Search
- Page Path
- HOME > Search
Systematic Review and Meta-analysis
- Cryotherapy versus radiofrequency ablation in the treatment of dysplastic Barrett’s esophagus with or without early esophageal neoplasia: a systematic review and meta-analysis
- Igor Logetto Caetité Gomes, Diogo Turiani Hourneaux de Moura, Igor Braga Ribeiro, Sérgio Barbosa Marques, Alexandre de Sousa Carlos, Beanie Conceição Medeiros Nunes, Bruno Salomão Hirsch, Guilherme Henrique Peixoto de Oliveira, Roberto Paolo Trasolini, Wanderley Marques Bernardo, Eduardo Guimarães Hourneaux de Moura
- Clin Endosc 2024;57(2):181-190. Published online January 17, 2024
- DOI: https://doi.org/10.5946/ce.2023.065
-
 Graphical Abstract
Graphical Abstract
 Abstract
Abstract
 PDF
PDF Supplementary Material
Supplementary Material PubReader
PubReader ePub
ePub 
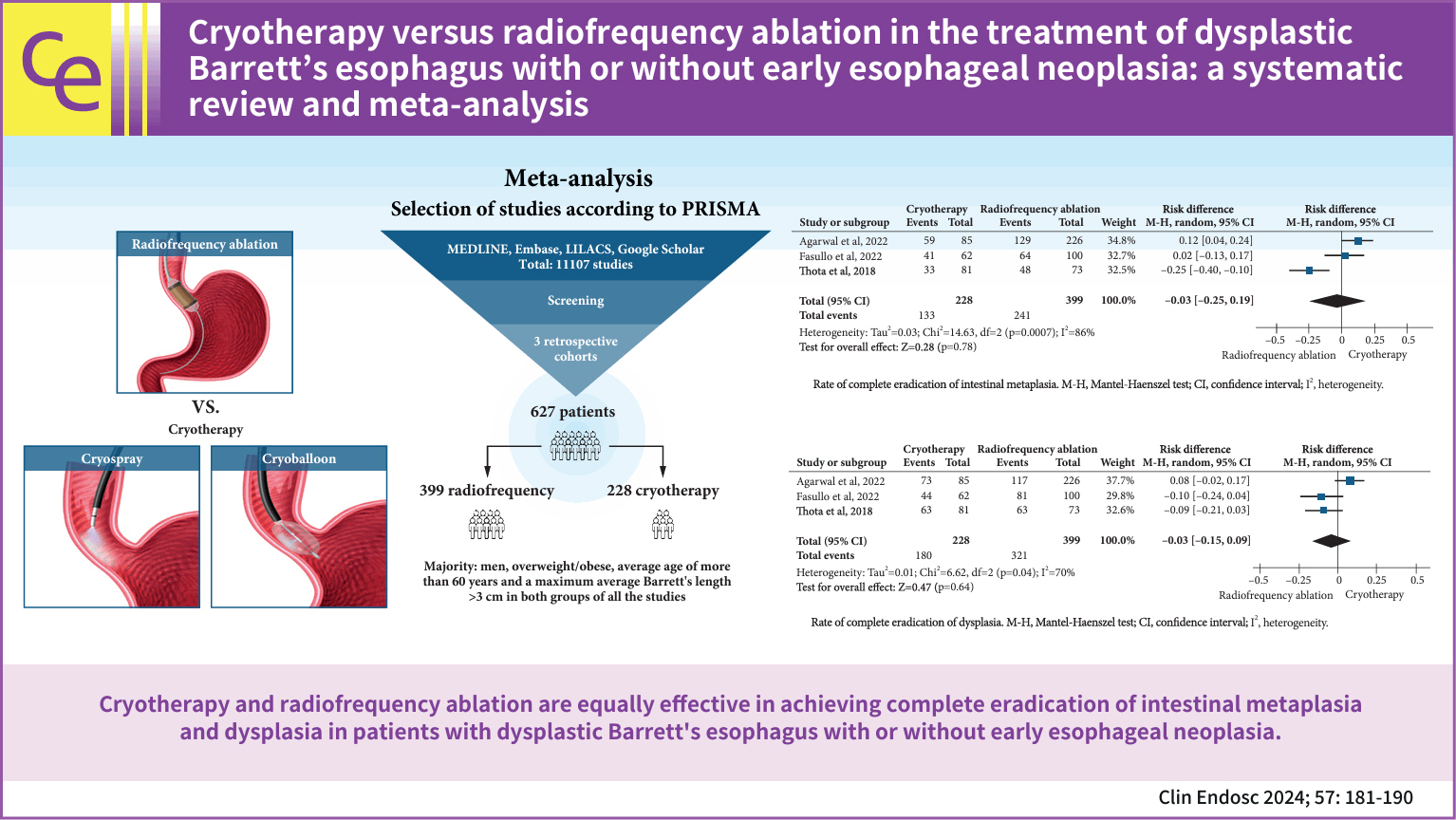
- Background
/Aims: Radiofrequency ablation (RFA) is the first-line therapy for dysplastic Barrett’s esophagus (BE). Therefore, cryotherapy has emerged as an alternative treatment option. This study aimed to compare the efficacies of these two techniques based on the rates of complete eradication of intestinal metaplasia (CE-IM) and dysplasia (CE-D). Adverse events and recurrence have also been reported.
Methods
An electronic search was conducted using the Medline (PubMed), Embase, LILACS, and Google Scholar databases until December 2022. Studies were included comparing cryotherapy and RFA for treating dysplastic BE with or without early esophageal neoplasia. This study was performed in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses guidelines.
Results
Three retrospective cohort studies involving 627 patients were included. Of these, 399 patients underwent RFA, and 228 were treated with cryotherapy. There was no difference in CE-IM (risk difference [RD], –0.03; 95% confidence interval [CI], –0.25 to 0.19; p=0.78; I2=86%) as well as in CE-D (RD, –0.03; 95% CI, –0.15 to 0.09; p=0.64; I2=70%) between the groups. The absolute number of adverse events was low, and there was no difference in the recurrence rate.
Conclusions
Cryotherapy and RFA were equally effective in treating dysplastic BE, with or without early esophageal neoplasia.
- 3,033 View
- 205 Download

Systematic Review and Meta-Analysises
- Stent versus Balloon Dilation for the Treatment of Dominant Strictures in Primary Sclerosing Cholangitis: A Systematic Review and Meta-Analysis
- Marina Tucci Gammaro Baldavira Ferreira, Igor Braga Ribeiro, Diogo Turiani Hourneaux de Moura, Thomas R. McCarty, Alberto Machado da Ponte Neto, Galileu Ferreira Ayala Farias, Antônio Afonso de Miranda Neto, Pedro Victor Aniz Gomes de Oliveira, Wanderley Marques Bernardo, Eduardo Guimarães Hourneaux de Moura
- Clin Endosc 2021;54(6):833-842. Published online July 1, 2021
- DOI: https://doi.org/10.5946/ce.2021.052

-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader ePub
ePub - Background
/Aims: The endoscopic management of primary sclerosing cholangitis (PSC)-associated dominant strictures remains challenging. This systematic review and meta-analysis aimed to compare balloon dilation and stent placement in the treatment of dominant strictures among PSC patients.
Methods
Literature searches on MEDLINE, EMBASE, Cochrane CENTRAL and Lilacs/Bireme were performed for studies published until December 2020. Measured outcomes included clinical efficacy, stricture recurrence, cumulative recurrencefree rate, transplant rate, 5-year survival rate, and adverse events (i.e., pancreatitis, cholangitis, bleeding, perforation and death).
Results
A total of 5 studies (n=467) were included. Based on pooled analyses, there were no differences in clinical efficacy (risk difference [RD], -0.13; 95% confidence interval [CI], -0.58 to 0.33; I2=93%) or transplant rates (RD, -0.09; 95% CI, -0.19 to 0.01; I2=0%); however, the risk of occurrence of adverse events was lower with balloon dilatation than with stent placement (RD,-0.34; 95% CI, -0.45 to -0.23; I2=61%). Among the types of adverse events reported, only the rates of cholangitis/bacteremia were significantly lower in balloon dilation patients (RD, -0.19; 95% CI, -0.25 to -0.13; I2=51%).
Conclusions
Compared to balloon dilation, stent placement for dominant strictures in PSC appeared to have higher complication rates without significant differences in efficacy. -
Citations
Citations to this article as recorded by- Treatment of Non-Anastomotic Biliary Strictures after Liver Transplantation: How Effective Is Our Current Treatment Strategy?
Florian A. Michael, Mireen Friedrich-Rust, Hans-Peter Erasmus, Christiana Graf, Olivier Ballo, Mate Knabe, Dirk Walter, Christoph D. Steup, Marcus M. Mücke, Victoria T. Mücke, Kai H. Peiffer, Esra Görgülü, Antonia Mondorf, Wolf O. Bechstein, Natalie Filma
Journal of Clinical Medicine.2023; 12(10): 3491. CrossRef - Treatment of primary sclerosing cholangitis combined with inflammatory bowel disease
You Sun Kim, Edward H. Hurley, Yoojeong Park, Sungjin Ko
Intestinal Research.2023; 21(4): 420. CrossRef - Liver Transplantation for Primary Sclerosing Cholangitis (PSC) With or Without Inflammatory Bowel Disease (IBD)—A European Society of Organ Transplantation (ESOT) Consensus Statement
M. Carbone, A. Della Penna, C. Mazzarelli, E. De Martin, C. Villard, A. Bergquist, P. D. Line, J. M. Neuberger, S. Al-Shakhshir, P. J. Trivedi, U. Baumann, L. Cristoferi, J. Hov, B. Fischler, N. H. Hadzic, D. Debray, L. D’Antiga, N. Selzner, L. S. Belli,
Transplant International.2023;[Epub] CrossRef - Primary Biliary Cholangitis and Primary Sclerosing Cholangitis: Current Knowledge of Pathogenesis and Therapeutics
Ji-Won Park, Jung-Hee Kim, Sung-Eun Kim, Jang Han Jung, Myoung-Kuk Jang, Sang-Hoon Park, Myung-Seok Lee, Hyoung-Su Kim, Ki Tae Suk, Dong Joon Kim
Biomedicines.2022; 10(6): 1288. CrossRef - Use a biodegradable stent in ERCP and it will never be forgotten
Jesús García-Cano, Eva de la Santa Belda, Francisco Domper
Revista Española de Enfermedades Digestivas.2022;[Epub] CrossRef - Endoscopic stenting of dominant strictures in patients with primary sclerosing cholangitis: When, how, and for how long?
