after Polypectomy
- Tae Oh Kim
- Clin Endosc 2016;49(4):359-363. Published online July 29, 2016
- DOI: https://doi.org/10.5946/ce.2016.080
-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader ePub
ePub - The detection and removal of adenomatous polyps and postpolypectomy surveillance are considered important for the control of colorectal cancer (CRC). Surveillance using colonoscopy is an effective tool for preventing CRC after colorectal polypectomy, especially if compliance is good. In current practice, the intervals between colonoscopies after polypectomy are variable. Different recommendations for recognizing at risk groups and defining surveillance intervals after an initial finding of colorectal adenomas have been published. However, high-grade dysplasia and the number and size of adenomas are known major cancer predictors. Based on this, a subgroup of patients that may benefit from intensive surveillance colonoscopy can be identified.
-
Citations
Citations to this article as recorded by- Gender disparities in colorectal polyps
A. K. Safiyeva
Klinicheskaia khirurgiia.2021; 88(1-2): 57. CrossRef - Three-year colonoscopy surveillance after polypectomy in Korea: a Korean Association for the Study of Intestinal Diseases (KASID) multicenter prospective study
Won Seok Choi, Dong Soo Han, Chang Soo Eun, Dong Il Park, Jeong-Sik Byeon, Dong-Hoon Yang, Sung-Ae Jung, Sang Kil Lee, Sung Pil Hong, Cheol Hee Park, Suck-Ho Lee, Jeong-Seon Ji, Sung Jae Shin, Bora Keum, Hyun Soo Kim, Jung Hye Choi, Sin-Ho Jung
Intestinal Research.2018; 16(1): 126. CrossRef
- Gender disparities in colorectal polyps
- 9,614 View
- 209 Download
- 2 Web of Science
- 2 Crossref

- Endocuff-Assisted Colonoscopy—A Novel Accessory in Improving Adenoma Detection Rate: A Review of the Literature
- Rashmee Patil, Mel A. Ona, Emmanuel Ofori, Madhavi Reddy
- Clin Endosc 2016;49(6):533-538. Published online May 2, 2016
- DOI: https://doi.org/10.5946/ce.2016.032
-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader ePub
ePub - Endocuff (Arc Medical Design) is a U.S. Food and Drug Administration-approved device that is attached like a cap to the distal tip of the colonoscope; it is used to improve adenoma detection rates during colonoscopy. The aim of this review was to summarize and evaluate the clinical and technical efficacy of Endocuff in improving adenoma detection rate. A comprehensive literature review was performed to identify studies describing this technique. In this review article, we have summarized case series and reports describing Endocuff use and results. The reported indications, results, limitations, and complications are discussed.
-
Citations
Citations to this article as recorded by- Endocuff Vision-Assisted Resection for Difficult Colonic Lesions—Preliminary Results of a Multicenter, Prospective Randomized Pilot Study
Rossella Palma, Gianluca Andrisani, Gianfranco Fanello, Augusto Lauro, Cristina Panetta, Chiara Eberspacher, Francesco Di Matteo, Samuele Vaccari, Noemi Zorzetti, Vito D’Andrea, Stefano Pontone
Journal of Clinical Medicine.2023; 12(15): 4980. CrossRef - Endocuff-assisted push enteroscopy increases the detection of proximal small-bowel gastrointestinal angiodysplasias
Christian S. Jackson, Chandrasekhar Kesavan, Anjali Das, Erick Imbertson, Richard M. Strong
Indian Journal of Gastroenterology.2022; 41(3): 300. CrossRef - Técnicas colonoscópicas para la detección de pólipos: un estudio egipcio
M. Abdelbary, S. Hamdy, H. Shehab, N. ElGarhy, M. Menesy, R. Marzaban
Revista de Gastroenterología de México.2021; 86(1): 36. CrossRef - Colonoscopic techniques in polyp detection: An Egyptian study
M. Abdelbary, S. Hamdy, H. Shehab, N. ElGarhy, M. Menesy, R. Marzaban
Revista de Gastroenterología de México (English Edition).2021; 86(1): 36. CrossRef - The Use of Attachment Devices to Aid in Adenoma Detection
Zoe Lawrence, Seth A. Gross
Current Treatment Options in Gastroenterology.2020; 18(1): 137. CrossRef - Number of significant polyps detected per six-minute withdrawal time at colonoscopy (SP6): a new measure of colonoscopy efficiency and quality
Rajaratnam Rameshshanker, Brian P Saunders
Frontline Gastroenterology.2020; 11(6): 491. CrossRef - EndoCuff‐assisted colonoscopy could improve adenoma detection rate: A meta‐analysis of randomized controlled trials
Hai Xu Jian, Bing Cheng Feng, Yan Zhang, Jun Yan Qu, Yue Yue Li, Xiu Li Zuo
Journal of Digestive Diseases.2019; 20(11): 578. CrossRef - Sessile Serrated Adenomas: How to Detect, Characterize and Resect
Michael X. Ma, Michael J. Bourke
Gut and Liver.2017; 11(6): 747. CrossRef - En este número
Enrique Murcio Pérez
Endoscopia.2016; 28(4): 135. CrossRef
- Endocuff Vision-Assisted Resection for Difficult Colonic Lesions—Preliminary Results of a Multicenter, Prospective Randomized Pilot Study
- 9,076 View
- 254 Download
- 8 Web of Science
- 9 Crossref

- Factors for Endoscopic Submucosal Dissection in Early Colorectal Neoplasms: A Single Center Clinical Experience in China
- Yu-Qi He, Xin Wang, Ai-Qin Li, Lang Yang, Jian Zhang, Qian Kang, Shan Tang, Peng Jin, Jian-Qiu Sheng
- Clin Endosc 2015;48(5):405-410. Published online September 30, 2015
- DOI: https://doi.org/10.5946/ce.2015.48.5.405
-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader ePub
ePub Background/Aims Early colorectal (CR) neoplasm can be cured by endoscopic submucosal dissection (ESD), but clinical experience and factors associated with complications from ESD for CR neoplasms in China have not been reported.
Methods Seventy-eight cases of early CR neoplasm treated with endoscopic resection performed between December 2012 and December 2013 at Beijing Military General Hospital were included. Factors associated with ESD complications and procedure times were evaluated.
Results The
en bloc resection rate was 88.5% (69/78), tumor size was 32.1±10.7 mm, and procedure time was 71.8±49.5 minutes. The major complication was perforation, which occurred in 8.97% of the ESD procedures. Multivariate logistic regression analysis indicated that only tumor size (p =0.022) was associated with ESD perforation. Tumor size (p <0.001) and the non-lifting sign (p =0.017) were independent factors for procedure time, and procedure time (p =0.016) was a key factor foren bloc resection. After a median 10 months (range, 4 to 16) of follow-up, no patients had local recurrence.Conclusions This study indicated that ESD is an applicable method for large early CR neoplasm in the colon and rectum. Tumor size and the non-lifting sign might be considerable factors for increased complication rate and procedural time of ESD.
-
Citations
Citations to this article as recorded by- Risk factors for unsuccessful colorectal endoscopic submucosal dissection: A systematic review and meta-analysis
Feng Gu, Wei Jiang, Jingyi Zhu, Lei Ma, Boyuan He, Huihong Zhai
Digestive and Liver Disease.2023;[Epub] CrossRef - A large multicenter cohort on the use of full-thickness resection device for difficult colonic lesions
Y. Ichkhanian, K. Vosoughi, D. L. Diehl, I. S. Grimm, T. W. James, A. W. Templeton, K. Hajifathalian, J. L. Tokar, J. B. Samarasena, N. El Hage Chehade, J. Lee, K. Chang, M. Mizrahi, M. Barawi, S. Irani, S. Friedland, P. Korc, A. A. Aadam, M. A. Al-Haddad
Surgical Endoscopy.2021; 35(3): 1296. CrossRef - Evolving management of colorectal polyps
Yervant Ichkhanian, Tobias Zuchelli, Andrew Watson, Cyrus Piraka
Therapeutic Advances in Gastrointestinal Endoscopy.2021; 14: 263177452110470. CrossRef - Risk factors for adverse events of colorectal endoscopic submucosal dissection: a systematic review and meta-analysis
Juliana B. Santos, Moacyr R.C. Nobre, Cleyton Z. Oliveira, Adriana V. Safatle-Ribeiro, Fabio Kawaguti, Bruno Martins, Sergio C. Nahas, Ulysses Ribeiro, Lanjing Zhang, Fauze Maluf-Filho
European Journal of Gastroenterology & Hepatology.2021; 33(1S): e33. CrossRef - Colo-rectal endoscopic full-thickness resection (EFTR) with the over-the-scope device (FTRD®): A multicenter Italian experience
G. Andrisani, P. Soriani, M. Manno, M. Pizzicannella, F. Pugliese, M. Mutignani, R. Naspetti, L. Petruzziello, F. Iacopini, C. Grossi, P. Lagoussis, S. Vavassori, F. Coppola, A. La Terra, S. Ghersi, P. Cecinato, G. De Nucci, R. Salerno, M. Pandolfi, G. Co
Digestive and Liver Disease.2019; 51(3): 375. CrossRef - Comparing outcomes for endoscopic submucosal dissection between Eastern and Western countries: A systematic review and meta-analysis
Dane Christina Daoud, Nicolas Suter, Madeleine Durand, Mickael Bouin, Bernard Faulques, Daniel von Renteln
World Journal of Gastroenterology.2018; 24(23): 2518. CrossRef - Colonoscopic full-thickness resection using an over-the-scope device: a prospective multicentre study in various indications
Arthur Schmidt, Torsten Beyna, Brigitte Schumacher, Alexander Meining, Hans-Juergen Richter-Schrag, Helmut Messmann, Horst Neuhaus, David Albers, Michael Birk, Robert Thimme, Andreas Probst, Martin Faehndrich, Thomas Frieling, Martin Goetz, Bettina Riecke
Gut.2018; 67(7): 1280. CrossRef - Why attempt en bloc resection of non-pedunculated colorectal adenomas? A systematic review of the prevalence of superficial submucosal invasive cancer after endoscopic submucosal dissection
Lorenzo Fuccio, Alessandro Repici, Cesare Hassan, Thierry Ponchon, Pradeep Bhandari, Rodrigo Jover, Konstantinos Triantafyllou, Daniele Mandolesi, Leonardo Frazzoni, Cristina Bellisario, Franco Bazzoli, Prateek Sharma, Thomas Rösch, Douglas K Rex
Gut.2018; 67(8): 1464. CrossRef - Clinical outcomes after endoscopic submucosal dissection for colorectal neoplasia: a systematic review and meta-analysis
Lorenzo Fuccio, Cesare Hassan, Thierry Ponchon, Daniele Mandolesi, Andrea Farioli, Alessandro Cucchetti, Leonardo Frazzoni, Pradeep Bhandari, Cristina Bellisario, Franco Bazzoli, Alessandro Repici
Gastrointestinal Endoscopy.2017; 86(1): 74. CrossRef
- Risk factors for unsuccessful colorectal endoscopic submucosal dissection: A systematic review and meta-analysis
- 6,494 View
- 88 Download
- 8 Web of Science
- 9 Crossref

- Colorectal Stents: Current Status
- Jeong-Mi Lee, Jeong-Sik Byeon
- Clin Endosc 2015;48(3):194-200. Published online May 29, 2015
- DOI: https://doi.org/10.5946/ce.2015.48.3.194
-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader ePub
ePub A self-expandable metal stent (SEMS) is an effective and safe method for the decompression of colon obstruction. Based on recent evidence, colorectal SEMS is now recommended for the palliation of patients with colonic obstruction from incurable colorectal cancer or extracolonic malignancy and also as a bridge to surgery in those who are a high surgical risk. Prophylactic SEMS insertion in patients with no obstruction symptoms is not recommended. Most colorectal SEMS are inserted endoscopically under fluoroscopic guidance. The technical and clinical success rates of colorectal SEMS are high, and the complication rate is acceptable. Advances in this technology will make the insertion of colorectal SEMS better and may expand the indications of colorectal SEMS in the future.
