AbstractBackground/AimsEndoscopic ultrasound-guided fine needle aspiration (EUS-FNA) is a highly accurate method for diagnosing pancreatic neuroendocrine tumors (PNETs); however, some PNETs are difficult to diagnose. Recently, the efficacy of needle-based confocal laser endomicroscopy (nCLE) in diagnosing solid pancreatic masses has been reported. However, the efficacy of nCLE in the diagnosis of PNETs remains unknown and only a small number of cases have been reported. Hence, this study aimed to evaluate the efficacy of nCLE in the diagnosis of PNETs.
MethodsThis single-center retrospective study evaluated 30 consecutive patients with suspected PNETs on contrast-enhanced computed tomography, who consented to nCLE combined with EUS-FNA and were diagnosed using EUS-FNA or surgical resection. The diagnostic criteria for PNETs using nCLE were based on the nesting and trabecular and glandular arrangement of tumor cell clusters surrounded by capillary vessels and fibrosis, as reported in previous studies.
INTRODUCTIONEndoscopic ultrasound-guided fine needle aspiration (EUS-FNA) is the gold standard technique for diagnosing pancreatic neuroendocrine tumors (PNETs). Typical PNETs can be differentiated from serous cystic neoplasms (SCNs), solid pseudopapillary neoplasms (SPNs), or hypervascular pancreatic metastases. Atypical PNETs and G3 need to be differentiated from normal pancreatic cancers and acinar cell carcinomas.1 The accuracy of EUS-FNA for the diagnosis of PNETs is reported to be between 83.3 and 99.9%.2-4 Recently, fine-needle biopsy (FNB) needles have been developed, and their utility has been shown in previous studies.5-10 EUS-FNA and FNB are highly accurate in diagnosing PNETs; however, some PNETs cases are challenging to diagnose. The location of the tumor in the pancreatic head and the presence of rich stromal fibrosis tend to be associated with a lower diagnostic yield on EUS-FNA.11
Confocal laser endomicroscopy (CLSM) is a novel endoscopic method that uses fluorescent dyes to enable a real-time in vivo histopathological evaluation without tissue sampling. Various types of probes have different resolutions and depths and have been developed for different organs. For pancreatic lesions, a novel needle-based confocal laser endomicroscopy (nCLE) mini-probe has been developed that can be passed through a 19-gauge EUS-FNA needle.12 The utility of EUS-FNA for small pancreatic tumors is limited. For tumors <1 cm, diagnosis using EUS-FNA is thought to be technically challenging not only in targeting the lesion, but also in obtaining an adequate specimen.13 nCLE is expected to overcome such problems. Recently, the efficacy of nCLE has been reported in solid pancreatic masses. With the use of nCLE, Giovannini et al. and Kongkam et al. reported an accuracy of pancreatic ductal adenocarcinoma diagnosis of 85.0% and 90.9%, respectively.14,15 In contrast, Karstensen et al.16 stated that nCLE is unable to distinguish benign from malignant solid lesions in the pancreas. However, the efficacy of nCLE in the diagnosis of PNETs remains unknown because these studies included only a few cases of PNETs. Here, we present the results of a retrospective study that evaluated the diagnostic performance of nCLE in PNETs.
METHODSPatientsThis single-center retrospective study evaluated 30 consecutive patients with suspected PNETs on contrast-enhanced computed tomography (CE-CT), who consented to nCLE combined with EUS-FNA and were diagnosed using EUS-FNA or surgical resection at Aichi Cancer Center, Nagoya, Japan, between March 1, 2017, and May 31, 2021. Magnetic resonance imaging (MRI) and contrast-enhanced endoscopic ultrasound (CE-EUS) findings were also considered as clinical factors affecting the accuracy of nCLE, but both were not performed in five of the 30 cases. Gastroenterological interpretation of the nCLE images was abstracted from the procedure notes in the electronic medical records.
nCLE combined with EUS-FNA procedureIn all patients, nCLE combined with EUS-FNA was performed under conscious sedation using 5 to 10 mg of intravenous midazolam (Astellas) and 35 mg of intravenous pethidine hydrochloride (Mitsubishi Tanabe Pharma). EUS was performed using a Prosound SSD ╬▒-10 (Hitachi Ltd.), EU-ME2 (Olympus Corporation), SU-1 (Fujifilm Corporation), or ARIETTA850 (Hitachi Ltd.) ultrasound systems with either a GF-UCT260 curved linear echoendoscope (Olympus Corporation) or EG-580UT curved linear echoendoscope (Fujifilm Corporation).