Il Sang Shin, Jong Ho Moon
Endoscopy International Open.2022; 10(09): E1169. CrossRef
- Treatment of Non-Anastomotic Biliary Strictures after Liver Transplantation: How Effective Is Our Current Treatment Strategy?
- 4,013 View
- 115 Download
- 8 Web of Science
- 6 Crossref

- Endoscopic Band Ligation Versus Argon Plasma Coagulation in the Treatment of Gastric Antral Vascular Ectasia: A Systematic Review and Meta-Analysis of Randomized Controlled Trials
- Bruno Salomão Hirsch, Igor Braga Ribeiro, Mateus Pereira Funari, Diogo Turiani Hourneaux de Moura, Sergio Eiji Matuguma, Sergio A. Sánchez-Luna, Fabio Catache Mancini, Guilherme Henrique Peixoto de Oliveira, Wanderley Marques Bernardo, Eduardo Guimarães Hourneaux de Moura
- Clin Endosc 2021;54(5):669-677. Published online May 31, 2021
- DOI: https://doi.org/10.5946/ce.2021.063

-
 Abstract
Abstract
 PDF
PDF Supplementary Material
Supplementary Material PubReader
PubReader ePub
ePub - Background
/Aims: Argon plasma coagulation (APC) is the most commonly used endoscopic treatment for gastric antral vascular ectasia (GAVE). Endoscopic band ligation (EBL) has emerged as an alternative therapy. Our goal was to evaluate the feasibility, efficacy, and safety of APC and EBL for the treatment of GAVE. This is the first systematic review that included only randomized controlled trials (RCTs) on this topic.
Methods
A comprehensive search was performed using electronic databases to identify RCTs comparing APC and EBL for the treatment of GAVE following the Preferred Reporting Items for Systematic Reviews and Meta-analyses guidelines.
Results
Four RCTs were included, with a total of 204 patients. EBL was related to higher endoscopic eradication rates risk difference [RD], 0.29; 95% confidence interval [CI] [0.14, 0.44]; I2=0%) and less bleeding recurrence than APC (RD, 0.29; 95% CI [0.15, 0.44]; I2=0%). Patients treated with EBL required fewer blood transfusions (mean difference [MD], 1.49; 95% CI [0.28, 2.71]; I2=96%) and hospitalizations (MD, 0.29; 95% CI [0.19, 0.39]; I2=0%). The number of sessions required for the obliteration of lesions was higher with APC. There was no difference in the incidence of adverse events.
Conclusions
EBL is superior to APC in the treatment of GAVE in terms of endoscopic eradication rates, recurrence of bleeding, and transfusion requirements. -
Citations
Citations to this article as recorded by- Review article: Upper gastrointestinal bleeding – review of current evidence and implications for management
Dennis L. Shung, Loren Laine
Alimentary Pharmacology & Therapeutics.2024; 59(9): 1062. CrossRef - Bevacizumab in combination with octreotide rescues a patient with liver cirrhosis, GAVE syndrome and refractory hemorrhage – a case report
Simon Johannes Gairing, Eva Maria Schleicher, Lukas Müller, Christian Labenz, Felix Darstein, Daniel Grimm, Visvakanth Sivanathan, Arndt Weinmann, Marcus-Alexander Wörns, Roman Kloeckner, Michael B. Pitton, Florian Thieringer, Khan Fareed Rahman, Peter Ro
Zeitschrift für Gastroenterologie.2023; 61(03): 275. CrossRef - A Practical Approach to the Management of Gastric Antral Vascular Ectasia
Matthew H. Meyers, Laura Rodriguez, Michael S. Kriss
American Journal of Gastroenterology.2023; 118(9): 1532. CrossRef - Endoscopic Advances in Hepatology
Emma Vanderschueren, Jonel Trebicka, Wim Laleman
Seminars in Liver Disease.2023; 43(02): 176. CrossRef - Comparisons Between Endoscopic Band Ligation, Radiofrequency Ablation and Endoscopic Thermal Therapy for Gastric Antral Vascular Ectasia: A Meta-Analysis
Cheng-Che Che, Sz-Iuan Shiu, Chung-Wang Ko, Yu-Kang Tu, Chung-Hsin Chang
Digestive Diseases and Sciences.2023; 68(9): 3534. CrossRef - Role of Endoscopy in the Diagnosis, Grading, and Treatment of Portal Hypertensive Gastropathy and Gastric Antral Vascular Ectasia
Ali Khalifa, Don C. Rockey
Gastrointestinal Endoscopy Clinics of North America.2023;[Epub] CrossRef - An update on the management of non-variceal upper gastrointestinal bleeding
Ali A Alali, Alan N Barkun
Gastroenterology Report.2022;[Epub] CrossRef - Endoscopic band ligation in the treatment of gastric antral vascular ectasia: a systematic review and meta-analysis
Babu P. Mohan, Gregory Toy, Lena L. Kassab, Suresh Ponnada, Saurabh Chandan, Sheeva Parbhu, Shaun Chandna, Douglas G. Adler
Gastrointestinal Endoscopy.2021; 94(6): 1021. CrossRef
- Review article: Upper gastrointestinal bleeding – review of current evidence and implications for management
- 4,453 View
- 219 Download
- 7 Web of Science
- 8 Crossref

Original Article
- Insufflation of Carbon Dioxide versus Air During Colonoscopy Among Pediatric Patients: A Systematic Review and Meta-Analysis of Randomized Controlled Trials
- John Alexander Lata Guacho, Diogo Turiani Hourneaux de Moura, Igor Braga Ribeiro, Bruna Furia Buzetti Hourneaux de Moura, Megui Marilia Mansilla Gallegos, Thomas McCarty, Ricardo Katsuya Toma, Eduardo Guimarães Hourneaux de Moura
- Clin Endosc 2021;54(2):242-249. Published online March 25, 2021
- DOI: https://doi.org/10.5946/ce.2020.275
-
 Abstract
Abstract
 PDF
PDF Supplementary Material
Supplementary Material PubReader
PubReader ePub
ePub - Background
/Aims: Carbon dioxide is increasingly used in insufflation during colonoscopy in adult patients; however, air insufflation remains the primary practice among pediatric gastroenterologists. This systematic review and meta-analysis aims to evaluate insufflation using CO2 versus air in colonoscopies in pediatric patients.
Methods
Individualized search strategies were performed using MEDLINE, Cochrane Library, EMBASE, and LILACS databases following Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines and Cochrane working methodology. Randomized control trials (RCTs) were selected for the present meta-analysis. Pooled proportions were calculated for outcomes including procedure time and abdominal pain immediately and 24 hours post-procedure.
Results
The initial search yielded 644 records, of which five RCTs with a total of 358 patients (CO2: n=178 versus air: n=180) were included in the final analysis. The procedure time was not different between the CO2 and air insufflation groups (mean difference, 10.84; 95% confidence interval [CI], -2.55 to 24.22; p=0.11). Abdominal pain immediately post-procedure was significantly lower in the CO2 group (risk difference, -0.15; 95% CI; -0.26 to -0.03; p=0.01) while abdominal pain at 24 hours post-procedure was similar (risk difference, -0.05; 95% CI; -0.11 to 0.01; p=0.11).
Conclusions
Based on this systematic review and meta-analysis of RCT data, CO2 insufflation reduced abdominal pain immediately following the procedure, while pain was similar at 24 hours post-procedure. These results suggest that CO2 is a preferred insufflation technique when performing colonoscopy in pediatric patients. -
Citations
Citations to this article as recorded by- Elevations in End-Tidal CO2 With CO2 Use During Pediatric Endoscopy With Airway Protection: Is This Physiologically Significant?
Chinenye R. Dike, Andrew Huang Pacheco, Elizabeth Lyden, David Freestone, Ojasvini Choudhry, Warren P. Bishop, Mohanad Shukry
Journal of Pediatric Gastroenterology & Nutrition.2023; 76(5): 660. CrossRef
- Elevations in End-Tidal CO2 With CO2 Use During Pediatric Endoscopy With Airway Protection: Is This Physiologically Significant?
- 5,246 View
- 150 Download
- 1 Web of Science
- 1 Crossref

Case Report
- Rare and Fatal Gastrointestinal Mucormycosis (Zygomycosis) in a COVID-19 Patient: A Case Report
- Epifanio Silvino do Monte Junior, Marcos Eduardo Lera dos Santos, Igor Braga Ribeiro, Gustavo de Oliveira Luz, Elisa Ryoka Baba, Bruno Salomão Hirsch, Mateus Pereira Funari, Eduardo Guimarães Hourneaux de Moura
- Clin Endosc 2020;53(6):746-749. Published online November 19, 2020
- DOI: https://doi.org/10.5946/ce.2020.180

-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader ePub
ePub - The novel coronavirus disease (COVID-19) quickly spread to all continents. However, data regarding all the signs and symptoms of COVID-19 are insufficient. Patients with COVID-19 might present higher susceptibility to fungal coinfections. Mucormycosis is a rare and often life-threatening fungal disease characterized by vascular invasion by hyphae, resulting in thrombosis and necrosis. This is the first case report of mucormycosis in a COVID-19 patient. An 86-year-old male patient was admitted to the emergency room with acute diarrhea, cough, dyspnea, and fever from 5 days prior. Blood tests revealed a hemoglobin level of 14.3 mg/dL. Five days following the admission, the patient presented with melena and a hemoglobin level of 5.6 mg/dL. A transfusion of three units of red blood cells was required. Esophagogastroduodenoscopy revealed two giant gastric ulcers with necrotic debris and a deep hemorrhagic base without active bleeding. Furthermore, biopsies confirmed mucormycosis. Despite intensive care, the patient died 36 hours after the esophagogastroduodenoscopy.