-
Citations
Citations to this article as recorded by- Oncologic impact of colonic stents for obstructive left-sided colon cancer
Hideyuki Suzuki, Shingo Tsujinaka, Yoshihiro Sato, Tomoya Miura, Chikashi Shibata
World Journal of Clinical Oncology.2023; 14(1): 1. CrossRef - Acute left-sided malignant colonic obstruction: Is there a role for endoscopic stenting?
Salvatore Russo, Rita Conigliaro, Francesca Coppini, Emanuela Dell'Aquila, Giuseppe Grande, Flavia Pigò, Santi Mangiafico, Marinella Lupo, Margherita Marocchi, Helga Bertani, Silvia Cocca
World Journal of Clinical Oncology.2023; 14(5): 190. CrossRef - Endometriosis as an Uncommon Cause of Intestinal Obstruction—A Comprehensive Literature Review
Florentina Mușat, Dan Nicolae Păduraru, Alexandra Bolocan, Alexandru Constantinescu, Daniel Ion, Octavian Andronic
Journal of Clinical Medicine.2023; 12(19): 6376. CrossRef - Are Thyroid Functions Affected in Multisystem Inflammatory Syndrome in Children?
Ayşegül Elvan-Tüz, İlkay Ayrancı, Yıldız Ekemen-Keleş, İnanç Karakoyun, Gönül Çatlı, Ahu Kara-Aksay, Eda Karadağ-Öncel, Bumin Nuri Dündar, Dilek Yılmaz
Journal of Clinical Research in Pediatric Endocrinology.2022; 14(4): 402. CrossRef - Colonic stent for bridge to surgery for acute left-sided malignant colonic obstruction: A review of the literature after 2020
Margherita Binetti, Augusto Lauro, Valeria Tonini
World Journal of Clinical Oncology.2022; 13(12): 957. CrossRef - Biopsy sampling during self-expandable metallic stent placement in acute malignant colorectal obstruction: a narrative review
Sigrid Skov Bennedsgaard, Lene Hjerrild Iversen
World Journal of Surgical Oncology.2021;[Epub] CrossRef - Management of obstructive colon cancer: Current status, obstacles, and future directions
Ri-Na Yoo, Hyeon-Min Cho, Bong-Hyeon Kye
World Journal of Gastrointestinal Oncology.2021; 13(12): 1850. CrossRef - Endoscopic Management of Benign Colonic Obstruction and Pseudo-Obstruction
Su Jin Jeong, Jongha Park
Clinical Endoscopy.2020; 53(1): 18. CrossRef - Stenting as a bridge to surgery for extra-colonic malignancy induced colorectal obstruction: preliminary experience
Eui Joo Kim, Sang Hoon Han, Kyoung Oh Kim, Jun-Won Chung, Dong Kyun Park, Kwang An Kwon, Jung Ho Kim
BMC Gastroenterology.2020;[Epub] CrossRef - Stent Placement for Palliative Treatment of Malignant Colorectal Obstruction: Extracolonic Malignancy Versus Primary Colorectal Cancer
Hyungwoo Ahn, Chang Jin Yoon, Jae Hwan Lee, Won Seok Choi
American Journal of Roentgenology.2020; 215(1): 248. CrossRef - Obstructing Left-Sided Colonic Cancer: Is Endoscopic Stenting a Bridge to Surgery or a Bridge to Nowhere?
Augusto Lauro, Margherita Binetti, Samuele Vaccari, Maurizio Cervellera, Valeria Tonini
Digestive Diseases and Sciences.2020; 65(10): 2789. CrossRef - Development and In Vitro Evaluation of 5-Fluorouracil-Eluting Stents for the Treatment of Colorectal Cancer and Cancer-Related Obstruction
Mohammad Arafat, Paris Fouladian, Anthony Wignall, Yunmei Song, Ankit Parikh, Hugo Albrecht, Clive A. Prestidge, Sanjay Garg, Anton Blencowe
Pharmaceutics.2020; 13(1): 17. CrossRef - Outcomes of stent insertion and mortality in obstructive stage IV colorectal cancer patients through 10 year duration
Yong Eun Park, Yehyun Park, Soo Jung Park, Jae Hee Cheon, Won Ho Kim, Tae Il Kim
Surgical Endoscopy.2019; 33(4): 1225. CrossRef - Drug-eluting non-vascular stents for localised drug targeting in obstructive gastrointestinal cancers
Mohammad Arafat, Paris Fouladian, Anton Blencowe, Hugo Albrecht, Yunmei Song, Sanjay Garg
Journal of Controlled Release.2019; 308: 209. CrossRef - Ten-year survival after endoscopic stent placement as a bridge to surgery in obstructing colon cancer
Bram Verstockt, Annelien Van Driessche, Marc De Man, Pieter van der Spek, Koen Hendrickx, Veerle Casneuf, Pieter Dobbels, Yves Van Molhem, Jo Vandervoort
Gastrointestinal Endoscopy.2018; 87(3): 705. CrossRef - Fracture of a Colonic Self-expandable Metallic Stent in Malignant Colonic Obstruction
Akinari Takao, Taku Tabata, Koichi Koizumi, Go Kuwata, Satomi Shibata, Makiko Mori, Kazuro Chiba, Sawako Kuruma, Tomoko Onishi, Takashi Fujiwara, Terumi Kamisawa, Junko Fujiwara, Takeo Arakawa, Kumiko Momma, Tatsu Shimoyama, Keiichi Takahashi
Internal Medicine.2018; 57(3): 329. CrossRef - Impacto de la utilización de stents colónicos como puente a la cirugía de neoplasias colorrectales oclusivas potencialmente curables sobre los resultados quirúrgicos y oncológicos
Antònia Crespí-Mir, Juan Manuel Romero-Marcos, Anabel de la Llave-Serralvo, Carlos Dolz-Abadía, José Andrés Cifuentes-Ródenas
Cirugía Española.2018; 96(7): 419. CrossRef - Impact on Surgical and Oncological Results of the Use of Colonic Stents as a Bridge to Surgery for Potentially Curable Occlusive Colorectal Neoplasms
Antònia Crespí-Mir, Juan Manuel Romero-Marcos, Anabel de la Llave-Serralvo, Carlos Dolz-Abadía, José Andrés Cifuentes-Ródenas
Cirugía Española (English Edition).2018; 96(7): 419. CrossRef - Use of covered self-expandable stents for benign colorectal disorders in children
Bettina Lange, Moritz Sold, Georg Kähler, Lucas M. Wessel, Rainer Kubiak
Journal of Pediatric Surgery.2017; 52(1): 184. CrossRef - Update on Enteral Stents
Emanuele Dabizzi, Paolo Giorgio Arcidiacono
Current Treatment Options in Gastroenterology.2016; 14(2): 178. CrossRef - Managing Malignant Colorectal Obstruction with Self-Expanding Stents. A Closer Look at Bowel Perforations and Failed Procedures
D. Gleditsch, O.K. Søreide, A. Nesbakken
Journal of Gastrointestinal Surgery.2016; 20(9): 1643. CrossRef - Intraoperative colonoscopy in patients with colorectal cancer: Review of recent developments
Kazushige Kawai, Yuuki Iida, Soichiro Ishihara, Hironori Yamaguchi, Hiroaki Nozawa, Keisuke Hata, Tomomichi Kiyomatsu, Toshiaki Tanaka, Takeshi Nishikawa, Koji Yasuda, Kensuke Otani, Koji Murono, Toshiaki Watanabe
Digestive Endoscopy.2016; 28(6): 633. CrossRef - UEG Week 2016 Oral Presentations
United European Gastroenterology Journal.2016; 4(5_suppl): A1. CrossRef - Endoprótesis colónica para el manejo paliativo de la obstrucción intestinal por cáncer. Reporte de caso
Víctor Manuel Noriega Usi, Leopoldo Gutiérrez Rodríguez, Carlos González de Cosío Corredor
Endoscopia.2015; 27(3): 129. CrossRef
- Oncologic impact of colonic stents for obstructive left-sided colon cancer
- 9,625 View
- 172 Download
- 20 Web of Science
- 24 Crossref

- Status and Literature Review of Self-Expandable Metallic Stents for Malignant Colorectal Obstruction
- Dae Young Cheung, Yong Kook Lee, Chang Heon Yang
- Clin Endosc 2014;47(1):65-73. Published online January 24, 2014
- DOI: https://doi.org/10.5946/ce.2014.47.1.65
-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader ePub
ePub Use of colorectal stents has increased dramatically over the last decades. Colorectal stents offer an alternative way to relieve fatal intestinal obstruction and can take place of emergency surgery, which associated with significant morbidity and mortality and a high incidence of stoma creation, to elective resection. Although there remain a few concerns regarding the use of stents as a bridge to surgical resection, use of self-expandable metallic stents for palliation in patients with unresectable disease has come to be generally accepted. Advantages of colorectal stents include acute restoration of luminal patency and allowance of time for proper staging and surgical optimization, and the well-known disadvantages are procedure-related complications including perforation, migration, and stent failure. General indications, procedures, and clinical outcomes as well as recent evidences regarding the use of colorectal stents will be discussed in this review.