Initially, the AQ-Flex 19 probe (Cellvizio; Mauna Kea Technologies) was preloaded into a 19-gauge EUS needle (Expect Slimline; Boston Scientific Corporation). The pancreatic tumor was punctured, and the nCLE probe was locked 2 mm above the tip. Sodium fluorescein was injected immediately after the target was punctured. The nCLE provided real-time images. The acquisition time was usually limited to 10 minutes. The needle position was changed using the fanning technique as appropriate to obtain specific images.
After the nCLE examination, EUS-FNA was performed using the slow-pull method. EUS-FNA was performed using 22- or 25-gauge needles (EZ shot 3 plus; Olympus Corporation or Acquire; Boston Scientific) as needed.
DefinitionsThe primary endpoint of this study was the accuracy of nCLE for PNETs diagnosis. The diagnosis of PNET was based on a larger series of nCLE in solid pancreatic masses that were presented at the United European Gastroenterology Week meeting in 2014. The typical findings of PNET are nesting, trabecular, and glandular arrangements of tumor cell clusters surrounded by capillary vessels, and fibrosis. These findings were consistent with the histological structure (Fig. 1). The secondary endpoints were adverse events and factors affecting nCLE accuracy. Adverse events that were possibly related to the procedure and that occurred after the procedure were described in accordance with the American Society for Gastrointestinal Endoscopy lexicon.17 The maximal section of the resected specimens was used to evaluate the degree of stromal fibrosis. ŌĆśRich fibrosisŌĆÖ was noted when stromal fibrosis occupied >30% of the total tumor area.18 Continuous variables were analyzed using the Mann-Whitney U-test. Categorical variables were analyzed using Fisher exact test. All statistical analyses were performed using EZR software (Saitama Medical Center, Jichi Medical University, Saitama, Japan).
RESULTSThe study included 15 males (50.0%) and 15 females (50.0%). The patientsŌĆÖ ages ranged from 38 to 76 years (median, 60 years). Pancreatic tumors were located in the body (n=13, 43.3%), head (n=9, 30.0%), or tail (n=8, 26.6%) of the organ. Median tumor size was 10.0 mm (range, 5.3ŌĆō60.0 mm). Seven lesions (23.3%) contained cystic components. The final diagnosis was achieved using surgical resection or EUS-FNA in 19 (63.3%) and 11 (36.7%) cases, respectively. Regarding grading, 27 PNETs were classified as G1 or G2 in 25 (83.3%) and 2 (6.7%) cases, respectively. The remaining three cases were diagnosed using EUS-FNA, but the grading was unclassified (Table 1).
The needles used for EUS-FNA were FNA needles in 10 patients (33.3%) and FNB needles in 20 patients (66.7%). The median number of punctures was 2 (range, 1ŌĆō5). The mean acquisition time for nCLE was 269 seconds (range, 90ŌĆō640 seconds). The accuracies of EUS-FNA were 93.3% and 90.0% with the FNA needle and 95.0% with the FNB needle. The accuracy of nCLE was 70.0%, which was significantly lower than that of EUS-FNA alone. However, in one of the two cases with inconclusive EUS-FNA results, the nCLE was able to diagnose PNET. The accuracy of nCLE combined with EUS-FNA was 96.7%. No adverse events were observed. Fluorescein was well tolerated by all patients (Table 2).
Among the 30 cases, nCLE results were classified as a misdiagnosis, non-diagnostic, and diagnostic in 3 (10%), 6 (20%), and 21 cases (70%), respectively. Among the patients, there was a 50-year-old male with an 8-mm pancreatic body lesion that was inconclusive on EUS-FNA (Fig. 2). The two misdiagnosed cases were diagnosed as SCNs due to a superficial vascular network-like findings observed on nCLE or a small number of cells in the vascular bundle. In the six non-diagnostic cases, there were findings indicating PNETs and other pancreatic tumors on nCLE; only isolated small dark cells or fine white fibrous bands were observed (Fig. 3). In four misdiagnosed and non-diagnostic cases, surgical resection was performed (Table 3), and the tumors in all cases had rich stromal fibrosis in the surgical specimens. For example, Case 1 from Table 3 is presented in Figure 4. Univariate analyses were conducted to identify the factors affecting the accuracy of nCLE. MRIŌĆōT2-weighted imaging (MRI-T2 WI) findings constituted a significant clinical factor affecting the accuracy of nCLE. Tumor location, tumor size, presence of cystic components, and CE-EUS findings were not found to be significant clinical factors (Table 4). Grading was a significant pathological factor affecting the accuracy of nCLE. The presence of a clear border, intraductal pancreatic extension, cystic components, and the degree of stromal fibrosis were not found to be significant pathological factors (Table 5).