-
Citations
Citations to this article as recorded by- The potential for rapid antigen testing for mucormycosis in the context of COVID-19
Christopher R. Thornton
Expert Review of Molecular Diagnostics.2024; 24(3): 161. CrossRef - A New Proposed Combined CT and MRI Staging System for Covid-19-Associated Rhino-Orbito-Cerebral Fungal Infection: A Multi-center Study with Pathological Correlation
Noha Yahia Ebaid, Haitham Foda, Doaa Khedr Mohamed Khedr, Ahmed Ebeed, Mahmoud Ahmed Ebada, Rabab Mohamed Abdelhay, Ali Awad, Amany Abd Al Badea, Basma Hamed Ibrahim, Emad Hassan Emara
Academic Radiology.2024; 31(3): 1055. CrossRef - Development of a Machine Learning Model to Predict Risk of Development of COVID-19-Associated Mucormycosis
Rajashri Patil, Sahjid Mukhida, Jyoti Ajagunde, Uzair Khan, Sameena Khan, Nageswari Gandham, Chanda Vyawhare, Nikunja K Das, Shahzad Mirza
Future Microbiology.2024; 19(4): 297. CrossRef - COVID-19 Second Wave with Mucormycosis, a Deadly Combination: A Systemic Review
Neetu Jain, Seema Bhadauria
Biomedical and Biotechnology Research Journal.2024; 8(1): 13. CrossRef - The cross-talk between mucormycosis, steroids and diabetes mellitus amidst the global contagion of COVID-19
Shrey Dwivedi, Princy Choudhary, Ayushi Gupta, Sangeeta Singh
Critical Reviews in Microbiology.2023; 49(3): 318. CrossRef - Magnetic resonance imaging spectrum of COVID-associated rhino-orbital-cerebral mucormycosis and assessment of anatomical severity
Ishan Kumar, Ashish Verma, Jyoti Dangwal, Pramod Kumar Singh, Ram Chandra Shukla, Jaya Chakravarty
The Neuroradiology Journal.2023; 36(4): 404. CrossRef - Mucormycosis and Its Upsurge During COVID-19 Epidemic: An Updated Review
Bharti Sharma, Skarma Nonzom
Current Microbiology.2023;[Epub] CrossRef - Mucormycosis: A hidden mystery of fungal infection, possible diagnosis, treatment and development of new therapeutic agents
Mohd Kamil Hussain, Shaista Ahmed, Andleeb Khan, Arif Jamal Siddiqui, Shahnaaz Khatoon, Sadaf Jahan
European Journal of Medicinal Chemistry.2023; 246: 115010. CrossRef -
cotH
Genes Are Necessary for Normal Spore Formation and Virulence in
Mucor lusitanicus
Csilla Szebenyi, Yiyou Gu, Teclegiorgis Gebremariam, Sándor Kocsubé, Sándor Kiss-Vetráb, Olivér Jáger, Roland Patai, Krisztina Spisák, Rita Sinka, Ulrike Binder, Mónika Homa, Csaba Vágvölgyi, Ashraf S. Ibrahim, Gábor Nagy, Tamás Papp, Anuradha Chowdhary
mBio.2023;[Epub] CrossRef - Post COVID-19: Risk Factors, Prevention, and Management of Black
Fungus
Aimen Salman, Suneela Dhaneshwar, Shaik Shafiulla
Anti-Infective Agents.2023; 21(1): 39. CrossRef - Surge of mucormycosis during the COVID-19 pandemic
Paulami Dam, Marlon H. Cardoso, Sukhendu Mandal, Octávio L. Franco, Pınar Sağıroğlu, Osman Ahmet Polat, Kerem Kokoglu, Rittick Mondal, Amit Kumar Mandal, Ismail Ocsoy
Travel Medicine and Infectious Disease.2023; 52: 102557. CrossRef - COVID-19 and Mucormycosis of Orofacial Region: A Scoping Review
Abhishek Banerjee, Moumalini Das, Pooja Verma, Abhishek Chatterjee, Karthikeyan Ramalingam, Kumar Chandan Srivastava
Cureus.2023;[Epub] CrossRef - Case Reports on Black Fungus of the Gastrointestinal Tract: A New Complication in COVID-19 Patients
Sachin Arora, Ashish Singh, Pallavi Prasad, Rahul, Rajneesh Singh
The Korean Journal of Gastroenterology.2023; 81(5): 221. CrossRef - Galangin for COVID-19 and Mucormycosis co-infection: a potential therapeutic strategy of targeting critical host signal pathways triggered by SARS-CoV-2 and Mucormycosis
Md. Imran Hasan, Md. Arju Hossain, Md Habibur Rahman, Md Sohel, Asif Ahsan, Md. Sadat Hossain Soikot, Md. Nazrul Islam, Mohammad Ruhul Amin, Deepak Kumar Jain
Network Modeling Analysis in Health Informatics and Bioinformatics.2023;[Epub] CrossRef - Opportunistic Fungal Invasion in COVID-19 Pandemic: A Critical Review in Diagnosis and Management
Abhishek Sharma, Gulnaz Bano, Abdul Malik, Yuman Rasool, Samrina Manzar, Tarun Singh, Manish Maity
Avicenna Journal of Medicine.2023; 13(03): 131. CrossRef - Effect of antifungal drugs against mucormycosis and impact on human health
Shivangi Giri, Sujata Sharma, Kumud Kant Awasthi, Lata Shahani
Materials Today: Proceedings.2023; 95: 43. CrossRef - Epidemiology, Risk Factors, Diagnosis and Treatment of Mucormycosis
(Black Fungus): A Review
Pragati Upadhayay, Keshav Bansal, Ahsas Goyal
Current Pharmaceutical Biotechnology.2023; 24(13): 1645. CrossRef - View of mucormycosis during the era of COVID-19 infection: A cross-sectional study
Ossama M. Zakaria, Dana W. Alkuwaity
Journal of Family Medicine and Primary Care.2023; 12(11): 2608. CrossRef - An Update on COVID‐19 Associated Mucormycosis Characteristics, Risk Factors, and Outcomes: a Systematic Review and Meta-Analysis
Kazem Khiabani, Mohammad Hosein Amirzade-Iranaq, Hanie Ahmadi
Current Fungal Infection Reports.2023; 17(4): 282. CrossRef - CT Findings of COVID-19–associated Pulmonary Mucormycosis: A Case Series and Literature Review
Mandeep Garg, Nidhi Prabhakar, Valliappan Muthu, Shameema Farookh, Harsimran Kaur, Vikas Suri, Ritesh Agarwal
Radiology.2022; 302(1): 214. CrossRef - Mucormycosis (black fungus) ensuing COVID-19 and comorbidity meets - Magnifying global pandemic grieve and catastrophe begins
Karthika Pushparaj, Haripriya Kuchi Bhotla, Vijaya Anand Arumugam, Manikantan Pappusamy, Murugesh Easwaran, Wen-Chao Liu, Utthapon Issara, Kannan R.R. Rengasamy, Arun Meyyazhagan, Balamuralikrishnan Balasubramanian
Science of The Total Environment.2022; 805: 150355. CrossRef - Mucormycosis: A Case Series of Patients Admitted in Non-COVID-19 Intensive Care Unit of a Tertiary Care Center during the Second Wave
Nikhil Kothari, Amit Goyal, Ankur Sharma, Shilpa Goyal, Pradeep K Bhatia, Sangam Yadav
Indian Journal of Critical Care Medicine.2022; 25(10): 1193. CrossRef - A systematic review on SARS‐CoV‐2‐associated fungal coinfections
Shringika Soni, Ramesh Namdeo Pudake, Utkarsh Jain, Nidhi Chauhan
Journal of Medical Virology.2022; 94(1): 99. CrossRef - Rhino-orbito-cerebral mucormycosis during the COVID-19 third wave in 2021: an Egyptian preliminary report from a single tertiary hospital
Taha K. Alloush, Osama Mansour, Adel T. Alloush, Tamer Roushdy, Eman Hamid, Mahmoud El-Shamy, Hossam M. Shokri
Neurological Sciences.2022; 43(2): 799. CrossRef - Cumulative Mortality and Factors Associated With Outcomes of Mucormycosis After COVID-19 at a Multispecialty Tertiary Care Center in India
Twinkle Choksi, Anamika Agrawal, Purva Date, Darshana Rathod, Anuja Gharat, Avinash Ingole, Bhushan Chaudhari, Nitin Pawar
JAMA Ophthalmology.2022; 140(1): 66. CrossRef - Using artificial intelligence-based models to predict the risk of mucormycosis among COVID-19 survivors: An experience from a public hospital in India
Shabbir Syed-Abdul, A. Shoban Babu, Raja Shekhar Bellamkonda, Ramaiah Itumalla, GVRK Acharyulu, Surya Krishnamurthy, Y. Venkat Santosh Ramana, Naresh Mogilicharla, Shwetambara Malwade, Yu-Chuan Li
Journal of Infection.2022; 84(3): 351. CrossRef - Bilateral Renal Mucor Mycosis Presenting as Anuria in a Covid 19 Recovered Patient: A Case Report and Review of Literature
Surya Prakash Vaddi, Seshu Mohan Khetavath, Dilip M. Babu, Nagarjuna Maturu, Bhulaxmi, Swathi, Krithika Mohan, Datta Prasad M, Jawahar B, Rajesh Reddy KRV