-
Citations
Citations to this article as recorded by- Colonic Stent as Bridge to Surgery for Malignant Obstruction Induces Gene Expressional Changes Associated with a More Aggressive Tumor Phenotype
Malene Broholm, Thea Helene Degett, Sara Furbo, Anne-Marie Kanstrup Fiehn, Mustafa Bulut, Thomas Litman, Jens Ole Eriksen, Jesper T. Troelsen, Lise Mette Rahbek Gjerdrum, Ismail Gögenur
Annals of Surgical Oncology.2021; 28(13): 8519. CrossRef - Outcomes of stent insertion and mortality in obstructive stage IV colorectal cancer patients through 10 year duration
Yong Eun Park, Yehyun Park, Soo Jung Park, Jae Hee Cheon, Won Ho Kim, Tae Il Kim
Surgical Endoscopy.2019; 33(4): 1225. CrossRef - Drug-eluting non-vascular stents for localised drug targeting in obstructive gastrointestinal cancers
Mohammad Arafat, Paris Fouladian, Anton Blencowe, Hugo Albrecht, Yunmei Song, Sanjay Garg
Journal of Controlled Release.2019; 308: 209. CrossRef - Endoscopic stenting of malignant, benign and iatrogenic colorectal disorders: a primer for radiologists
Massimo Tonolini, Emilia Bareggi, Raffaele Salerno
Insights into Imaging.2019;[Epub] CrossRef - Comparison of through-the-scope stent insertion with standard stent insertion for the management of malignant colorectal obstruction: a prospective study
Y. Wan, Y.-Q. Zhu, N.-W. Chen, Z.-G. Wang, Y.-S. Cheng, J. Shi
Techniques in Coloproctology.2016; 20(10): 707. CrossRef - Simulated training in colonoscopic stenting of colonic strictures: validation of a cadaver model
F. Iordache, J. C. Bucobo, D. Devlin, K. You, R. Bergamaschi
Colorectal Disease.2015; 17(7): 627. CrossRef - Surgical failure after colonic stenting as a bridge to surgery
Jung Ho Kim
World Journal of Gastroenterology.2014; 20(33): 11826. CrossRef - Endoscopic stenting for recurrence-related colorectal anastomotic site obstruction: Preliminary experience
Jung Ho Kim
World Journal of Gastroenterology.2014; 20(38): 13936. CrossRef
- Colonic Stent as Bridge to Surgery for Malignant Obstruction Induces Gene Expressional Changes Associated with a More Aggressive Tumor Phenotype
- 8,381 View
- 65 Download
- 10 Web of Science
- 8 Crossref

- Debates on Colorectal Endoscopic Submucosal Dissection - Traction for Effective Dissection: Gravity Is Enough
- Bo-In Lee
- Clin Endosc 2013;46(5):467-471. Published online September 30, 2013
- DOI: https://doi.org/10.5946/ce.2013.46.5.467
-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader ePub
ePub Colorectal endoscopic submucosal dissection (ESD) still remains a technically difficult procedure. The maintenance of tissue tension and good submucosal exposure during dissection is one of the most important factors for an effective and safe dissection. Although various traction methods have been developed, traction by gravity is one of the most useful method for colorectal ESD. Traction using adjunctive devices can thus be reserved for extremely difficult cases or for endoscopists in their learning periods for colorectal ESD.
-
Citations
Citations to this article as recorded by- Conventional Versus Traction Endoscopic Submucosal Dissection for Colorectal Tumors
Sahib Singh, Babu P. Mohan, Saurabh Chandan, Neil Sharma, Rakesh Vinayek, Sudhir Dutta, Sergey V. Kantsevoy, Michelle Le, Douglas G. Adler
Journal of Clinical Gastroenterology.2024;[Epub] CrossRef - Water pressure method for endoscopic submucosal dissection of a rectal tumor on the gravitational side close to the dentate line
Tao Dong, Hanying Wang, Lin Jing, Xuan Zhou, Yaohui Wang, Jun Xiao
Endoscopy International Open.2024; 12(04): E532. CrossRef - A new traction device (S-O clip) facilitating the endoscopic submucosal dissection of tumors in the ileocecal valve region
Shangrui Yu, Pengfei Wang, Yanhu Feng
Zeitschrift für Gastroenterologie.2023; 61(04): 394. CrossRef - Magnetic anchor technique assisted endoscopic submucosal dissection for early esophageal cancer
Min Pan, Miao-Miao Zhang, Shu-Qin Xu, Yi Lyu, Xiao-Peng Yan
World Journal of Gastrointestinal Endoscopy.2023; 15(10): 584. CrossRef - ESD with elastic ring traction is more effective and safer than conventional ESD in large proximal colon neoplastic lesions: a retrospective cohort study (with video)
Sikong Yinhe, Jiao Yang, Zhang Aijun, Li Ruyuan
Surgical Endoscopy.2023; 37(12): 9658. CrossRef - Training program using a traction device improves trainees’ learning curve of colorectal endoscopic submucosal dissection
Yuki Mitsuyoshi, Daisuke Ide, Tomohiko Richard Ohya, Mitsuaki Ishihoka, Chihiro Yasue, Akiko Chino, Masahiro Igarashi, Akio Nakashima, Shoichi Saito, Junko Fujisaki, Masayuki Saruta
Surgical Endoscopy.2022; 36(6): 4462. CrossRef - A novel flexible auxiliary single-arm transluminal endoscopic robot facilitates endoscopic submucosal dissection of gastric lesions (with video)
Xiao-Xiao Yang, Shi-Chen Fu, Rui Ji, Li-Xiang Li, Yan-Qing Li, Xiu-Li Zuo
Surgical Endoscopy.2022; 36(7): 5510. CrossRef - Comparison Between Preincision Traction and On-Demand Traction in Assisting Colorectal Endoscopic Submucosal Dissection
Jun Li, Yunlei Wei, Di Zhang, Xiaojia Hou, Ming Shen, Kan Chen, Ruijin Wu, Kangsheng Peng, Feng Liu
Clinical and Translational Gastroenterology.2022; 13(12): e00539. CrossRef - Endoscopic resection of large (≥ 4 cm) upper gastrointestinal subepithelial tumors originating from the muscularis propria layer: a single-center study of 101 cases (with video)
Yu Zhang, Jin-Bang Peng, Xin-Li Mao, Hai-Hong Zheng, Shen-Kang Zhou, Lin-Hong Zhu, Li-Ping Ye
Surgical Endoscopy.2021; 35(3): 1442. CrossRef - Effectiveness of S-O Clip-Assisted Colorectal Endoscopic Submucosal Dissection
Haruka Fujinami, Akira Teramoto, Saeko Takahashi, Takayuki Ando, Shinya Kajiura, Ichiro Yasuda
Journal of Clinical Medicine.2021; 11(1): 141. CrossRef - Review on colorectal endoscopic submucosal dissection focusing on the technical aspect
Tak Lit Derek Fung, Chi Woo Samuel Chow, Pak Tat Chan, Kam Hung Kwok
Surgical Endoscopy.2020; 34(9): 3766. CrossRef - Novel approach to endoscopic submucosal dissection of a large gastroesophageal junction mass by use of the mucosal bridge technique
Salmaan Jawaid, Dennis Yang, Peter V. Draganov
VideoGIE.2019; 4(6): 249. CrossRef - Magnetic bead-assisted endoscopic submucosal dissection: a gravity-based traction method for treating large superficial colorectal tumors
Liansong Ye, Xianglei Yuan, Maoyin Pang, Johannes Bethge, Mark Ellrichmann, Jiang Du, Xianhui Zeng, Chengwei Tang, Stefan Schreiber, Bing Hu
Surgical Endoscopy.2019; 33(6): 2034. CrossRef - Advanced Endoscopic Treatment for Early Colorectal Cancers - Traction Assisted Endoscopic Submucosal Dissection for Colorectal Tumors -
NAOTO SAKAMOTO, TARO OSADA, AKIHITO NAGAHARA
Juntendo Medical Journal.2019; 65(5): 426. CrossRef - Efficacy of traction‐assisted colorectal endoscopic submucosal dissection using a clip‐and‐thread technique: A prospective randomized study
Yasushi Yamasaki, Yoji Takeuchi, Noriya Uedo, Takashi Kanesaka, Minoru Kato, Kenta Hamada, Yusuke Tonai, Noriko Matsuura, Tomofumi Akasaka, Noboru Hanaoka, Koji Higashino, Ryu Ishihara, Hiroyuki Okada, Hiroyasu Iishi
Digestive Endoscopy.2018; 30(4): 467. CrossRef - Magnetic anchor guidance for endoscopic submucosal dissection and other endoscopic procedures
Mohamed Mortagy, Neal Mehta, Mansour A Parsi, Seiichiro Abe, Tyler Stevens, John J Vargo, Yutaka Saito, Amit Bhatt
World Journal of Gastroenterology.2017; 23(16): 2883. CrossRef - Clinical outcomes of endoscopic submucosal dissection for large colorectal neoplasms: a comparison of protruding and laterally spreading tumors
Jung Ho Bae, Dong-Hoon Yang, Jae Yeon Lee, Jae Seung Soh, Seohyun Lee, Ho-Su Lee, Hyo Jeong Lee, Sang Hyoung Park, Kyung-Jo Kim, Byong Duk Ye, Seung-Jae Myung, Suk-Kyun Yang, Jin-Ho Kim, Jeong-Sik Byeon
Surgical Endoscopy.2016; 30(4): 1619. CrossRef - A Flexible Surgical Robotic System for Removal of Early-Stage Gastrointestinal Cancers by Endoscopic Submucosal Dissection
Ka Chun Lau, Esther Yun Yee Leung, Philip Wai Yan Chiu, Yeung Yam, James Yun Wong Lau, Carmen Chung Yan Poon
IEEE Transactions on Industrial Informatics.2016; 12(6): 2365. CrossRef - Advanced endoscopic submucosal dissection with traction
Hiroyuki Imaeda
World Journal of Gastrointestinal Endoscopy.2014; 6(7): 286. CrossRef - Endoscopic Submucosal Dissection (ESD) in Colorectal Tumors
Franz Ludwig Dumoulin, Bernd Sido, Reinhard Bollmann, Malte Sauer
Visceral Medicine.2014; 30(1): 39. CrossRef
- Conventional Versus Traction Endoscopic Submucosal Dissection for Colorectal Tumors
- 6,378 View
- 75 Download
- 20 Crossref

- Diagnostic Accuracy and Interobserver Agreement in Predicting the Submucosal Invasion of Colorectal Tumors Using Gross Findings, Pit Patterns, and Microvasculatures
- Hye Jung Choi, Bo-In Lee, Hwang Choi, Kyu Yong Choi, Sang-Woo Kim, Joo Yong Song, Jeong Seon Ji, Byung-Wook Kim
- Clin Endosc 2013;46(2):168-171. Published online March 31, 2013
- DOI: https://doi.org/10.5946/ce.2013.46.2.168
-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader ePub
ePub Background/Aims Depth of invasion is one of the most important factors for establishing treatment strategy for colorectal tumors.