DISCUSSIONThis retrospective study investigated the diagnostic performance and safety of EUS-guided nCLE for PNET. To our knowledge, this is the first report to evaluate the diagnostic yield of nCLE for PNETs in a relatively large sample of patients.
Regarding the safety of nCLE combined with EUS-FNA, because nCLE is performed using FNA needles, no problems beyond the known adverse events related to EUS-FNA should be expected. Additionally, adverse events associated with the intravenous administration of fluorescein are considered mild and transient. No adverse events were found in this study, similar to previous studies on nCLE for solid pancreatic masses.14-16 Although nCLE requires exclusive equipment and is costly, it can be performed easily and safely in combination with EUS-FNA.
In this study, the accuracy of nCLE for the diagnosis of PNETs was 70.0%, which was not satisfactory. Typical PNETs are detected as hypervascular tumors on contrast-enhanced CT. Hence, differentiating them from SCNs and SPNs is of crucial importance. Typical findings of SCNs and SPNs are superficial vascular networks and small cells with white stromal bands, respectively.19,20 In the two misdiagnosed cases of SCNs, the hypervascular areas were identified as superficial vascular networks. In one misdiagnosed SPN case, areas of rich stromal fibrosis and a few tumor cells were identified as small cells with white stromal bands. It is critical to consider that even in PNETs, nCLE images characteristic of SPNs and SCNs may be observed. In surgical cases among the misdiagnosed and non-diagnostic cases, the characteristic arrangement of tumor cells was only observed in small areas due to the presence of rich stromal fibrosis, and nCLE was unable to detect the findings. The diagnosis of PNET by EUS-FNA is based on the presence of small round cells on hematoxylin and eosin staining in combination with immunohistochemistry showing the expression of chromogranin A and synaptophysin. Therefore, even cases with rich stromal fibrosis can be diagnosed if the tumor cells are collected. Conversely, nCLE cannot be diagnosed unless characteristic arrangements of tumor cell clusters are observed. In this study, to evaluate the association between the diagnostic performance of nCLE and stromal fibrosis, we examined the degree of stromal fibrosis in surgical specimens as a pathological factor and the findings of MRI-T2 WI as a clinical factor. We considered the findings of MRI-T2 WI as a clinical factor because it has been reported that most PNETs are hyperintense on MRI-T2 WI, but PNETs with rich stromal fibrosis appear isointense or hypointense.21,22 The present results suggest that the findings of MRI-T2 WI were a significant clinical factor affecting the accuracy of nCLE, and the accuracy of nCLE in cases with isointense or hypointense findings was lower than that in cases with hyperintense findings. The degree of stromal fibrosis was not a significant pathological factor affecting the accuracy of nCLE. However, the accuracy of nCLE in cases with poor stromal fibrosis was 100%, whereas the accuracy of nCLE in cases with rich stromal fibrosis was 63.6%, which may not have been a significant factor owing to the small number of cases. Hijioka et al. reported that tumors with rich stromal fibrosis have a lower diagnostic yield on EUS-FNA than tumors with minimal fibrosis,11 which may be more pronounced in nCLE. How can the diagnostic performance of nCLE be improved in such cases? The fanning technique is useful for obtaining diagnostic findings from nCLE. However, the fanning technique is difficult to apply for small lesions.