Urology.2022; 161: 12. CrossRef - Fungal Infections Other Than Invasive Aspergillosis in COVID-19 Patients
Kerri Basile, Catriona Halliday, Jen Kok, Sharon C-A. Chen
Journal of Fungi.2022; 8(1): 58. CrossRef - Gastrointestinal mucormycosis: A periodic systematic review of case reports from 2015 to 2021
Mojtaba Didehdar, Zahra chegini, Alireza Moradabadi, Ali Arash Anoushirvani, Seidamir Pasha Tabaeian, Milad Yousefimashouf, Aref Shariati
Microbial Pathogenesis.2022; 163: 105388. CrossRef - Gastrointestinal Mucormycosis: A Challenge during COVID-19 Pandemic
Jagdish Chander
Journal of Gastrointestinal Infections.2022; 11(1): 30. CrossRef - COVID-19 associated mucormycosis – An emerging threat
Chien-Ming Chao, Chih-Cheng Lai, Wen-Liang Yu
Journal of Microbiology, Immunology and Infection.2022; 55(2): 183. CrossRef - The emergence of COVID-19 associated mucormycosis: a review of cases from 18 countries
Martin Hoenigl, Danila Seidel, Agostinho Carvalho, Shivaprakash M Rudramurthy, Amir Arastehfar, Jean-Pierre Gangneux, Nosheen Nasir, Alexandro Bonifaz, Javier Araiza, Nikolai Klimko, Alexandra Serris, Katrien Lagrou, Jacques F Meis, Oliver A Cornely, John
The Lancet Microbe.2022; 3(7): e543. CrossRef - Fatal allograft mucormycosis complicating severe COVID‐19 infection and bacterial pyelonephritis
Abhilash Chandra, Namrata Rao S., Kiran Preet Malhotra
Transplant Infectious Disease.2022;[Epub] CrossRef - Mucormycosis in COVID-19 pandemic: study at tertiary hospital in India
Reshma P. Chavan, Shivraj M. Ingole, Hamna Abdul Nazir, Wilson V. Desai, Gajanan S. Kanchewad
European Archives of Oto-Rhino-Laryngology.2022; 279(6): 3201. CrossRef - Mucormycosis and COVID-19 pandemic: Clinical and diagnostic approach
Asim Azhar, Wajihul Hasan Khan, Parvez Anwar Khan, Khaled Alhosaini, Mohammad Owais, Aijaz Ahmad
Journal of Infection and Public Health.2022; 15(4): 466. CrossRef - COVID19 associated mucormycosis: A review
PriyadharsiniR Palanisamy, Dhivya Elango
Journal of Family Medicine and Primary Care.2022; 11(2): 418. CrossRef - Mucormycosis: risk factors, diagnosis, treatments, and challenges during COVID-19 pandemic
Ayushi Sharma, Anjana Goel
Folia Microbiologica.2022; 67(3): 363. CrossRef - Retracted: Mucormycosis infection in patients with COVID‐19: A systematic review
SeyedAhmad SeyedAlinaghi, Amirali Karimi, Alireza Barzegary, Zahra Pashaei, Amir Masoud Afsahi, Sanam Alilou, Nazanin Janfaza, Alireza Shojaei, Fatemeh Afroughi, Parsa Mohammadi, Yasna Soleimani, Newsha Nazarian, Ava Amiri, Marcarious M. Tantuoyir, Shahra
Health Science Reports.2022;[Epub] CrossRef - Epidemiology, clinical presentation and management of COVID‐19 associated mucormycosis: A single centre experience from Pune, Western India
Ameet Dravid, Reema Kashiva, Zafer Khan, Balasaheb Bande, Danish Memon, Aparna Kodre, Milind Mane, Vishal Pawar, Dattatraya Patil, Suraj Kalyani, Prathamesh Raut, Madhura Bapte, Charlotte Saldanha, Dinesh Chandak, Teerthagouda Patil, Sateesh Reddy, Krushn
Mycoses.2022; 65(5): 526. CrossRef - Rhino-Orbital-Cerebral Mucormycosis in a Post-COVID-19 Patient from Peru
Linda Ponce-Rosas, Jose Gonzales-Zamora, Nelson Diaz-Reyes, Oliver Alarco-Cadillo, Jorge Alave-Rosas, Mohd Adnan
Case Reports in Infectious Diseases.2022; 2022: 1. CrossRef - Mucormycosis: A Lethal Disease
Pawan N. Karwa, Jyoti K. Soundarmal, Pallavi S. Shinde, Swapna R. Jalde
Asian Journal of Pharmacy and Technology.2022; : 41. CrossRef - An overview of COVID-19 related to fungal infections: what do we know after the first year of pandemic?
R. G. Vitale, J. Afeltra, S. Seyedmousavi, S. L. Giudicessi, S. M. Romero
Brazilian Journal of Microbiology.2022; 53(2): 759. CrossRef - Mucormycosis: A new threat to Coronavirus disease 2019 with special emphasis on India
Deganta Ghosh, Sagardeep Dey, Himanko Chakraborty, Sneha Mukherjee, Ankita Halder, Akash Sarkar, Pallab Chakraborty, Rajdeep Ghosh, Joy Sarkar
Clinical Epidemiology and Global Health.2022; 15: 101013. CrossRef - First Reported Cases of COVID-19-Associated Mucormycosis in Tunisia
Rim Khemakhem, Ichrak Bougharriou, Nesrine Kallel, Anis Bafoun, Feten Mahmoudi, Samy Kammoun
Electronic Journal of Medical and Dental Studies.2022; 12(1): em0097. CrossRef - Iranian patients co-infected with COVID-19 and mucormycosis: the most common predisposing factor, clinical outcomes, laboratory markers and diagnosis, and drug therapies
Hamideh Molaei, Ehsan Shojaeefar, Eghlim Nemati, Leila Khedmat, Sayed Yousef Mojtahedi, Nematollah Jonaidi Jafari, Morteza Izadi, Behzad Einollahi
Infectious Diseases.2022; 54(8): 600. CrossRef - COVID-19 and Plethora of Fungal Infections
Reetu Kundu, Nidhi Singla
Current Fungal Infection Reports.2022; 16(2): 47. CrossRef - A Comprehensive Review on the Management of COVID-19-Associated Mucormycosis (CAM): The New Basics
Divyam Girdhar, Ekta Manocha
BioMed.2022; 2(2): 181. CrossRef - Mucormycosis and COVID-19-Associated Mucormycosis: Insights of a Deadly but Neglected Mycosis
Laura C. García-Carnero, Héctor M. Mora-Montes
Journal of Fungi.2022; 8(5): 445. CrossRef - COVID-19-Associated Fungal Infections: An Urgent Need for Alternative Therapeutic Approach?
Marianna Domán, Krisztián Bányai
Frontiers in Microbiology.2022;[Epub] CrossRef - Current Treatment Options for COVID-19 Associated Mucormycosis: Present Status and Future Perspectives
Yasasve Madhavan, Kadambari Vijay Sai, Dilip Kumar Shanmugam, Aashabharathi Manimaran, Karthigadevi Guruviah, Yugal Kishore Mohanta, Divyambika Catakapatri Venugopal, Tapan Kumar Mohanta, Nanaocha Sharma, Saravanan Muthupandian
Journal of Clinical Medicine.2022; 11(13): 3620. CrossRef - Fungal Infection in Co-infected Patients With COVID-19: An Overview of Case Reports/Case Series and Systematic Review
Sima Sadat Seyedjavadi, Parmida Bagheri, Mohammad Javad Nasiri, Mehdi Razzaghi-Abyaneh, Mehdi Goudarzi
Frontiers in Microbiology.2022;[Epub] CrossRef - COVID-19-associated fungal infections in Iran: A systematic review
Tina Nazari, Fatemeh Sadeghi, Alireza Izadi, Setayesh Sameni, Shahram Mahmoudi, Felix Bongomin
PLOS ONE.2022; 17(7): e0271333. CrossRef - Effect of Indoor Bioaerosols (Fungal) Exposure on the Health of Post-COVID-19 Patients and Possible Mitigation Strategies
Yogesh Kumar Vishwakarma, Amrita Shahi, Ram Sharan Singh
COVID.2022; 2(7): 940. CrossRef - Mucormycosis in patients with COVID-19 in Russia: the results of a prospective multi-center study
S. N. Khostelidi, V. A. Zaytsev, S. A. Vartanyan, N. A. Nikitin, G. N. Evtukh, M. N. Gilalov, G. V. Portnov, A. A. Zubareva, I. B. Baranova, T. S. Bogomolova, Yu. L. Avdeenko, O. V. Shadrivova, E. A. Desyatik, E. V. Shagdileeva, Yu. V. Borzova, Yu. A. Kri
Journal Infectology.2022; 14(2): 116. CrossRef - Development of a Monoclonal Antibody and a Serodiagnostic Lateral-Flow Device Specific to Rhizopus arrhizus (Syn. R. oryzae), the Principal Global Agent of Mucormycosis in Humans
Genna E. Davies, Christopher R. Thornton
Journal of Fungi.2022; 8(7): 756. CrossRef - Mucormycosis co-infection in COVID-19 patients: An update
Abdullah S. Alkhamiss, Ahmed A. Ahmed, Zafar Rasheed, Ruqaih Alghsham, Ali Shariq, Thamir Alsaeed, Sami A. Althwab, Suliman Alsagaby, Abdullah S. M. Aljohani, Fahad A. Alhumaydhi, Sharifa K. Alduraibi, Alaa K. Alduraibi, Homaidan T. Alhomaidan, Khaled S.