Methods Three blinded experts reviewed electronic photos and video clips of 33 early colorectal cancer-like lesions. They estimated the depth of invasion based on conventional white light endoscopy (CWE), magnifying chromoendoscopy (MCE), and magnifying narrow band imaging endoscopy (MNE).
Results The lesions included nine mucosal low-grade neoplasias, 16 mucosal high grade neoplasias, and eight carcinomas with invasion to the submucosal layer or beyond. The diagnostic accuracy for submucosal invasion by CWE ranged from 67% to 82%, while those by MCE and MNE ranged from 85% to 88% and 85% to 88%, respectively. The diagnostic accuracy significantly differed between CWE and MCE (
p =0.034) and between CWE and MNE (p =0.039). The kappa values for CWE, MCE, and MNE among the endoscopists were 0.564, 0.673, and 0.673, respectively.Conclusions The estimation of submucosal invasion for early colorectal cancer-like lesions based on MCE or MNE is more accurate than CWE. MCE and MNE were demonstrated to have substantial agreement for predicting submucosal invasion.
-
Citations
Citations to this article as recorded by- Estimation of Invasion Depth: The First Key to Successful Colorectal ESD
Bo-In Lee, Takahisa Matsuda
Clinical Endoscopy.2019; 52(2): 100. CrossRef - Comparison of endoscopic ultrasonography and magnifying endoscopy for assessment of the invasion depth of shallow gastrointestinal neoplasms: a systematic review and meta-analysis
Zhang Tao, Chen Yan, He Zhao, Jiawei Tsauo, Xiaowu Zhang, Bing Qiu, Yanqing Zhao, Xiao Li
Surgical Endoscopy.2017; 31(12): 4923. CrossRef - Use of confocal laser endomicroscopy with a fluorescently labeled fatty acid to diagnose colorectal neoplasms
Feihong Deng, Yuan Fang, Zhiyong Shen, Wei Gong, Tao Liu, Jing Wen, Wanling Zhang, Xianjun Zhu, Hui Zhong, Tong Wang, Fachao Zhi, Biao Nie
Oncotarget.2017; 8(35): 58934. CrossRef - Higher net change of index of hemoglobin values between colon polyp and nonpolyp mucosa correlates with the presence of an advanced colon adenoma
Wei‐Chun Cheng, Hsiu‐Chi Cheng, Po‐Jun Chen, Jui‐Wen Kang, Er‐Hsiang Yang, Bor‐Shyang Sheu, Wei‐Ying Chen
Advances in Digestive Medicine.2016; 3(4): 161. CrossRef - Brief Education on Microvasculature and Pit Pattern for Trainees Significantly Improves Estimation of the Invasion Depth of Colorectal Tumors
Joon Sung Kim, Bo-In Lee, Hwang Choi, Bong Koo Kang, Jong In Kim, Hae Mi Lee, Eun-Joo Im, Byung-Wook Kim, Sang-Woo Kim, Myung-Gyu Choi, Kyu Yong Choi
Gastroenterology Research and Practice.2014; 2014: 1. CrossRef - Clinical Usefulness of Magnifying Chromoendoscopy and Magnifying Narrow Band Imaging Endoscopy for Predicting the Submucosal Invasion of Early Colorectal Cancers
Kwang An Kwon, Yang Suh Ku
Clinical Endoscopy.2013; 46(2): 113. CrossRef
- Estimation of Invasion Depth: The First Key to Successful Colorectal ESD
- 7,192 View
- 44 Download
- 6 Crossref

- Indications, Knives, and Electric Current: What's the Best?
- Bo-In Lee
- Clin Endosc 2012;45(3):285-287. Published online August 22, 2012
- DOI: https://doi.org/10.5946/ce.2012.45.3.285
-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader ePub
ePub Endoscopic submucosal dissection (ESD) was developed to overcome the limitations of conventional endoscopic mucosal resection (EMR), and ESD has been also applied for large colorectal neoplasms. Since colorectal ESD is still associated with higher perforation rate, a longer procedure time, and increased technical difficulty, the indications should be strictly considered. Generally, colorectal tumors without deep submucosal invasion or minimal possibility of lymph node metastasis, for which en bloc resection using conventional EMR is difficult, are good candidates for colorectal ESD. The ideal knife for colorectal ESD should avoid making perforations but can make a clean cut of optimal depth at one time. The ideal current for ESD differs depending on the procedure used, the surgical devices used, the tissue to be dissected, and the operator's preference. Application of the optimal indications and improvements in the technical skill and surgical devices are required for easier and safer colorectal ESD.
-
Citations
Citations to this article as recorded by- Review on colorectal endoscopic submucosal dissection focusing on the technical aspect
Tak Lit Derek Fung, Chi Woo Samuel Chow, Pak Tat Chan, Kam Hung Kwok
Surgical Endoscopy.2020; 34(9): 3766. CrossRef - International Digestive Endoscopy Network to Strengthen Network for Lower Gastrointestinal Diseases Including Inflammatory Bowel Disease and Colorectal Cancer
Kwang An Kwon
Clinical Endoscopy.2012; 45(3): 251. CrossRef
- Review on colorectal endoscopic submucosal dissection focusing on the technical aspect
- 4,945 View
- 62 Download
- 2 Crossref

- Korean Guidelines for Postpolypectomy Colonoscopy Surveillance
- Dong-Hoon Yang, Sung Noh Hong, Young-Ho Kim, Sung Pil Hong, Sung Jae Shin, Seong-Eun Kim, Bo In Lee, Suck-Ho Lee, Dong Il Park, Hyun-Soo Kim, Suk-Kyun Yang, Hyo Jong Kim, Se Hyung Kim, Hyun Jung Kim, Multi-Society Task Force for Development of Guidelines for Colorectal Polyp Screening, Surveillance and Management
- Clin Endosc 2012;45(1):44-61. Published online March 31, 2012
- DOI: https://doi.org/10.5946/ce.2012.45.1.44
-
 Abstract
Abstract
 PDF
PDF Supplementary Material
Supplementary Material PubReader
PubReader ePub
ePub Postpolypectomy surveillance has become a major indication for colonoscopy as a result of increased use of screening colonoscopy in Korea. In this report, a careful analytic approach was used to address all available evidences to delineate the predictors for advanced neoplasia at surveillance colonoscopy and we elucidated the high risk findings of the index colonoscopy as follows: 3 or more adenomas, any adenoma larger than 10 mm, any tubulovillous or villous adenoma, any adenoma with high-grade dysplasia, and any serrated polyps larger than 10 mm. Surveillance colonoscopy should be performed five years after the index colonoscopy for those without any high-risk findings and three years after the index colonoscopy for those with one or more high risk findings. However, the surveillance interval can be shortened considering the quality of the index colonoscopy, the completeness of polypectomy, the patient's general condition, and family and medical history.