In contrast, when the tumor is too small to allow for inadequate sampling by EUS-FNA, nCLE is useful because tissue sampling is not required. Such a case was presented in this study. Furthermore, cystic PNETs are considered good candidates for nCLE for the same reason. Although there were no cases with strong cystic changes in this study, the usefulness of nCLE in cystic neuroendocrine tumors that cannot be diagnosed by EUS-FNA has been reported.23,24 In univariate analyses of factors affecting the accuracy of nCLE, the location of the tumor, size of the tumor, and presence of cystic components were not found to be significant factors that may enable the diagnosis of pancreatic lesions without tissue sampling. Grading was a significant independent factor affecting the accuracy of nCLE; however, there were only two G2 cases, and these two cases were characterized by rich stromal fibrosis. It is possible that nCLE could overcome the limitations of EUS-FNA. However, further studies with larger numbers of cases are required to confirm this hypothesis.
Currently, probe-based confocal laser endomicroscopy (pCLE) is widely used in the gastrointestinal tract, and its efficacy has been reported in several studies.25-30 In contrast, the diagnostic performance of nCLE for pancreatic cystic lesions and solid pancreatic masses has not yet reached the level of pCLE. nCLE has a smaller outer diameter and lower resolution than pCLE because it is performed through the FNA needle. In addition, nCLE is susceptible to respiratory variability and intratumor heterogeneity of tumor cells. Each of these issues can be addressed to some extent by abdominal compression and changes in the puncture line; however, there are some limitations to this approach. Further developments in the resolution and optimization of diagnostic criteria are warranted to improve the diagnostic performance of combined nCLE.
The limitations of this study include its retrospective design and the fact that it was performed at a single center with a small number of patients. Another limitation is that the final diagnosis was achieved not only by surgical resection but also by EUS-FNA. In addition, because all the cases were PNETs, the specificity and positive/negative predictive values could not be evaluated. In fact, there were cases with typical findings of PNETs on nCLE that could not be diagnosed or were diagnosed as another tumor by EUS-FNA and were followed up without surgical resection. If such cases were diagnosed as PNETs by surgical resection, the diagnostic yield of nCLE would have a different outcome.