Open Life Sciences.2022; 17(1): 917. CrossRef - COVID-19-Associated Mucormycosis: A Matter of Concern Amid the SARS-CoV-2 Pandemic
Pankaj Chandley, Priyanka Subba, Soma Rohatgi
Vaccines.2022; 10(8): 1266. CrossRef - Antifungal therapy in the management of fungal secondary infections in COVID-19 patients: A systematic review and meta-analysis
Sujit Kumar Sah, Atiqulla Shariff, Niharika Pathakamuri, Subramanian Ramaswamy, Madhan Ramesh, Krishna Undela, Malavalli Siddalingegowda Srikanth, Teggina Math Pramod Kumar, Joy Sturtevant
PLOS ONE.2022; 17(7): e0271795. CrossRef - The role of SARS-CoV-2 immunosuppression and the therapy used to manage COVID-19 disease in the emergence of opportunistic fungal infections: A review
Nahid Akhtar, Atif Khurshid Wani, Surya Kant Tripathi, Ajit Prakash, M. Amin-ul Mannan
Current Research in Biotechnology.2022; 4: 337. CrossRef - Mortality-Related Risk Factors for Coronavirus Disease (COVID-19)-Associated Mucormycosis: a systematic review and meta-analysis
Vahid Reza Ostovan, Reza Tabrizi, Hanieh Bazrafshan, Zahra Bahrami, Hajar Khazraei, Samaneh Khazraei, Afshin Borhani-Haghighi, Mohsen Moghadami, Matthew Grant
Current Fungal Infection Reports.2022; 16(4): 143. CrossRef - Comparative risk assessment of COVID‐19 associated mucormycosis and aspergillosis: A systematic review
Prodip Kumar Baral, Md. Abdul Aziz, Mohammad Safiqul Islam
Health Science Reports.2022;[Epub] CrossRef - COVID-19 and Fungal infections: a double debacle
Sara Mina, Hajar Yaakoub, Cédric Annweiler, Vincent Dubée, Nicolas Papon
Microbes and Infection.2022; 24(8): 105039. CrossRef - Wave of Invasive Fungal Disease on the Shores of COVID-19: A Case Series of COVID-19 Associated Rhino-Orbital Fungal Rhinosinusitis and Literature Review
Sandeep Trehan, Neena Chaudhary, Ashwin Bhasarkar
Indian Journal of Otolaryngology and Head & Neck Surgery.2022; 74(S2): 3359. CrossRef - A study of rhino-orbito-cerebral mucormycosis with COVID-19: A new challenge in North West of Rajasthan
Surendra Kumar, Harish Kumar, Manoj Mali, BabuLal Meena
Annals of African Medicine.2022; 21(4): 383. CrossRef - Is the production of reactive oxygen and nitrogen species by macrophages associated with better infectious control in female mice with experimentally disseminated and pulmonary mucormycosis?
Amanda Ribeiro dos Santos, Thais Fernanda Fraga-Silva, Débora de Fátima Almeida-Donanzam, Angela Carolina Finatto, Camila Marchetti, Maria Izilda Andrade, Olavo Speranza de Arruda, Maria Sueli Parreira de Arruda, James Venturini, Michal A Olszewski
PLOS ONE.2022; 17(12): e0270071. CrossRef - Mucormycosis, COVID-19 Pandemic and the Lessons Learnt: A Review
Anila Varghese, Anita Upadhyay, RoyA Daniel, Twinkle Sharma, MShyam Mohan, Balaji Susindran, Priyanka Singh, Chandrakant Lahariya
Journal of Medical Evidence.2022; 3(3): 256. CrossRef - Gastritis enfisematosa secundaria a mucormicosis gástrica en paciente con COVID-19. Reporte de un caso
Martín Islas Torres, Ana Laura Castillo Luna, José Juan Rodríguez Moreno, Valeria Priscilla Rendón Muñoz, José Gerardo Zamora Inzuna, Albert Antonio Ibarra Trejo
Cirujano General.2022; 44(2): 87. CrossRef - A Fatal Case of Rhizopus azygosporus Pneumonia Following COVID-19
Anubhav Kanwar, Alex Jordan, Scott Olewiler, Kurt Wehberg, Michael Cortes, Brendan R. Jackson
Journal of Fungi.2021; 7(3): 174. CrossRef - The double‐edged sword of systemic corticosteroid therapy in viral pneumonia: A case report and comparative review of influenza‐associated mucormycosis versus COVID‐19 associated mucormycosis
Kazem Ahmadikia, Seyed Jamal Hashemi, Sadegh Khodavaisy, Muhammad Ibrahim Getso, Neda Alijani, Hamid Badali, Hossein Mirhendi, Mohammadreza Salehi, Azin Tabari, Mojtaba Mohammadi Ardehali, Mohammad Kord, Emmanuel Roilides, Sassan Rezaie
Mycoses.2021; 64(8): 798. CrossRef - Antibacterials/hydrocortisone/oseltamivir
Reactions Weekly.2021; 1845(1): 43. CrossRef - Mucormycosis after Coronavirus disease 2019 infection in a heart transplant recipient – Case report and review of literature
Akshay Khatri, Kai-Ming Chang, Ilan Berlinrut, Frances Wallach
Journal of Medical Mycology.2021; 31(2): 101125. CrossRef - Research and Management of Rare Diseases in the COVID-19 Pandemic Era: Challenges and Countermeasures
Sanjana Fatema Chowdhury, Syed Muktadir Al Sium, Saeed Anwar
Frontiers in Public Health.2021;[Epub] CrossRef - Mucormycosis in COVID-19: A systematic review of cases reported worldwide and in India
Awadhesh Kumar Singh, Ritu Singh, Shashank R. Joshi, Anoop Misra
Diabetes & Metabolic Syndrome: Clinical Research & Reviews.2021; 15(4): 102146. CrossRef - Rhinocerebral mucormycosis in COVID-19 patient with diabetes a deadly trio: Case series from the north-western part of India
Hardeva Ram Nehara, Inder Puri, Vipin Singhal, Sunil IH, Bhagirath Ram Bishnoi, Pramendra Sirohi
Indian Journal of Medical Microbiology.2021; 39(3): 380. CrossRef - COVID 19 infection and mucormycosis—a dangerously increasing combination
Satvinder Singh Bakshi, Vinoth Kumar Kalidoss
The Egyptian Journal of Otolaryngology.2021;[Epub] CrossRef - Autoptic identification of disseminated mucormycosis in a young male presenting with cerebrovascular event, multi-organ dysfunction and COVID-19 infection
Vidya Krishna, Jaymin Morjaria, Rona Jalandari, Fatima Omar, Sundeep Kaul
IDCases.2021; 25: e01172. CrossRef - Does COVID 19 generate a milieu for propagation of mucormycosis?
Deepak Pandiar, N. Siva Kumar, Rahul Anand, Mala Kamboj, Anjali Narwal, P.M. Shameena
Medical Hypotheses.2021; 152: 110613. CrossRef - Mucormycosis associated with COVID‐19 in two kidney transplant patients
Carolt Arana, Rafael E. Cuevas Ramírez, Marc Xipell, Joaquim Casals, Asunción Moreno, Sabina Herrera, Marta Bodro, Frederic Cofan, Fritz Diekmann, Núria Esforzado
Transplant Infectious Disease.2021;[Epub] CrossRef - COVID-19 associated mucormycosis: the urgent need to reconsider the indiscriminate use of immunosuppressive drugs
Alfonso J. Rodriguez-Morales, Ranjit Sah, Jose Millan-Oñate, Angel Gonzalez, Juan J. Montenegro-Idrogo, Sias Scherger, Carlos Franco-Paredes, Andrés F. Henao-Martínez
Therapeutic Advances in Infectious Disease.2021; 8: 204993612110270. CrossRef - COVID‐19‐associated mucormycosis: An updated systematic review of literature
Rimesh Pal, Birgurman Singh, Sanjay Kumar Bhadada, Mainak Banerjee, Ranjitpal Singh Bhogal, Neemu Hage, Ashok Kumar
Mycoses.2021; 64(12): 1452. CrossRef - Rare case of gastrointestinal mucormycosis with colonic perforation in an immunocompetent patient with COVID-19
Ravinder Pal Singh, Nishkarsh Gupta, Tanudeep Kaur, Anju Gupta
BMJ Case Reports.2021; 14(7): e244096. CrossRef - The double trouble: COVID-19 associated mucormycosis a focused review and future perspectives
Arun Kumar Agnihotri, Monika Vij, Okezie I. Aruoma, Vipul D Yagnik, Theeshan Bahorun, Maria Elena Villamil, Godfred A. Menezes, Vineet Gupta
Global Journal of Medical, Pharmaceutical, and Biomedical Update.2021; 16: 4. CrossRef - Mucormycosis: An opportunistic pathogen during COVID-19
Iyer Mahalaxmi, Kaavya Jayaramayya, Dhivya Venkatesan, Mohana Devi Subramaniam, Kaviyarasi Renu, Padmavathi Vijayakumar, Arul Narayanasamy, Abilash Valsala Gopalakrishnan, Nachimuthu Senthil Kumar, Palanisamy Sivaprakash, Krothapalli R.S. Sambasiva Rao, B
Environmental Research.2021; 201: 111643. CrossRef - Epidemiology of Systemic Mycoses in the COVID-19 Pandemic
María Guadalupe Frías-De-León, Rodolfo Pinto-Almazán, Rigoberto Hernández-Castro, Eduardo García-Salazar, Patricia Meza-Meneses, Carmen Rodríguez-Cerdeira, Roberto Arenas, Esther Conde-Cuevas, Gustavo Acosta-Altamirano, Erick Martínez-Herrera
Journal of Fungi.2021; 7(7): 556. CrossRef - EFFICACY OF ENDOSCOPIC TOPICAL MITOMYCIN C APPLICATION IN CAUSTIC ESOPHAGEAL STRICTURES IN THE PEDIATRIC POPULATION: A SYSTEMATIC REVIEW AND META-ANALYSIS OF RANDOMIZED CONTROLLED TRIALS
Marcelo Mochate FLOR, Igor Braga RIBEIRO, Diogo Turiani Hourneaux DE MOURA, Sérgio Barbosa MARQUES, Wanderley Marques BERNARDO, Eduardo Guimarães Hourneaux DE MOURA