-
Citations
Citations to this article as recorded by- Post-colonoscopy colorectal cancers in a national fecal immunochemical test-based colorectal cancer screening program
Pieter H. A. Wisse, Sybrand Y. de Boer, Marco Oudkerk Pool, Jochim S Terhaar sive Droste, Claudia Verveer, Gerrit A. Meijer, Evelien Dekker, Manon C. W. Spaander
Endoscopy.2024; 56(05): 364. CrossRef - Recurrence rates of advanced colorectal neoplasia (ACN) in subjects with baseline ACN followed up at different surveillance intervals
Martin C.S. Wong, Eman Yee-man Leung, Sam C.C. Chun, Yunyang Deng, Thomas Lam, Raymond S.Y. Tang, Junjie Huang
Digestive and Liver Disease.2023; 55(12): 1742. CrossRef - Risk of recurrent advanced colorectal neoplasia in individuals with baseline non‐advanced neoplasia followed up at 5 vs 7–10 years
Martin C. S. Wong, Eman Yee‐man Leung, Sam C. C. Chun, Yunyang Deng, Thomas Lam, Raymond S. Y. Tang, Junjie Huang
Journal of Gastroenterology and Hepatology.2023; 38(12): 2122. CrossRef - Third Asia-Pacific consensus recommendations on colorectal cancer screening and postpolypectomy surveillance
Joseph J Y Sung, Han-Mo Chiu, David Lieberman, Ernst J Kuipers, Matthew D Rutter, Finlay Macrae, Khay-Guan Yeoh, Tiing Leong Ang, Vui Heng Chong, Sneha John, Jingnan Li, Kaichun Wu, Simon S M Ng, Govind K Makharia, Murdani Abdullah, Nozomu Kobayashi, Masa
Gut.2022; 71(11): 2152. CrossRef - Optimization of the surveillance strategy in patients with colorectal adenomas: A combination of clinical parameters and index colonoscopy findings
Chan Hyuk Park, Yoon Suk Jung, Nam Hee Kim, Jung Ho Park, Dong Il Park, Chong Il Sohn
Journal of Gastroenterology and Hepatology.2021; 36(4): 974. CrossRef - Increasing changes in visceral adiposity is associated with higher risk for colorectal adenoma: Multilevel analysis in a prospective cohort
Jung Min Moon, Jong Pil Im, Donghee Kim, Yoo Min Han, Hosim Soh, Ji Hyun Song, Sun Young Yang, Young Sun Kim, Jeong Yoon Yim, Seon Hee Lim, Joo Sung Kim
Journal of Gastroenterology and Hepatology.2021; 36(7): 1836. CrossRef - Strategies for colorectal cancer screening and post-polypectomy surveillance for young adults under age 50
Yoon Suk Jung
Precision and Future Medicine.2021; 5(2): 69. CrossRef - Adherence to Surveillance Guidelines after the Removal of Colorectal Polyps: A Multinational, Multicenter, Prospective Survey
Chang Kyo Oh, Satimai Aniwan, Panida Piyachaturawat, Zhiqin Wong, Thida Soe, Bayasgalan Luvsandagva, Quang Trung Tran, Achmad Fauzi, Jeong-Sik Byeon, Young-Seok Cho
Gut and Liver.2021; 15(6): 878. CrossRef - Colon Polyp Detection in Primary Health Care Institutions of Korea: Detection Rate and Issues with Following the Guidelines
Sang Hyun Park, Kwang Il Hong, Hyun Chul Park, Young Sun Kim, Gene Hyun Bok, Kyung Ho Kim, Dong Suk Shin, Jae Yong Han, Young Kwan Kim, Yeun Jong Choi, Soo Hoon Eun, Byung Hoon Lim, Kyeong Kun Kwack
The Korean Journal of Gastroenterology.2021; 78(6): 328. CrossRef - Effect of Cotinine-Verified Change in Smoking Status on Risk of Metachronous Colorectal Neoplasia After Polypectomy
Yoon Suk Jung, Nam Hee Kim, Mi Yeon Lee, Jung Ho Park, Dong Il Park, Chong Il Sohn
Clinical Gastroenterology and Hepatology.2020; 18(1): 163. CrossRef - Risk of developing metachronous advanced colorectal neoplasia after resection of low-risk diminutive versus small adenomas
Nam Hee Kim, Yoon Suk Jung, Jung Ho Park, Dong Il Park, Chong Il Sohn
Gastrointestinal Endoscopy.2020; 91(3): 622. CrossRef - Colorectal sessile serrated lesion with large size or synchronous neoplasm: a prospective study
Laxmi B. Chavali, Kun Hu, Anish Sheth, Nan Gao, Wei Xiong, Lanjing Zhang
European Journal of Gastroenterology & Hepatology.2020; 32(2): 199. CrossRef - Colorectal Polyp Prevalence According to Alcohol Consumption, Smoking and Obesity
Kyujin Lee, Yong Hwan Kim
International Journal of Environmental Research and Public Health.2020; 17(7): 2387. CrossRef - Comparative systematic review and meta-analysis of 1- to 5-mm versus 6- to 9-mm adenomas on the risk of metachronous advanced colorectal neoplasia
Yoon Suk Jung, Tae Jun Kim, Eunwoo Nam, Chan Hyuk Park
Gastrointestinal Endoscopy.2020; 92(3): 692. CrossRef - The current capacity and quality of colonoscopy in Korea
Jae Ho Choi, Jae Myung Cha, Jin Young Yoon, Min Seob Kwak, Jung Won Jeon, Hyun Phil Shin
Intestinal Research.2019; 17(1): 119. CrossRef - Appropriate Surveillance Interval after Colonoscopic Polypectomy in Patients Younger than 50 Years
Yoon Suk Jung, Nam Hee Kim, Jung Ho Park, Dong Il Park, Chong Il Sohn
Journal of Korean Medical Science.2019;[Epub] CrossRef - Terminology, Molecular Features, Epidemiology, and Management of Serrated Colorectal Neoplasia
Seth D. Crockett, Iris D. Nagtegaal
Gastroenterology.2019; 157(4): 949. CrossRef - Risk of Developing Metachronous Advanced Colorectal Neoplasia After Polypectomy in Patients With Multiple Diminutive or Small Adenomas
Nam Hee Kim, Yoon Suk Jung, Mi Yeon Lee, Jung Ho Park, Dong Il Park, Chong Il Sohn
American Journal of Gastroenterology.2019; 114(10): 1657. CrossRef - Visceral obesity as a risk factor for colorectal adenoma occurrence in surveillance colonoscopy
Jong Pil Im, Donghee Kim, Su Jin Chung, Eun Hyo Jin, Yoo Min Han, Min Jung Park, Ji Hyun Song, Sun Young Yang, Young Sun Kim, Jeong Yoon Yim, Seon Hee Lim, Joo Sung Kim
Gastrointestinal Endoscopy.2018; 88(1): 119. CrossRef - Guideline Adherence to Colonoscopic Surveillance Intervals after Polypectomy in Korea: Results from a Nationwide Survey
Seri Hong, Mina Suh, Kui Son Choi, Boyoung Park, Jae Myung Cha, Hyun-Soo Kim, Jae Kwan Jun, Dong Soo Han
Gut and Liver.2018; 12(4): 426. CrossRef - Risk factors of nonadherence to colonoscopy surveillance after polypectomy and its impact on clinical outcomes: a KASID multicenter study
Chung Hyun Tae, Chang Mo Moon, Seong-Eun Kim, Sung-Ae Jung, Chang Soo Eun, Jae Jun Park, Geom Seog Seo, Jae Myung Cha, Sung Chul Park, Jaeyoung Chun, Hyun Jung Lee, Yunho Jung, Jin Oh Kim, Young-Eun Joo, Dong Il Park
Journal of Gastroenterology.2017; 52(7): 809. CrossRef - Unusual Local Recurrence with Distant Metastasis after Successful Endoscopic Submucosal Dissection for Colorectal Mucosal Cancer
Hyo Jeong Lee, Byong Duk Ye, Jeong-Sik Byeon, Jihun Kim, Young Soo Park, Yong Sang Hong, Yong Sik Yoon, Dong-Hoon Yang
Clinical Endoscopy.2017; 50(1): 91. CrossRef - Determining the optimal surveillance interval after a colonoscopic polypectomy for the Korean population?
Jung Lok Lee, Jae Myung Cha, Hye Min Lee, Jung Won Jeon, Min Seob Kwak, Jin Young Yoon, Hyun Phil Shin, Kwang Ro Joo, Joung Il Lee, Dong Il Park
Intestinal Research.2017; 15(1): 109. CrossRef - Advanced Colonic Neoplasia at Follow-up Colonoscopy According to Risk Components and Adenoma Location at Index Colonoscopy: A Retrospective Study of 1,974 Asymptomatic Koreans
Su Jung Baik, Hyojin Park, Jae Jun Park, Hyun Ju Lee, So Young Jo, Yoo Mi Park, Hye Sun Lee
Gut and Liver.2017; 11(5): 667. CrossRef - The Serrated Polyp Pathway: Is It Time to Alter Surveillance Guidelines?
Brendon O’Connell, Nazar Hafiz, Seth Crockett
Current Gastroenterology Reports.2017;[Epub] CrossRef - Optimizing post‐polypectomy surveillance: A practical guide for the endoscopist
Roel Bogie, Silvia Sanduleanu
Digestive Endoscopy.2016; 28(3): 348. CrossRef - Cap-assisted EMR for rectal neuroendocrine tumors: comparisons with conventional EMR and endoscopic submucosal dissection (with videos)
Dong-Hoon Yang, Yangsoon Park, Sang Hyoung Park, Kyung-Jo Kim, Byong Duk Ye, Jeong-Sik Byeon, Seung-Jae Myung, Suk-Kyun Yang
Gastrointestinal Endoscopy.2016; 83(5): 1015. CrossRef - Colorectal cancer screening of the general population in East Asia
Yasushi Sano, Jeong‐Sik Byeon, Xiao‐Bo Li, Martin C.S. Wong, Han‐Mo Chiu, Rungsun Rerknimitr, Takahiro Utsumi, Santa Hattori, Wataru Sano, Mineo Iwatate, Philip Chiu, Joseph Sung
Digestive Endoscopy.2016; 28(3): 243. CrossRef - Surveillance colonoscopy after endoscopic treatment for colorectal neoplasia: From the standpoint of the Asia–Pacific region
Takahisa Matsuda, Han‐Mo Chiu, Yasushi Sano, Takahiro Fujii, Akiko Ono, Yutaka Saito
Digestive Endoscopy.2016; 28(3): 342. CrossRef - Long-Term Outcome and Surveillance Colonoscopy after Successful Endoscopic Treatment of Large Sessile Colorectal Polyps
Bun Kim, A Ra Choi, Soo Jung Park, Jae Hee Cheon, Tae Il Kim, Won Ho Kim, Sung Pil Hong
Yonsei Medical Journal.2016; 57(5): 1106. CrossRef - Probability of High-Risk Colorectal Neoplasm Recurrence Based on the Results of Two Previous Colonoscopies
Hye Won Park, Seungbong Han, Ji Young Lee, Hye-Sook Chang, Jaewon Choe, Yunsik Choi, Hoonsub So, Dong-Hoon Yang, Seung-Jae Myung, Suk-Kyun Yang, Jin-Ho Kim, Jeong-Sik Byeon
Digestive Diseases and Sciences.2015; 60(1): 226. CrossRef - Distribution of the Colonoscopic Adenoma Detection Rate According to Age: Is Recommending Colonoscopy Screening for Koreans Over the Age of 50 Safe?