In conclusion, nCLE combined with EUS-FNA can be performed safely and easily for PNETs. Although the diagnostic performance of EUS-FNA for PNETs is high, nCLE may be a diagnostic option in cases of inconclusive EUS-FNA findings.
NOTESConflicts of Interest
Dr. Mizuno reports the following grants, none of which are connected to the submitted work: grants from Yakult Honsha, Novartis, MSD, ASLAN Pharmaceuticals, Incyte, Ono Pharmaceutical, Seagen, Taiho Pharmaceutical, and Dainippon Sumitomo Pharma; and personal fees from Yakult Honsha, AstraZeneca, Novartis, FUJIFILM Toyama Chemical, MSD, and Taiho Pharmaceutical.
Fig.┬Ā1.Typical needle-based confocal laser endomicroscopy images of pancreatic neuroendocrine tumors (PNETs). (A) Typical findings of PNETs. (B) Nesting, trabecular, and glandular arrangements of tumor cell clusters. (C) Surrounding capillary vessels and fibrosis. (D) These findings are consistent with the histological structure (hematoxylin and eosin staining, ├Ś400). 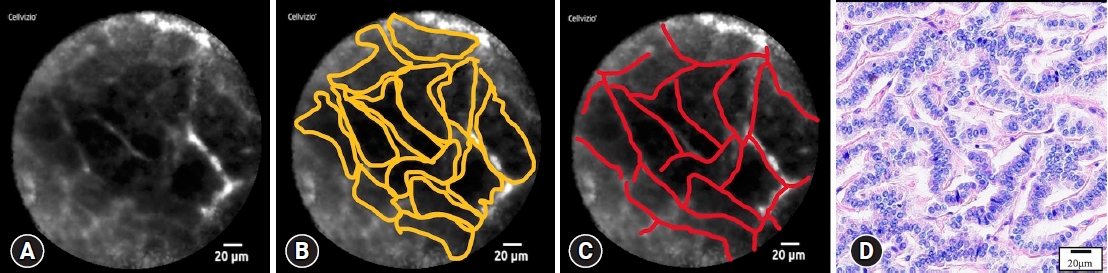
Fig.┬Ā2.A case of inconclusive endoscopic ultrasound-guided fine needle aspiration (EUS-FNA). (A, B) The lesion was detected in the pancreatic body using enhanced computed tomography and EUS. (C) The needle-based confocal laser endomicroscopy image shows typical findings of pancreatic neuroendocrine tumor (PNET). (D) EUS-FNA showed no tumor cells in the cell block (├Ś100). (E) Histopathological view of the surgical specimen (hematoxylin and eosin staining, ├Ś20). (F) The pathological diagnosis was PNET, G1. F-1: hematoxylin and eosin staining, ├Ś100; F-2: synaptophysin was positive, ├Ś100; F-3: chromogranin A was positive, ├Ś100; F-4; Ki-index was <1%, ├Ś100. 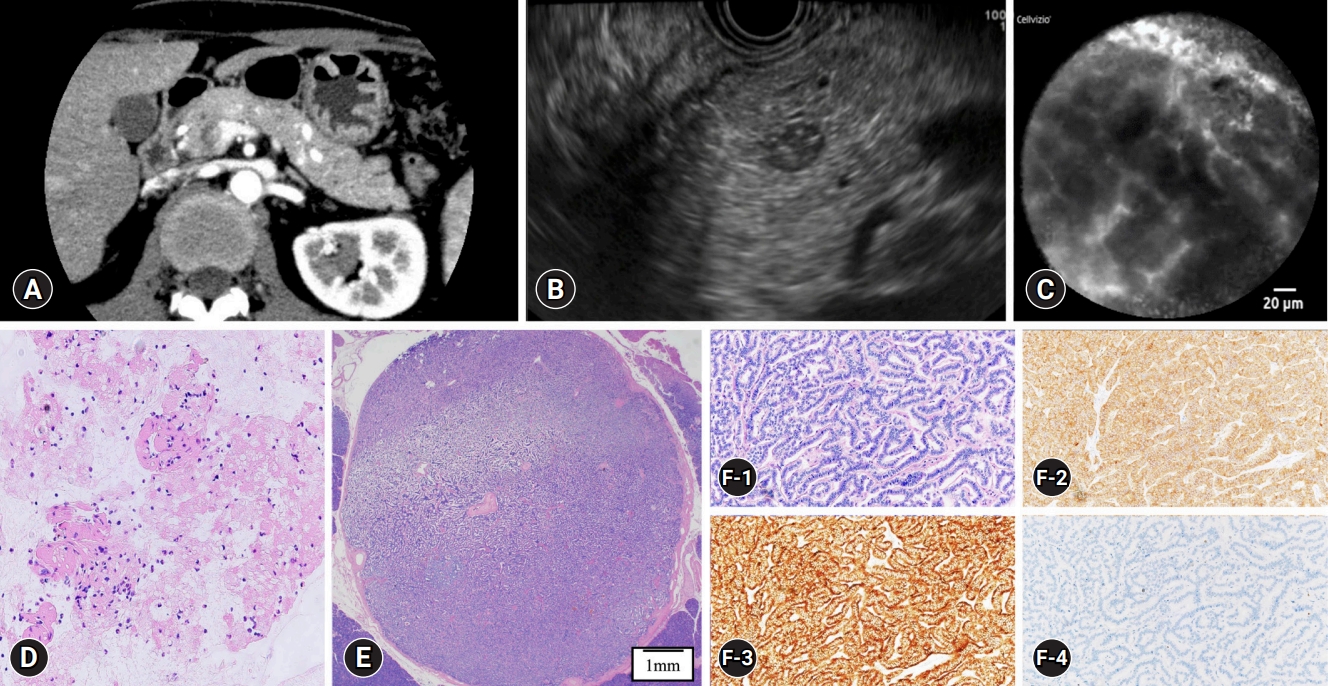
Fig.┬Ā3.Needle-based confocal laser endomicroscopy images of misdiagnosed and non-diagnostic cases. (A) Superficial vascular network. (B) Small cells with vascular bundles. (C) Small dark cells that are isolated. (D) Fine white fibrous bands. 