Arquivos de Gastroenterologia.2021; 58(2): 253. CrossRef - Coronavirus Disease-Associated Mucormycosis from a Tertiary Care Hospital in India: A Case Series
Yudhyavir Singh, Venkata Ganesh, Shailendra Kumar, Nishant Patel, Richa Aggarwala, Kapil Dev Soni, Anjan Trikha
Cureus.2021;[Epub] CrossRef - Coronavirus Disease (Covid-19) Associated Mucormycosis (CAM): Case Report and Systematic Review of Literature
Deepak Garg, Valliappan Muthu, Inderpaul Singh Sehgal, Raja Ramachandran, Harsimran Kaur, Ashish Bhalla, Goverdhan D. Puri, Arunaloke Chakrabarti, Ritesh Agarwal
Mycopathologia.2021; 186(2): 289. CrossRef - Mucormycosis and COVID‐19: An epidemic within a pandemic in India
Lav Selarka, Suktara Sharma, Dinesh Saini, Sanjay Sharma, Amit Batra, Vishal T. Waghmare, Pratibha Dileep, Sanket Patel, Monarch Shah, Tejas Parikh, Prakash Darji, Amit Patel, Gaurav Goswami, Anand Shah, Sandeep Shah, Harsh Lathiya, Moksha Shah, Pranita S
Mycoses.2021; 64(10): 1253. CrossRef - COVID-19 and mucormycosis superinfection: the perfect storm
Jaffar A. Al-Tawfiq, Saad Alhumaid, Abeer N. Alshukairi, Mohamad-Hani Temsah, Mazin Barry, Abbas Al Mutair, Ali A. Rabaan, Awadh Al-Omari, Raghavendra Tirupathi, Manaf AlQahtani, Salma AlBahrani, Kuldeep Dhama
Infection.2021; 49(5): 833. CrossRef - COVID-19 Associated Rhino-Orbital Mucormycosis Complicated by Gangrenous and Bone Necrosis—A Case Report from Honduras
Elsa Yolanda Palou, María Auxiliadora Ramos, Emec Cherenfant, Adoni Duarte, Itzel Carolina Fuentes-Barahona, Lysien I. Zambrano, Fausto Muñoz-Lara, Sandra Aracely Montoya-Ramirez, Alex Francisco Cardona-Ortiz, Jorge Alberto Valle-Reconco, Juan J. Monteneg
Vaccines.2021; 9(8): 826. CrossRef - A rare case of knee joint mucormycosis with pathological fracture after COVID-19 infection
Sergiu Andrei Iordache, Adrian Cursaru, Bogdan Şerban, Mihnea Ioan Gabriel Popa
Romanian Journal of Orthopaedic Surgery and Traumatology.2021; 4(1): 9. CrossRef - Epidemiology and Pathophysiology of COVID-19-Associated Mucormycosis: India Versus the Rest of the World
Valliappan Muthu, Shivaprakash M. Rudramurthy, Arunaloke Chakrabarti, Ritesh Agarwal
Mycopathologia.2021; 186(6): 739. CrossRef - COVID-19-associated mucormycosis: Case report and systematic review
Ahmet Dilek, Resat Ozaras, Sevket Ozkaya, Mustafa Sunbul, Elif Itir Sen, Hakan Leblebicioglu
Travel Medicine and Infectious Disease.2021; 44: 102148. CrossRef - COVID-19 and mucormycosis in Latin America – An emerging concern
Alfonso J. Rodriguez-Morales, Carlos S. Mamani-García, Janeth N. Nuñez-Lupaca, Darwin A. León-Figueroa, Mely Olarte-Durand, Robinson A. Yrene-Cubas, Diana M. Ticona, Sebastian Abanto-Urbano
Travel Medicine and Infectious Disease.2021; 44: 102156. CrossRef - Overview on the Prevalence of Fungal Infections, Immune Response, and Microbiome Role in COVID-19 Patients
Maryam Roudbary, Sunil Kumar, Awanish Kumar, Lucia Černáková, Fatemeh Nikoomanesh, Célia F. Rodrigues
Journal of Fungi.2021; 7(9): 720. CrossRef - A case report of rhino-facial mucormycosis in a non-diabetic patient with COVID-19: a systematic review of literature and current update
Faezeh Mohammadi, Milad Badri, Shapoor Safari, Nima Hemmat
BMC Infectious Diseases.2021;[Epub] CrossRef - Mucormycosis: A manifestation in COVID-19 infection
Abhishek Sharma, Gulnaz Bano, Abdul Malik
Indian Journal of Pharmacy and Pharmacology.2021; 8(3): 189. CrossRef - A Review of Coronavirus Disease Covid-19
Swapnali Zore
International Journal of Advanced Research in Science, Communication and Technology.2021; : 104. CrossRef - COVID-19 Associated Mucormycosis: A Systematic Review from Diagnostic Challenges to Management
Farah Yasmin, Hala Najeeb, Aisha Naeem, Kartik Dapke, Rachana Phadke, Muhammad Sohaib Asghar, Syed Muhammad Ismail Shah, Domenico De Berardis, Irfan Ullah
Diseases.2021; 9(4): 65. CrossRef - COVID-19-Associated Mucormycosis (CAM): An Updated Evidence Mapping
Salman Hussain, Harveen Baxi, Abanoub Riad, Jitka Klugarová, Andrea Pokorná, Simona Slezáková, Radim Líčeník, Abul Kalam Najmi, Miloslav Klugar
International Journal of Environmental Research and Public Health.2021; 18(19): 10340. CrossRef - Rhino-orbital Mucormycosis as a complication of severe COVID-19 pneumonia
Mohammed A. Alamin, Mohammed Abdulgayoom, Sushil Niraula, Elabbass Abdelmahmuod, Ashraf O. Ahmed, Mohammed I. Danjuma
IDCases.2021; 26: e01293. CrossRef - COVID-19-Associated Mucormycosis (CAM): Case-Series and Global Analysis of Mortality Risk Factors
Abanoub Riad, Alshaimaa Ahmed Shabaan, Julien Issa, Sally Ibrahim, Hatem Amer, Yossef Mansy, Islam Kassem, Amira Bisher Kassem, Hans-Peter Howaldt, Miloslav Klugar, Sameh Attia
Journal of Fungi.2021; 7(10): 837. CrossRef - Sinoorbital Mucormycosis Associated with Corticosteroid Therapy in COVID-19 Infection
Zeinab Mehrabi, Maryam Salimi, Kianoush Niknam, Farzaneh Mohammadi, Hesan Jelodari Mamaghani, Mohammad Reza Sasani, Mohammad Javad Ashraf, Amirhossein Salimi, Mohammad Hassan Zahedroozegar, Zohreh Erfani, Huban Atilla
Case Reports in Ophthalmological Medicine.2021; 2021: 1. CrossRef - Mucormycosis infection in severe COVID‐19 patient with multiple underlying health conditions
Zahra Heydarifard, Moslem Safaei, Sevrin Zadheidar, Soroush Ehsan, Nazanin Zahra Shafiei‐Jandaghi
Clinical Case Reports.2021;[Epub] CrossRef - Invasive Fungal Infections Complicating COVID-19: A Narrative Review
Giacomo Casalini, Andrea Giacomelli, Annalisa Ridolfo, Cristina Gervasoni, Spinello Antinori
Journal of Fungi.2021; 7(11): 921. CrossRef - Coronavirus Disease 2019–Associated Invasive Fungal Infection
John W Baddley, George R Thompson, Sharon C -A Chen, P Lewis White, Melissa D Johnson, M Hong Nguyen, Ilan S Schwartz, Andrej Spec, Luis Ostrosky-Zeichner, Brendan R Jackson, Thomas F Patterson, Peter G Pappas
Open Forum Infectious Diseases.2021;[Epub] CrossRef - Manifestations and risk factors of COVID-19 and mucormycosis: A mini-review
Jugal Sutradhar, BapiRay Sarkar
Journal of Acute Disease.2021; 10(6): 221. CrossRef - Invasive Mucormycosis – An Enigma
Anil Prasad, Minakshi Mishra, Kaushik Saha
Cureus.2021;[Epub] CrossRef - Salix spp. Bark Hot Water Extracts Show Antiviral, Antibacterial, and Antioxidant Activities—The Bioactive Properties of 16 Clones
Jenni Tienaho, Dhanik Reshamwala, Tytti Sarjala, Petri Kilpeläinen, Jaana Liimatainen, Jinze Dou, Anneli Viherä-Aarnio, Riikka Linnakoski, Varpu Marjomäki, Tuula Jyske
Frontiers in Bioengineering and Biotechnology.2021;[Epub] CrossRef - Mucormycosis – resurgence of a deadly opportunist during COVID-19 pandemic: Four case reports
Shalini Upadhyay, Tanisha Bharara, Manisha Khandait, Ankit Chawdhry, Bharat Bhushan Sharma
World Journal of Clinical Cases.2021; 9(36): 11338. CrossRef - Mucormycosis following COVID19: clinical case and literature review
Sofya N. Khostelidi, V.A. Zaytsev, E.V. Pelikh, E.V. Yashina, O.N. Rodionova, T.S. Bogomolova, Yu.L. Avdeenko, Nikolay N. Klimko
Clinical Microbiology and Antimicrobial Chemotherapy.2021; 23(3): 255. CrossRef
- The potential for rapid antigen testing for mucormycosis in the context of COVID-19
- 7,751 View
- 256 Download
- 107 Web of Science
- 111 Crossref

Reviews
- Endoscopic Ultrasound-Guided Fine Needle Aspiration and Endoscopic Retrograde Cholangiopancreatography-Based Tissue Sampling in Suspected Malignant Biliary Strictures: A Meta-Analysis of Same-Session Procedures
- Diogo Turiani Hourneax de Moura, Marvin Ryou, Eduardo Guimarães Hourneaux de Moura, Igor Braga Ribeiro, Wanderlei Marques Bernardo, Christopher C. Thompson
- Clin Endosc 2020;53(4):417-428. Published online November 5, 2019
- DOI: https://doi.org/10.5946/ce.2019.053
-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader ePub
ePub - Background
/Aims: The diagnosis of biliary strictures can be challenging. There are no systematic reviews studying same-session endoscopic retrograde cholangiopancreatography (ERCP)-based tissue sampling and endoscopic ultrasound-guided fine needle aspiration (EUS-FNA) for the diagnosis of biliary strictures.