Taeseok Bae, Yunhyung Ha, Changkyun Kim, Jihyun Lee, Kwangil Ha, Sanghyun Shin, Youngcheol Lee, Yoonsik Kang
Annals of Coloproctology.2015; 31(2): 46. CrossRef - Risk of adenomas with high‐risk characteristics based on two previous colonoscopy
Kang‐Heum Suh, Ja Seol Koo, Jong Jin Hyun, Jungsoon Choi, Jang Soo Han, Seung Young Kim, Sung Woo Jung, Yoon Tae Jeen, Sang Woo Lee, Jai Hyun Choi
Journal of Gastroenterology and Hepatology.2014; 29(12): 1985. CrossRef - Only the Size of Resected Polyps Is an Independent Risk Factor for Delayed Postpolypectomy Hemorrhage: A 10-Year Single-Center Case-Control Study
Hee Seok Moon, Sun Wook Park, Dong Hwan Kim, Sun Hyung Kang, Jae Kyu Sung, Hyun Yong Jeong
Annals of Coloproctology.2014; 30(4): 182. CrossRef - Prevalence of and Risk Factors for Gastrointestinal Diseases in Korean Americans and Native Koreans Undergoing Screening Endoscopy
Hee Sun Kim, Su Jung Baik, Kyung Hee Kim, Cho Rong Oh, Jung Hyun Lee, Wan Jae Jo, Hye Kyoung Kim, Eun Young Kim, Min Jung Kim
Gut and Liver.2013; 7(5): 539. CrossRef - Proximal colon cancer and serrated adenomas – hunting the missing 10%
Pelvender Gill, Hannah Rafferty, David Munday, Adam Bailey, Lai Mun Wang, James E East, Runjan Chetty, Simon J Leedham
Clinical Medicine.2013; 13(6): 557. CrossRef - Relationship of Non-Alcoholic Fatty Liver Disease to Colorectal Neoplasia
Jue Yong Lee, Ja Won Kim
Korean Journal of Medicine.2013; 84(3): 363. CrossRef - Highlights of International Digestive Endoscopy Network 2013
Kwang An Kwon, Il Ju Choi, Eun Young Kim, Seok Ho Dong, Ki Baik Hahm
Clinical Endoscopy.2013; 46(5): 425. CrossRef
- Post-colonoscopy colorectal cancers in a national fecal immunochemical test-based colorectal cancer screening program
- 10,175 View
- 161 Download
- 38 Crossref

- Korean Guidelines for Colorectal Cancer Screening and Polyp Detection
- Bo-In Lee, Sung Pil Hong, Seong-Eun Kim, Se Hyung Kim, Hyun-Soo Kim, Sung Noh Hong, Dong-Hoon Yang, Sung Jae Shin, Suck-Ho Lee, Dong Il Park, Young-Ho Kim, Hyun Jung Kim, Suk-Kyun Yang, Hyo Jong Kim, Hae Jeong Jeon, Multi-Society Task Force for Development of Guidelines for Colorectal Polyp Screening, Surveillance and Management
- Clin Endosc 2012;45(1):25-43. Published online March 31, 2012
- DOI: https://doi.org/10.5946/ce.2012.45.1.25
-
 Abstract
Abstract
 PDF
PDF Supplementary Material
Supplementary Material PubReader
PubReader ePub
ePub Now colorectal cancer is the second most common cancer in males and the fourth most common cancer in females in Korea. Since most of colorectal cancers occur after the prolonged transformation of adenomas into carcinomas, early detection and removal of colorectal adenomas are one of the most effective methods to prevent colorectal cancer. Considering the increasing incidence of colorectal cancer and polyps in Korea, it is very important to establish Korean guideline for colorectal cancer screening and polyp detection. The guideline was developed by the Korean Multi-Society Take Force and we tried to establish the guideline by evidence-based methods. Parts of the statements were draw by systematic reviews and meta-analyses. Herein we discussed epidemiology of colorectal cancers and adenomas in Korea and optimal methods for screening of colorectal cancer and detection of adenomas including fecal occult blood tests, radiologic tests, and endoscopic examinations.
-
Citations
Citations to this article as recorded by- Colorectal cancer screening guidelines for average-risk and high-risk individuals: A systematic review
Caroline Tanadi, Kevin Tandarto, Maureen Miracle Stella, Kenny Wijaya Sutanto, Mario Steffanus, Riki Tenggara, Muhammad Begawan Bestari
Romanian Journal of Internal Medicine.2024; 62(2): 101. CrossRef - Trends in colorectal cancer incidence according to an increase in the number of colonoscopy cases in Korea
Ga Hee Kim, Yeong Chan Lee, Tae Jun Kim, Sung Noh Hong, Dong Kyung Chang, Young-Ho Kim, Dong-Hoon Yang, Chang Mo Moon, Kyunga Kim, Hyun Gun Kim, Eun-Ran Kim
World Journal of Gastrointestinal Oncology.2024; 16(1): 51. CrossRef - Evaluation of the “Burgenland PREvention trial of colorectal cancer Disease with ImmunologiCal Testing” (B-PREDICT)—a population-based colorectal cancer screening program
Stefanie BREZINA, Gernot LEEB, Andreas BAIERL, Evelyn GRÄF, Monika HACKL, Philipp HOFER, Harald LANG, Michaela KLEIN, Karl MACH, Remy SCHWARZER, Wilhelm WLASSITS, Andreas PÜSPÖK, Andrea GSUR
BMC Gastroenterology.2024;[Epub] CrossRef - 2022 Seoul Consensus on Clinical Practice Guidelines for Functional Constipation
Young Sin Cho, Yoo Jin Lee, Jeong Eun Shin, Hye-Kyung Jung, Seon-Young Park, Seung Joo Kang, Kyung Ho Song, Jung-Wook Kim, Hyun Chul Lim, Hee Sun Park, Seong-Jung Kim, Ra Ri Cha, Ki Bae Bang, Chang Seok Bang, Sung Kyun Yim, Seung-Bum Ryoo, Bong Hyeon Kye,
Journal of Neurogastroenterology and Motility.2023; 29(3): 271. CrossRef - Association of HLA-G 3′UTR polymorphisms and haplotypes with colorectal cancer susceptibility and prognosis
Sabrine Dhouioui, Ahmed-Baligh Laaribi, Nadia Boujelbene, Refka Jelassi, Hamza Ben Salah, Hedia Bellali, Hadda-Imene Ouzari, Amel Mezlini, Inès Zemni, Hanene Chelbi, Inès Zidi
Human Immunology.2022; 83(1): 39. CrossRef - An updated review of the methods, guidelines of, and controversies on screening for colorectal cancer
Sameh Hany Emile, Samer Hani Barsom, Steven D. Wexner
The American Journal of Surgery.2022; 224(1): 339. CrossRef - Disparities in Recommendations for Colorectal Cancer Screening Among Average-Risk Individuals: An Ecobiosocial Approach
Sharifah Saffinas Syed Soffian, Azmawati Mohammed Nawi, Rozita Hod, Mohd Rizal Abdul Manaf, Huan-Keat Chan, Muhammad Radzi Abu Hassan
Risk Management and Healthcare Policy.2022; Volume 15: 1025. CrossRef - Apurinic/apyrimidinic endonuclease 1 is associated with poor prognosis after curative resection followed by adjuvant chemotherapy in patients with stage III colon cancer
Ji Hyeong Song, Myung Sun Lee, Eun Young Cha, Kyung Ha Lee, Ji Yeon Kim, Jin Soo Kim
Korean Journal of Clinical Oncology.2022; 18(1): 1. CrossRef - Systematic review of shared decision‐making in guidelines about colorectal cancer screening
Marta Maes‐Carballo, Manuel García‐García, Yolanda Gómez‐Fandiño, Carlos Roberto Estrada‐López, Andrés Iglesias‐Álvarez, Aurora Bueno‐Cavanillas, Khalid Saeed Khan
European Journal of Cancer Care.2022;[Epub] CrossRef - Real-World Use of Colonoscopy in an Older Population: A Nationwide Standard Cohort Study Using a Common Data Model
Ha Il Kim, Jin Young Yoon, Min Seob Kwak, Jae Myung Cha
Digestive Diseases and Sciences.2021; 66(7): 2227. CrossRef - Cecal intubation time in screening colonoscopy
Hyun Young Kim
Medicine.2021; 100(19): e25927. CrossRef - Gastrointestinal and Nongastrointestinal Complications of Esophagogastroduodenoscopy and Colonoscopy in the Real World: A Nationwide Standard Cohort Using the Common Data Model Database
Ha Il Kim, Jin Young Yoon, Min Seob Kwak, Jae Myung Cha
Gut and Liver.2021; 15(4): 569. CrossRef - Optimal postoperative surveillance strategies for stage III colorectal cancer
Min Young Park, In Ja Park, Hyo Seon Ryu, Jay Jung, Minsung Kim, Seok-Byung Lim, Chang Sik Yu, Jin Cheon Kim
World Journal of Gastrointestinal Surgery.2021; 13(9): 1012. CrossRef - Tumor Size >5 cm and Harvested LNs <12 Are the Risk Factors for Recurrence in Stage I Colon and Rectal Cancer after Radical Resection
Hye-Sol Jung, Seung-Bum Ryoo, Han-Ki Lim, Min Jung Kim, Sang Hui Moon, Ji Won Park, Seung-Yong Jeong, Kyu Joo Park
Cancers.2021; 13(21): 5294. CrossRef - Colon Polyp Detection in Primary Health Care Institutions of Korea: Detection Rate and Issues with Following the Guidelines
Sang Hyun Park, Kwang Il Hong, Hyun Chul Park, Young Sun Kim, Gene Hyun Bok, Kyung Ho Kim, Dong Suk Shin, Jae Yong Han, Young Kwan Kim, Yeun Jong Choi, Soo Hoon Eun, Byung Hoon Lim, Kyeong Kun Kwack
The Korean Journal of Gastroenterology.2021; 78(6): 328. CrossRef - Evaluation of the Cut-Off Value of Fecal Immunochemical Test for Colorectal Cancer Screening during Health Checkups
Hye-Ran Park, Eun-Hee Nah, Jong-Seon Lee, Seon Cho
Journal of Laboratory Medicine and Quality Assurance.2021; 43(4): 190. CrossRef - Risk of developing metachronous advanced colorectal neoplasia after resection of low-risk diminutive versus small adenomas
Nam Hee Kim, Yoon Suk Jung, Jung Ho Park, Dong Il Park, Chong Il Sohn
Gastrointestinal Endoscopy.2020; 91(3): 622. CrossRef - Factors Associated With Colonoscopy Compliance Based on Health Belief Model in a Community-Based Colorectal Cancer Screening Program Shanghai, China
Lihua He, Shuna Gao, Sha Tao, Weiyi Li, Juan Du, Yunfang Ji, Yejing Wang
International Quarterly of Community Health Education.2020; 41(1): 25. CrossRef - Prevalence of and Risk Factors for Colorectal Neoplasia in Asymptomatic Young Adults (20–39 Years Old)