Fig.┬Ā4.Examples of misdiagnosed and non-diagnostic cases (case 1 from Table 3). (A, B) Lesions were detected in the pancreatic tail using contrast-enhanced computed tomography and endoscopic ultrasound. (C) The needle-based confocal laser endomicroscopy image shows only fine white fibrous bands. (D) Histopathological examination of the surgical specimen showed rich stromal fibrosis in the tumor (hematoxylin and eosin staining, ├Ś20). 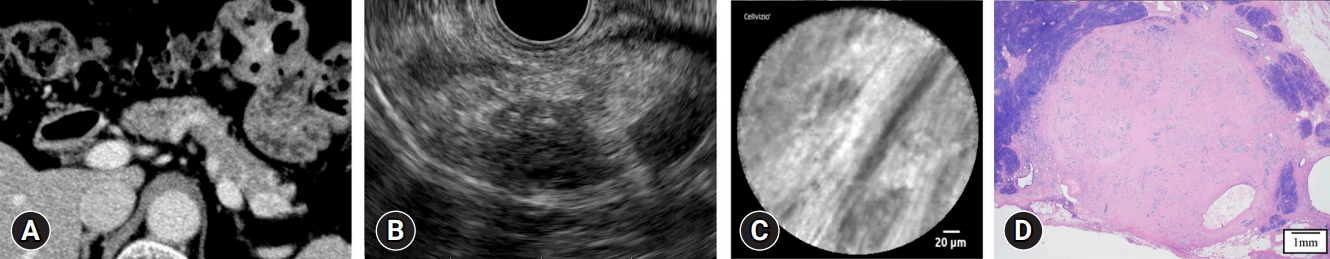
Table┬Ā1.Patient characteristics (n=30) Table┬Ā2.Details and results of EUS-FNA and nCLE (n=30) Table┬Ā3.Detailed characteristics of misdiagnosed and non-diagnostic cases Table┬Ā4.Univariate analyses of clinical factors affecting the accuracy of nCLE
Table┬Ā5.Univariate analyses of pathological factors affecting the accuracy of needle-based confocal laser endomicroscopy REFERENCES1. Ishii T, Katanuma A, Toyonaga H, et al. Role of endoscopic ultrasound in the diagnosis of pancreatic neuroendocrine neoplasms. Diagnostics (Basel) 2021;11:316.
2. Ito T, Hijioka S, Masui T, et al. Advances in the diagnosis and treatment of pancreatic neuroendocrine neoplasms in Japan. J Gastroenterol 2017;52:9ŌĆō18.
3. Krishna SG, Bhattacharya A, Li F, et al. Diagnostic differentiation of pancreatic neuroendocrine tumor from other neoplastic solid pancreatic lesions during endoscopic ultrasound-guided fine-needle aspiration. Pancreas 2016;45:394ŌĆō400.
4. Heidsma CM, Tsilimigras DI, Rocha F, et al. Clinical relevance of performing endoscopic ultrasound-guided fine-needle biopsy for pancreatic neuroendocrine tumors less than 2ŌĆēcm. J Surg Oncol 2020;122:1393ŌĆō1400.
5. Hedenstr├Čm P. The best approach for sampling of pancreatic neuroendocrine tumors: EUS-FNA or EUS-FNB? Endosc Int Open 2019;7:E1400ŌĆōE1402.
6. Eusebi LH, Thorburn D, Toumpanakis C, et al. Endoscopic ultrasound-guided fine-needle aspiration vs fine-needle biopsy for the diagnosis of pancreatic neuroendocrine tumors. Endosc Int Open 2019;7:E1393ŌĆōE1399.
7. Witt BL, Factor RE, Chadwick BE, et al. Evaluation of the SharkCore┬« needle for EUS-guided core biopsy of pancreatic neuroendocrine tumors. Endosc Ultrasound 2018;7:323ŌĆō328.
8. Leeds JS, Nayar MK, Bekkali NL, et al. Endoscopic ultrasound-guided fine-needle biopsy is superior to fine-needle aspiration in assessing pancreatic neuroendocrine tumors. Endosc Int Open 2019;7:E1281ŌĆōE1287.
9. Di Leo M, Poliani L, Rahal D, et al. Pancreatic neuroendocrine tumours: the role of endoscopic ultrasound biopsy in diagnosis and grading based on the WHO 2017 classification. Dig Dis 2019;37:325ŌĆō333.
10. Kamata K, Ashida R, Yasukawa S, et al. Histological diagnosis and grading of pancreatic neuroendocrine tumor by endoscopic ultrasound-guided fine needle biopsy using a 25-gauge needle with a core trap: a multicenter prospective trial. Pancreatology 2020;20:1428ŌĆō1433.
11. Hijioka S, Hara K, Mizuno N, et al. Diagnostic performance and factors influencing the accuracy of EUS-FNA of pancreatic neuroendocrine neoplasms. J Gastroenterol 2017;52:264.