Methods
A systematic review was conducted on studies analyzing same-session EUS and ERCP for tissue diagnosis of suspected malignant biliary strictures. The primary outcome was the accuracy of each method individually compared to the two methods combined. The secondary outcome was the accuracy of each method in pancreatic and biliary etiologies. In the meta-analysis, we used Forest plots, summary receiver operating characteristic curves, and estimates of the area under the curve for intention-to-treat analysis.
Results
Of the 12,132 articles identified, six were included, resulting in a total of 497 patients analyzed. The sensitivity, specificity, positive likelihood ratio, negative likelihood ratio, and accuracy of the association between the two methods were: 86%, 98%, 12.50, 0.17, and 96.5%, respectively. For the individual analysis, the sensitivity, specificity and accuracy of EUS-FNA were 76%, 100%, and 94.5%, respectively; for ERCP-based tissue sampling, the sensitivity, specificity, and accuracy were 58%, 98%, and 78.1%, respectively. For pancreatic lesions, EUS-FNA was superior to ERCP-based tissue sampling. However, for biliary lesions, both methods had similar sensitivities.
Conclusions
Same-session EUS-FNA and ERCP-based tissue sampling is superior to either method alone in the diagnosis of suspected malignant biliary strictures. Considering these results, combination sampling should be performed when possible. -
Citations
Citations to this article as recorded by- British Society of Gastroenterology guidelines for the diagnosis and management of cholangiocarcinoma
Simon M Rushbrook, Timothy James Kendall, Yoh Zen, Raneem Albazaz, Prakash Manoharan, Stephen P Pereira, Richard Sturgess, Brian R Davidson, Hassan Z Malik, Derek Manas, Nigel Heaton, K Raj Prasad, John Bridgewater, Juan W Valle, Rebecca Goody, Maria Hawk
Gut.2024; 73(1): 16. CrossRef - Contrast-enhanced guided endoscopic ultrasound procedures
Marcel Ioan Gheorghiu, Andrada Seicean, Cristina Pojoga, Claudia Hagiu, Radu Seicean, Zeno Sparchez
World Journal of Gastroenterology.2024; 30(17): 2311. CrossRef - ACG Clinical Guideline: Diagnosis and Management of Biliary Strictures
B. Joseph Elmunzer, Jennifer L. Maranki, Victoria Gómez, Anna Tavakkoli, Bryan G. Sauer, Berkeley N. Limketkai, Emily A. Brennan, Elaine M. Attridge, Tara J. Brigham, Andrew Y. Wang
American Journal of Gastroenterology.2023; 118(3): 405. CrossRef - Endoscopic Ultrasound in the Diagnosis of Extrahepatic Cholangiocarcinoma: What Do We Know in 2023?
Rares Ilie Orzan, Cristina Pojoga, Renata Agoston, Radu Seicean, Andrada Seicean
Diagnostics.2023; 13(6): 1023. CrossRef - Endoscopic evaluation of indeterminate biliary strictures: Cholangioscopy, endoscopic ultrasound, or both?
Raymond S. Y. Tang
Digestive Endoscopy.2023;[Epub] CrossRef - Brush Cytology, Forceps Biopsy, or Endoscopic Ultrasound-Guided Sampling for Diagnosis of Bile Duct Cancer: A Meta-Analysis
Seung Bae Yoon, Sung-Hoon Moon, Sung Woo Ko, Hyun Lim, Ho Suk Kang, Jong Hyeok Kim
Digestive Diseases and Sciences.2022; 67(7): 3284. CrossRef - Managing adverse events after endoscopic ultrasound‐guided drainage of the biliary tract and pancreatic fluid collections: Narrative review (with video)
Mateus Pereira Funari, Igor Braga Ribeiro, Marcos Eduardo Lera dos Santos, Sergio Eiji Matuguma, Eduardo Guimarães Hourneaux de Moura
Digestive Endoscopy.2022; 34(2): 359. CrossRef - Endoscopic Ultrasound for the Diagnosis and Staging of Biliary Malignancy
Martin Coronel, Jeffrey H. Lee, Emmanuel Coronel
Clinics in Liver Disease.2022; 26(1): 115. CrossRef - Endoscopic Management of Pancreatobiliary Malignancies
Dong Wook Lee, Eun Young Kim
Digestive Diseases and Sciences.2022; 67(5): 1635. CrossRef - IgG4-related sclerosing cholangitis involving the gallbladder mimicking a hilar cholangiocarcinoma
Yun Chae Lee, Hyung Ku Chon, Keum Ha Choi
Endoscopy.2022; 54(12): E739. CrossRef - Promising Genomic Testing for Biliary Tract Cancer Using Endoscopic Ultrasound-Guided Fine-Needle Aspiration/Biopsy Specimens
Masaki Kuwatani, Kazumichi Kawakubo, Naoya Sakamoto
Diagnostics.2022; 12(4): 900. CrossRef - Endoscopic Ultrasound Plus Endoscopic Retrograde Cholangiopancreatography Based Tissue Sampling for Diagnosis of Proximal and Distal Biliary Stenosis Due to Cholangiocarcinoma: Results from a Retrospective Single-Center Study
Edoardo Troncone, Fabio Gadaleta, Omero Alessandro Paoluzi, Cristina Maria Gesuale, Vincenzo Formica, Cristina Morelli, Mario Roselli, Luca Savino, Giampiero Palmieri, Giovanni Monteleone, Giovanna Del Vecchio Blanco
Cancers.2022; 14(7): 1730. CrossRef - The Role of Cholangioscopy and EUS in the Evaluation of Indeterminate Biliary Strictures
Wilson Siu, Raymond S. Y. Tang
Gastroenterology Insights.2022; 13(2): 192. CrossRef - Current endoscopic approaches to biliary strictures
Tatsuya Sato, Yousuke Nakai, Mitsuhiro Fujishiro
Current Opinion in Gastroenterology.2022; 38(5): 450. CrossRef - Acute cholecystitis caused by gallbladder metastasis from non-small cell lung cancer: a case report
Kouki Imaoka, Daisuke Satoh, Ko Oshita, Takuya Yano, Tetsushi Kubota, Michihiro Ishida, Yasuhiro Choda, Masanori Yoshimitsu, Kanyu Nakano, Masao Harano, Hiroyoshi Matsukawa, Hitoshi Idani, Shigehiro Shiozaki, Masazumi Okajima
Clinical Journal of Gastroenterology.2021; 14(1): 351. CrossRef - Current Status and Research Progress of ERCP in the Diagnosis and Treatment of Biliary and Pancreatic System Diseases
跃华 李
Advances in Clinical Medicine.2021; 11(07): 3123. CrossRef - Same day endoscopic retrograde cholangio-pancreatography immediately after endoscopic ultrasound for choledocholithiasis is feasible, safe and cost-effective
Wisam Sbeit, Anas Kadah, Amir Shahin, Tawfik Khoury
Scandinavian Journal of Gastroenterology.2021; 56(10): 1243. CrossRef - Editors' Choice of Noteworthy Clinical Endoscopy Publications in the First Decade
Gwang Ha Kim, Kwang An Kwon, Do Hyun Park, Jimin Han
Clinical Endoscopy.2021; 54(5): 633. CrossRef - Tips and tricks for the diagnosis and management of biliary stenosis-state of the art review
Giovanna Del Vecchio Blanco, Michelangela Mossa, Edoardo Troncone, Renato Argirò, Andrea Anderloni, Alessandro Repici, Omero Alessandro Paoluzi, Giovanni Monteleone
World Journal of Gastrointestinal Endoscopy.2021; 13(10): 473. CrossRef - Stent versus Balloon Dilation for the Treatment of Dominant Strictures in Primary Sclerosing Cholangitis: A Systematic Review and Meta-Analysis
Marina Tucci Gammaro Baldavira Ferreira, Igor Braga Ribeiro, Diogo Turiani Hourneaux de Moura, Thomas R. McCarty, Alberto Machado da Ponte Neto, Galileu Ferreira Ayala Farias, Antônio Afonso de Miranda Neto, Pedro Victor Aniz Gomes de Oliveira, Wanderley
Clinical Endoscopy.2021; 54(6): 833. CrossRef - Endoscopic ultrasound fine needle aspiration vs fine needle biopsy in solid lesions: A multi-center analysis
Diogo Turiani Hourneaux Moura, Thomas R McCarty, Pichamol Jirapinyo, Igor Braga Ribeiro, Galileu Ferreira Ayala Farias, Antonio Coutinho Madruga-Neto, Marvin Ryou, Christopher C Thompson
World Journal of Clinical Cases.2021; 9(34): 10507. CrossRef - Efficacy of digital single-operator cholangioscopy in the visual interpretation of indeterminate biliary strictures: a systematic review and meta-analysis
Pedro Victor Aniz Gomes de Oliveira, Diogo Turiani Hourneaux de Moura, Igor Braga Ribeiro, Ahmad Najdat Bazarbashi, Tomazo Antonio Prince Franzini, Marcos Eduardo Lera dos Santos, Wanderley Marques Bernardo, Eduardo Guimarães Hourneaux de Moura
Surgical Endoscopy.2020; 34(8): 3321. CrossRef - Role of pancreatography in the endoscopic management of encapsulated pancreatic collections – review and new proposed classification
Igor Mendonça Proença, Marcos Eduardo Lera dos Santos, Diogo Turiani Hourneaux de Moura, Igor Braga Ribeiro, Sergio Eiji Matuguma, Spencer Cheng, Thomas R McCarty, Epifanio Silvino do Monte Junior, Paulo Sakai, Eduardo Guimarães Hourneaux de Moura
World Journal of Gastroenterology.2020; 26(45): 7104. CrossRef
- British Society of Gastroenterology guidelines for the diagnosis and management of cholangiocarcinoma
- 5,822 View
- 205 Download
- 21 Web of Science
- 23 Crossref

- Role of Cardiac Septal Occluders in the Treatment of Gastrointestinal Fistulas: A Systematic Review
- Diogo Turiani Hourneaux De Moura, Alberto Baptista, Pichamol Jirapinyo, Eduardo Guimarães Hourneaux De Moura, Christopher Thompson
- Clin Endosc 2020;53(1):37-48. Published online July 9, 2019
- DOI: https://doi.org/10.5946/ce.2019.030
-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader ePub
ePub - Treating gastrointestinal (GI) fistulas endoscopically is challenging owing to an established epithelial tract. The variety of endoscopic approaches is transforming endoscopy into a first-line therapy. However, many sessions are often required, with variable success rates. Owing to these limitations, the off-label use of cardiac septal occluders (CSOs) has been reported. We searched for articles related to CSOs in the MEDLINE, EMBASE, Cochrane Library, and LILACS databases and gray literature. The primary outcomes included technical success, clinical success, and safety of CSOs in GI fistula management. A total of 25,574 records were identified, and 19 studies ultimately satisfied the inclusion criteria. Technical success was achieved in all cases. Of the 22 fistulas, 77.27% had successful closure, with a mean follow-up period of 32.02 weeks. The adverse event rate was 22.72%, with no associated mortality. Univariable and multivariable regression analyses showed no significant difference in the success of closure and adverse events in relation to several variables among the subgroups. The use of CSOs appeared to be technically feasible, effective, and safe in the treatment of GI fistulas. The satisfactory results derived from this sparse literature suggest that it can be an option in the management of GI fistulas.