Nam Hee Kim, Yoon Suk Jung, Hyo-Joon Yang, Soo-Kyung Park, Jung Ho Park, Dong Il Park, Chong Il Sohn
Clinical Gastroenterology and Hepatology.2019; 17(1): 115. CrossRef - Optimal colonoscopy surveillance interval period for the adenoma patients who had an adequate polypectomy at baseline colonoscopy
Jian Dong, Minman Wu, Jiarong Miao, Tao Zhi, Tianmei Zhang, Gang Yang, Yarong Chen, Lei Zhang, Qiong Nan
European Journal of Cancer Prevention.2019; 28(1): 10. CrossRef - Impact of family history of colorectal cancer on age‐specific prevalence of colorectal neoplasia
Chan Hyuk Park, Nam Hee Kim, Jung Ho Park, Dong Il Park, Chong Il Sohn, Yoon Suk Jung
Journal of Gastroenterology and Hepatology.2019; 34(3): 537. CrossRef - A Novel Blood‐Based Colorectal Cancer Diagnostic Technology Using Electrical Detection of Colon Cancer Secreted Protein‐2
Minhong Jeun, Hyo Jeong Lee, Sungwook Park, Eun‐ju Do, Jaewon Choi, You‐Na Sung, Seung‐Mo Hong, Sang‐Yeob Kim, Dong‐Hee Kim, Ja Young Kang, Hye‐Nam Son, Jinmyoung Joo, Eun Mi Song, Sung Wook Hwang, Sang Hyoung Park, Dong‐Hoon Yang, Byong Duk Ye, Jeong‐Sik
Advanced Science.2019;[Epub] CrossRef - Increasing Trend in Young-Onset Colorectal Cancer in Asia: More Cancers in Men and More Rectal Cancers
Joseph J.Y. Sung, Han-Mo Chiu, Kyu-Won Jung, Jae Kwan Jun, Masau Sekiguchi, Takahisa Matsuda, Moe H. Kyaw
American Journal of Gastroenterology.2019; 114(2): 322. CrossRef - Bowel preparation for colonoscopy may decrease the levels of testosterone in Korean men
Soo-Hyun Lee, Seung Geon Park, Moon-Jong Kim, Hyejin Chun, Doo-Yeoun Cho, Doohee Hong, Young-Sang Kim
Scientific Reports.2019;[Epub] CrossRef - A Comparative Study Evaluating the Incidence of Colorectal Neoplasia(s) in Candidates for Bariatric Surgery by Screening Colonoscopy, 40–49 Versus 50–65 Years Old: a Preliminary Study
Toygar Toydemir, Görkem Özgen, İsmail Çalıkoğlu, Özdal Ersoy, Mehmet Ali Yerdel
Obesity Surgery.2019; 29(8): 2430. CrossRef - Appropriate Surveillance Interval after Colonoscopic Polypectomy in Patients Younger than 50 Years
Yoon Suk Jung, Nam Hee Kim, Jung Ho Park, Dong Il Park, Chong Il Sohn
Journal of Korean Medical Science.2019;[Epub] CrossRef - The Rise of Colorectal Cancer in Asia: Epidemiology, Screening, and Management
Elias F. Onyoh, Wen-Feng Hsu, Li-Chun Chang, Yi-Chia Lee, Ming-Shiang Wu, Han-Mo Chiu
Current Gastroenterology Reports.2019;[Epub] CrossRef - Colonic Intramucosal Cancer in the Interposed Colon Treated with Endoscopic Mucosal Resection: A Case Report and Review of Literature
Seung-Ho Baek, Jang-Ho Lee, Dong Ryeol Yoo, Hye Yeong Kim, Meihua Jin, Ah-reum Jang, Dong-Hoon Yang, Jeong-Sik Byeon
Clinical Endoscopy.2019; 52(4): 377. CrossRef - IMMUNOCHROMATOGRAPHIC TEST FOR DETECTION OF FECAL OCCULT BLOOD
Anna Viktorovna Nikitina, Y. A. Akinshina, N. E. Nishchakova, E. A. Amelina, S. G. Mardanly
Russian Clinical Laboratory Diagnostics.2019; 64(9): 536. CrossRef - Abdominal Obesity is More Predictive of Advanced Colorectal Neoplasia Risk Than Overall Obesity in Men
Nam Hee Kim, Yoon Suk Jung, Jung Ho Park, Dong Il Park, Chong Il Sohn
Journal of Clinical Gastroenterology.2019; 53(7): e284. CrossRef - Stool-Based miR-92a and miR-144* as Noninvasive Biomarkers for Colorectal Cancer Screening
Hyun Ho Choi, Young-Seok Cho, Ji Hye Choi, Hyung-Keun Kim, Sung Soo Kim, Hiun-Suk Chae
Oncology.2019; 97(3): 173. CrossRef - Association between family history of colorectal cancer and the risk of metachronous colorectal neoplasia following polypectomy in patients aged < 50 years
Nam Hee Kim, Yoon Suk Jung, Jung Ho Park, Dong Il Park, Chong Il Sohn
Journal of Gastroenterology and Hepatology.2019; 34(2): 383. CrossRef - Risk of metachronous neoplasia on surveillance colonoscopy in young patients with colorectal neoplasia
Hyun Gun Kim, Young-Seok Cho, Jae Myung Cha, Jeong Eun Shin, Kyeong Ok Kim, Hyo-Joon Yang, Hoon Sup Koo, Young-Eun Joo, Sun-Jin Boo
Gastrointestinal Endoscopy.2018; 87(3): 666. CrossRef - Systematic review of colorectal cancer screening guidelines for average-risk adults: Summarizing the current global recommendations
Florence Bénard, Alan N Barkun, Myriam Martel, Daniel von Renteln
World Journal of Gastroenterology.2018; 24(1): 124. CrossRef - Stages of Adoption for Fecal Occult Blood Test and Colonoscopy Tests for Colorectal Cancer Screening in Korea
Nhung Cam Bui, Ha Na Cho, Yoon Young Lee, Mina Suh, Boyoung Park, Jae Kwan Jun, Yeol Kim, Kui Son Choi
Cancer Research and Treatment.2018; 50(2): 416. CrossRef - Anatomical distribution and detection rate of colorectal neoplasms according to age in the colonoscopic screening of a Korean population
Suk-young Lee, Wan Hee Song, Sang Cheul Oh, Byung-Wook Min, Sun Il Lee
Annals of Surgical Treatment and Research.2018; 94(1): 36. CrossRef - The prognostic implications of primary tumor location on recurrence in early-stage colorectal cancer with no associated risk factors
Sung Il Kang, Duck-Woo Kim, Yoonjin Kwak, Hye-Seung Lee, Min Hyun Kim, Myung Jo Kim, Heung-Kwon Oh, Sung-Bum Kang
International Journal of Colorectal Disease.2018; 33(6): 719. CrossRef - A combination of clinical risk stratification and fecal immunochemical test is useful for identifying persons with high priority of early colonoscopy
Yoon Suk Jung, Chan Hyuk Park, Nam Hee Kim, Jung Ho Park, Dong Il Park, Chong Il Sohn
Digestive and Liver Disease.2018; 50(3): 254. CrossRef - Guideline Adherence to Colonoscopic Surveillance Intervals after Polypectomy in Korea: Results from a Nationwide Survey
Seri Hong, Mina Suh, Kui Son Choi, Boyoung Park, Jae Myung Cha, Hyun-Soo Kim, Jae Kwan Jun, Dong Soo Han
Gut and Liver.2018; 12(4): 426. CrossRef - Risk of developing metachronous advanced colorectal neoplasia after colonoscopic polypectomy in patients aged 30 to 39 and 40 to 49 years
Nam Hee Kim, Yoon Suk Jung, Jung Ho Park, Dong Il Park, Chong Il Sohn
Gastrointestinal Endoscopy.2018; 88(4): 715. CrossRef - A simple scoring model for advanced colorectal neoplasm in asymptomatic subjects aged 40–49 years
Yoo Mi Park, Hee Sun Kim, Jae Jun Park, Su Jung Baik, Young Hoon Youn, Jie-Hyun Kim, Hyojin Park
BMC Gastroenterology.2017;[Epub] CrossRef - The fecal immunochemical test has high accuracy for detecting advanced colorectal neoplasia before age 50
Nam Hee Kim, Jung Ho Park, Dong Il Park, Chong Il Sohn, Kyuyong Choi, Yoon Suk Jung
Digestive and Liver Disease.2017; 49(5): 557. CrossRef - Colorectal cancer screening with the fecal immunochemical test in persons aged 30 to 49 years: focusing on the age for commencing screening
Yoon Suk Jung, Chan Hyuk Park, Nam Hee Kim, Jung Ho Park, Dong Il Park, Chong Il Sohn
Gastrointestinal Endoscopy.2017; 86(5): 892. CrossRef - Biomedical optical spectroscopy for the early diagnosis of gastrointestinal neoplasms
Qin-Si Wan, Ting Wang, Kun-He Zhang
Tumor Biology.2017; 39(7): 101042831771798. CrossRef - Training in Endoscopy: Colonoscopy
Hyun Joo Jang
Clinical Endoscopy.2017; 50(4): 322. CrossRef - Impact of Age on the Risk of Advanced Colorectal Neoplasia in a Young Population: An Analysis Using the Predicted Probability Model
Yoon Suk Jung, Chan Hyuk Park, Nam Hee Kim, Mi Yeon Lee, Dong Il Park
Digestive Diseases and Sciences.2017; 62(9): 2518. CrossRef - Screening of colorectal cancer: present and future
Marcello Maida, Fabio Salvatore Macaluso, Gianluca Ianiro, Francesca Mangiola, Emanuele Sinagra, Georgina Hold, Carlo Maida, Giovanni Cammarota, Antonio Gasbarrini, Giuseppe Scarpulla
Expert Review of Anticancer Therapy.2017; 17(12): 1131. CrossRef - Advanced Colonic Neoplasia at Follow-up Colonoscopy According to Risk Components and Adenoma Location at Index Colonoscopy: A Retrospective Study of 1,974 Asymptomatic Koreans
Su Jung Baik, Hyojin Park, Jae Jun Park, Hyun Ju Lee, So Young Jo, Yoo Mi Park, Hye Sun Lee
Gut and Liver.2017; 11(5): 667. CrossRef - Risk Factors Such as Male Sex, Smoking, Metabolic Syndrome, Obesity, and Fatty Liver Do Not Justify Screening Colonoscopies Before Age 45
Yoon Suk Jung, Kyung Eun Yun, Yoosoo Chang, Seungho Ryu, Dong Il Park
Digestive Diseases and Sciences.2016; 61(4): 1021. CrossRef - Risk of developing advanced colorectal neoplasia after removing high‐risk adenoma detected at index colonoscopy in young patients: A KASID study
Soo‐Kyung Park, Nam Hee Kim, Yoon Suk Jung, Won Hee Kim, Chang Soo Eun, Bong Min Ko, Geom Seog Seo, Jae Myung Cha, Jae Jun Park, Kyeong Ok Kim, Chang Mo Moon, Yoonho Jung, Eun Soo Kim, Seong Ran Jeon, Chang Kyun Lee, Dong Il Park
Journal of Gastroenterology and Hepatology.2016; 31(1): 138. CrossRef - Colorectal cancer screening of the general population in East Asia
Yasushi Sano, Jeong‐Sik Byeon, Xiao‐Bo Li, Martin C.S. Wong, Han‐Mo Chiu, Rungsun Rerknimitr, Takahiro Utsumi, Santa Hattori, Wataru Sano, Mineo Iwatate, Philip Chiu, Joseph Sung
Digestive Endoscopy.2016; 28(3): 243. CrossRef - Does Low Threshold Value Use Improve Proximal Neoplasia Detection by Fecal Immunochemical Test?