12. Konda VJ, Meining A, Jamil LH, et al. A pilot study of in vivo identification of pancreatic cystic neoplasms with needle-based confocal laser endomicroscopy under endosonographic guidance. Endoscopy 2013;45:1006ŌĆō1013.
13. Haba S, Yamao K, Bhatia V, et al. Diagnostic ability and factors affecting accuracy of endoscopic ultrasound-guided fine needle aspiration for pancreatic solid lesions: Japanese large single center experience. J Gastroenterol 2013;48:973ŌĆō981.
14. Kongkam P, Pittayanon R, Sampatanukul P, et al. Endoscopic ultrasound-guided needle-based confocal laser endomicroscopy for diagnosis of solid pancreatic lesions (ENES): a pilot study. Endosc Int Open 2016;4:E17ŌĆōE23.
15. Giovannini M, Caillol F, Monges G, et al. Endoscopic ultrasound-guided needle-based confocal laser endomicroscopy in solid pancreatic masses. Endoscopy 2016;48:892ŌĆō898.
16. Karstensen JG, C├ór┼Ż├ón─ā T, Constantinescu C, et al. Endoscopic ultrasound guided needle-based confocal laser endomicroscopy in solid pancreatic masses: a prospective validation study. Endosc Int Open 2018;6:E78ŌĆōE85.
17. Cotton PB, Eisen GM, Aabakken L, et al. A lexicon for endoscopic adverse events: report of an ASGE workshop. Gastrointest Endosc 2010;71:446ŌĆō454.
18. McCall CM, Shi C, Klein AP, et al. Serotonin expression in pancreatic neuroendocrine tumors correlates with a trabecular histologic pattern and large duct involvement. Hum Pathol 2012;43:1169ŌĆō1176.
19. Nakai Y, Iwashita T, Park DH, et al. Diagnosis of pancreatic cysts: EUS-guided, through-the-needle confocal laser-induced endomicroscopy and cystoscopy trial: DETECT study. Gastrointest Endosc 2015;81:1204ŌĆō1214.
20. Okuno N, Hara K, Obata M. Novel method of diagnosing solid pseudopapillary neoplasms of the pancreas: Needle-based confocal laser endomicroscopy. Dig Endosc 2019;31:461.
21. Thoeni RF, Mueller-Lisse UG, Chan R, et al. Detection of small, functional islet cell tumors in the pancreas: selection of MR imaging sequences for optimal sensitivity. Radiology 2000;214:483ŌĆō490.
22. Lewis RB, Lattin GE Jr, Paal E. Pancreatic endocrine tumors: radiologic-clinicopathologic correlation. Radiographics 2010;30:1445ŌĆō1464.
23. Krishna SG, Brugge WR, Dewitt JM, et al. Needle-based confocal laser endomicroscopy for the diagnosis of pancreatic cystic lesions: an international external interobserver and intraobserver study (with videos). Gastrointest Endosc 2017;86:644ŌĆō654.
24. Haq I, Krishna SG, Patel B, et al. The impact of repeating endosonography with confocal endomicroscopy for the diagnosis of cystic neuroendocrine tumor. Case Rep Gastrointest Med 2019;2019:5187874.
25. Sharma P, Meining AR, Coron E, et al. Real-time increased detection of neoplastic tissue in Barrett's esophagus with probe-based confocal laser endomicroscopy: final results of an international multicenter, prospective, randomized, controlled trial. Gastrointest Endosc 2011;74:465ŌĆō472.
26. Bertani H, Frazzoni M, Dabizzi E, et al. Improved detection of incident dysplasia by probe-based confocal laser endomicroscopy in a Barrett's esophagus surveillance program. Dig Dis Sci 2013;58:188ŌĆō193.
27. Bok GH, Jeon SR, Cho JY, et al. The accuracy of probe-based confocal endomicroscopy versus conventional endoscopic biopsies for the diagnosis of superficial gastric neoplasia (with videos). Gastrointest Endosc 2013;77:899ŌĆō908.
28. Lim LG, Yeoh KG, Srivastava S, et al. Comparison of probe-based confocal endomicroscopy with virtual chromoendoscopy and white-light endoscopy for diagnosis of gastric intestinal metaplasia. Surg Endosc 2013;27:4649ŌĆō4655.
|
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