-
Citations
Citations to this article as recorded by- Endoscopic closure of a recto-pelvic fistula with a cardiac septal occluder device
Ayowumi A. Adekolu, Ethan M. Cohen, Sardar Momin Shah-Khan, Soban Maan, Joyce Foryoung, Ademola Ajibade, Shyam Thakkar, Shailendra Singh
VideoGIE.2024; 9(1): 31. CrossRef - Cardiac Septal Occluder for Refractory Anastamotic Leak
Marcel Tomaszewski, Cameron McAlister, Janarthanan Sathananthan, Fergal Donnellan
Journal of the Canadian Association of Gastroenterology.2023; 6(5): 153. CrossRef - Use of atrial septal occluder in the treatment of chronic fistula following post-esophagectomy anastomotic leak
Manisha Daminda Kariyawasam, Jonathan Liang Yap, Zehao Tan, Tiffany Lye, Weng Hoong Chan, Jeremy Tian Hui Tan, Chin Hong Lim
Endoscopy.2023; 55(S 01): E1005. CrossRef - Closure of a Bronchoesophageal Fistula After Lung Transplantation With an Amplatzer Occluder Device
Erik J. Orozco-Hernandez, David McGiffin, Gregory Von Mering, Mustafa Ahmed, Joseph Thachuthara-George, Kondal R. Kyanam-Kabir-Baig, Charles W. Hoopes
Annals of Thoracic Surgery Short Reports.2023; 1(2): 332. CrossRef - Choosing the best endoscopic approach for post-bariatric surgical leaks and fistulas: Basic principles and recommendations
Victor Lira de Oliveira, Alexandre Moraes Bestetti, Roberto Paolo Trasolini, Eduardo Guimarães Hourneaux de Moura, Diogo Turiani Hourneaux de Moura
World Journal of Gastroenterology.2023; 29(7): 1173. CrossRef - Endoscopic closure of refractory upper GI–tracheobronchial fistulas with a novel occluder: a prospective, single-arm, single-center study (with video)
Lurong Li, Yun Wang, Chang Zhu, Jianyu Wei, Weifeng Zhang, Huaiming Sang, Han Chen, Haisheng Qian, Miao Xu, Jiahao Liu, Shuxian Jin, Yu Jin, Wangjian Zha, Wei Song, Yi Zhu, Jiwang Wang, Simon K. Lo, Guoxin Zhang
Gastrointestinal Endoscopy.2023; 97(5): 859. CrossRef - Endoscopic Treatment of Non-malignant Esophageal Perforation: Time to Go Vacuum?
Diogo Turiani Hourneaux de Moura, Bruno Salomão Hirsch, Heli Clóvis de Medeiros Neto, Victor Lira de Oliveira, Alexandre Moraes Bestetti, Bruna Furia Buzetti Hourneaux de Moura, Mouen A. Khashab, Eduardo Guimarães Hourneaux de Moura
Current Treatment Options in Gastroenterology.2023; 21(2): 95. CrossRef - Technical Review on Endoscopic Treatment Devices for Management of Upper Gastrointestinal Postsurgical Leaks
Renato Medas, Eduardo Rodrigues-Pinto, Eiji Sakai
Gastroenterology Research and Practice.2023; 2023: 1. CrossRef - Endoscopic Management of a Chronic Gastrocutaneous Fistula after Bariatric Revisional Surgery Using a Novel Cardiac Septal Occluder
Mariana Kumaira Fonseca, Nelson Heitor Vieira Coelho, João Luiz Langer Manica, Rafael Ramos Ramblo, Ingrid Elisa Spier, Artur Pacheco Seabra
GE - Portuguese Journal of Gastroenterology.2023; 30(Suppl. 1): 52. CrossRef - Catheter-based deployment of vascular plugs for the management of challenging gastric fistulae
Prashanth Rau, Philip McNamara, Ikechukwu Achebe, Dimitri Belkin, Odel Zadeh, Neil B. Marya
VideoGIE.2023; 8(12): 497. CrossRef - Long-term endoscopic follow-up after closure of a post-bariatric surgery fistula with a cardiac septal defect occluder
Diogo Turiani Hourneaux de Moura, Mateus Bond Boghossian, Bruno Salomão Hirsch, Thomas R. McCarty, Alberto Jose Baptista, Eduardo Guimarães Hourneaux de Moura
Endoscopy.2022; 54(03): E127. CrossRef - Status of bariatric endoscopy–what does the surgeon need to know? A review
Diogo Turiani Hourneaux de Moura, Anna Carolina Batista Dantas, Igor Braga Ribeiro, Thomas R McCarty, Flávio Roberto Takeda, Marco Aurelio Santo, Sergio Carlos Nahas, Eduardo Guimarães Hourneaux de Moura
World Journal of Gastrointestinal Surgery.2022; 14(2): 185. CrossRef - Tailored endoscopic treatment of tracheo-oesophageal fistula using preoperative holographic assessment and a cardiac septal occluder
Stefano Siboni, Angelo Fabio D'Aiello, Massimo Chessa, Luigi Bonavina
BMJ Case Reports.2022; 15(3): e248981. CrossRef - Closure of recurrent colovaginal fistulas using AMPLATZER occluder device
Joseph Simmons, Ahmed Sherif, Jason Mader, Saba Altarawneh, Mehiar El-Hamdani, Wesam Frandah
BMJ Open Gastroenterology.2022; 9(1): e000921. CrossRef - Acquired Benign Tracheoesophageal Fistula
Hasnain S. Bawaadam, Matthew Russell, Yaron B. Gesthalter
Journal of Bronchology & Interventional Pulmonology.2022; 29(3): e38. CrossRef - Adequate Management of Postoperative Complications after Esophagectomy: A Cornerstone for a Positive Outcome
Imad Kamaleddine, Alexander Hendricks, Magdalena Popova, Clemens Schafmayer
Cancers.2022; 14(22): 5556. CrossRef - Colovaginal fistula closure using a cardiac septal defect occluder
Omar Sadiq, Stephen Simmer, Andrew Watson, Marvin Eng, Tiberio Frisoli, Tobias Zuchelli
VideoGIE.2021; 6(1): 41. CrossRef - Cardiac septal occluder for closure of persistent gastrogastric fistula
Ki-Yoon Kim, Matthew J. Skinner
VideoGIE.2021; 6(7): 294. CrossRef - Role and possibilities of endoscopic Vacuum Therapy in the treatment of transmural defects of upper gastrointestinal tract
Vladimir Alekseevich Porkhanov, Stanislav Nikolayevich Pyatakov, Alexander Gennadievich Baryshev, Denis Mikhailovich Melnik, Maxim Sergeevich Shevchenko, Mikhail Ilyich Bykov, Svetlana Nikolaevna Pyatakova
Hirurg (Surgeon).2021; (1): 5. CrossRef - A Comprehensive Review of Endoscopic Management of Sleeve Gastrectomy Leaks
Mihajlo Gjeorgjievski, Zaid Imam, Mitchell S. Cappell, Laith H. Jamil, Michel Kahaleh
Journal of Clinical Gastroenterology.2021; 55(7): 551. CrossRef
- Endoscopic closure of a recto-pelvic fistula with a cardiac septal occluder device
- 7,616 View
- 272 Download
- 14 Web of Science
- 20 Crossref


 KSGE
KSGE


 First
First Prev
Prev