Nam Hee Kim, Hyo-Joon Yang, Soo-Kyung Park, Jung Ho Park, Dong Il Park, Chong Il Sohn, Kyuyong Choi, Yoon Suk Jung
Digestive Diseases and Sciences.2016; 61(9): 2685. CrossRef - High levels of carcinoembryonic antigen and smoking might be markers of colorectal adenoma in Korean males aged 40-49 years
In Cheol Yoon, Jeong Hyeon Cho, Heejin Choi, Young Hoon Choi, Kyu Min Lim, Sung Hwa Choi, Jae Ho Han, Hyeon Ju Jeong, Hong Sub Lee
Yeungnam University Journal of Medicine.2016; 33(1): 13. CrossRef - Endoscopic Resection for Small Rectal Neuroendocrine Tumors: Comparison of Endoscopic Submucosal Resection with Band Ligation and Endoscopic Submucosal Dissection
Byoung Wook Bang, Jin Seok Park, Hyung Kil Kim, Yong Woon Shin, Kye Sook Kwon, Joon Mee Kim
Gastroenterology Research and Practice.2016; 2016: 1. CrossRef - Is a Preoperative Gastrointestinal Endoscopy for Second Primary Cancer Detection in Head and Neck Cancer Necessary? Ten-year Registry Data
Gyeong Mi Heo, Mi Hee Kim, Jin Hwan Kim, Young Soo Rho, Woon Geon Shin
The Korean Journal of Gastroenterology.2016; 68(1): 23. CrossRef - Effect of Previous Gastrectomy on the Performance of Postoperative Colonoscopy
Sunghwan Kim, Jeongmin Choi, Tae Han Kim, Seong-Ho Kong, Yun-Suhk Suh, Jong Pil Im, Hyuk-Joon Lee, Sang Gyun Kim, Seung-Yong Jeong, Joo Sung Kim, Han-Kwang Yang
Journal of Gastric Cancer.2016; 16(3): 167. CrossRef - Diagnostic Value of Methylated Septin9 for Colorectal Cancer Screening: A Meta-Analysis
Shirong Yan, Zijing Liu, Shuang Yu, Yixi Bao
Medical Science Monitor.2016; 22: 3409. CrossRef - Characteristics of and risk factors for colorectal neoplasms in young adults in a screening population
Seung Eun Lee, Hee Bum Jo, Won Gun Kwack, Yun Jin Jeong, Yeo-Jin Yoon, Hyoun Woo Kang
World Journal of Gastroenterology.2016; 22(10): 2981. CrossRef - Background and significance of Korean national cancer screening guideline revision
Won-Chul Lee, Yeol Kim
Journal of the Korean Medical Association.2015; 58(4): 274. CrossRef - Effect of different reconstruction algorithms on computer-aided diagnosis (CAD) performance in ultra-low dose CT colonography
Eun Sun Lee, Se Hyung Kim, Jong Pil Im, Sang Gyun Kim, Cheong-il Shin, Joon Koo Han, Byung Ihn Choi
European Journal of Radiology.2015; 84(4): 547. CrossRef - Comparing the effectiveness of competing tests for reducing colorectal cancer mortality: a network meta-analysis
B. Joseph Elmunzer, Amit G. Singal, Jeremy B. Sussman, Amar R. Deshpande, Daniel A. Sussman, Marisa L. Conte, Ben A. Dwamena, Mary A.M. Rogers, Philip S. Schoenfeld, John M. Inadomi, Sameer D. Saini, Akbar K. Waljee
Gastrointestinal Endoscopy.2015; 81(3): 700. CrossRef - Risk factors for colorectal neoplasia in persons aged 30 to 39 years and 40 to 49 years
Yoon Suk Jung, Seungho Ryu, Yoosoo Chang, Kyung Eun Yun, Jung Ho Park, Hong Joo Kim, Yong Kyun Cho, Chong Il Sohn, Woo Kyu Jeon, Byung Ik Kim, Dong Il Park
Gastrointestinal Endoscopy.2015; 81(3): 637. CrossRef - Non-invasive screening for colorectal cancer in Asia
Han-Mo Chiu, Li-Chun Chang, Wen-Feng Hsu, Chu-Kuang Chou, Ming-Shiang Wu
Best Practice & Research Clinical Gastroenterology.2015; 29(6): 953. CrossRef - Risk Factors for Delayed Post-Polypectomy Bleeding
Min Jung Kwon, You Sun Kim, Song I Bae, Young Il Park, Kyung Jin Lee, Jung Hwa Min, Soo Yeon Jo, Mi Young Kim, Hye Jin Jung, Seong Yeon Jeong, Won Jae Yoon, Jin Nam Kim, Jeong Seop Moon
Intestinal Research.2015; 13(2): 160. CrossRef - Distribution of the Colonoscopic Adenoma Detection Rate According to Age: Is Recommending Colonoscopy Screening for Koreans Over the Age of 50 Safe?
Taeseok Bae, Yunhyung Ha, Changkyun Kim, Jihyun Lee, Kwangil Ha, Sanghyun Shin, Youngcheol Lee, Yoonsik Kang
Annals of Coloproctology.2015; 31(2): 46. CrossRef - The Korean guideline for colorectal cancer screening
Dae Kyung Sohn, Min Ju Kim, Younhee Park, Mina Suh, Aesun Shin, Hee Young Lee, Jong Pil Im, Hyoen-Min Cho, Sung Pil Hong, Baek-hui Kim, Yongsoo Kim, Jeong Wook Kim, Hyun-Soo Kim, Chung Mo Nam, Dong Il Park, Jun Won Um, Soon Nam Oh, Hwan Sub Lim, Hee Jin C
Journal of the Korean Medical Association.2015; 58(5): 420. CrossRef - Age-specific prevalence of serrated lesions and their subtypes by screening colonoscopy: a retrospective study
Hyun Young Kim, Seon Mie Kim, Ji-Hyun Seo, Eun-Ha Park, Nayoung Kim, Dong Ho Lee
BMC Gastroenterology.2014;[Epub] CrossRef - Clinical features and course of ulcerative colitis diagnosed in asymptomatic subjects
Soo-Kyung Park, Byong Duk Ye, Suk-Kyun Yang, Seon-Ok Kim, Jihun Kim, Jong Wook Kim, Sang Hyoung Park, Dong-Hoon Yang, Kee Wook Jung, Kyung-Jo Kim, Jeong-Sik Byeon, Seung-Jae Myung, Jin-Ho Kim
Journal of Crohn's and Colitis.2014; 8(10): 1254. CrossRef - Do We Need Colonoscopy Following Acute Diverticulitis Detected on Computed Tomography to Exclude Colorectal Malignancy?
Young Hoon Choi, Seong-Joon Koh, Ji Won Kim, Byeong Gwan Kim, Kook Lae Lee, Jong Pil Im, Joo Sung Kim, Hyun Chae Jung
Digestive Diseases and Sciences.2014; 59(9): 2236. CrossRef - A Survey of Colonoscopic Surveillance After Polypectomy
Dae Kyung Sohn
Annals of Coloproctology.2014; 30(2): 88. CrossRef - The Prevalence of Colorectal Adenomas in Asymptomatic Korean Men and Women
Moon Hee Yang, Sanjay Rampal, Jidong Sung, Yoon-Ho Choi, Hee Jung Son, Jun Haeng Lee, Young-Ho Kim, Dong Kyung Chang, Poong-Lyul Rhee, Jong Chul Rhee, Eliseo Guallar, Juhee Cho
Cancer Epidemiology, Biomarkers & Prevention.2014; 23(3): 499. CrossRef - Circulating Methylated Septin 9 Nucleic Acid in the Plasma of Patients with Gastrointestinal Cancer in the Stomach and Colon
Hye Seung Lee, Sang Mee Hwang, Taek Soo Kim, Duck-Woo Kim, Do Joong Park, Sung-Bum Kang, Hyung-Ho Kim, Kyoung Un Park
Translational Oncology.2013; 6(3): 290. CrossRef
- Colorectal cancer screening guidelines for average-risk and high-risk individuals: A systematic review
- 12,710 View
- 164 Download
- 72 Crossref


 KSGE
KSGE


 First
First Prev
Prev



