Search
- Page Path
- HOME > Search
Original Article
- Performance comparison between two computer-aided detection colonoscopy models by trainees using different false positive thresholds: a cross-sectional study in Thailand
- Kasenee Tiankanon, Julalak Karuehardsuwan, Satimai Aniwan, Parit Mekaroonkamol, Panukorn Sunthornwechapong, Huttakan Navadurong, Kittithat Tantitanawat, Krittaya Mekritthikrai, Salin Samutrangsi, Peerapon Vateekul, Rungsun Rerknimitr
- Clin Endosc 2024;57(2):217-225. Published online February 7, 2024
- DOI: https://doi.org/10.5946/ce.2023.145

-
 Graphical Abstract
Graphical Abstract
 Abstract
Abstract
 PDF
PDF Supplementary Material
Supplementary Material PubReader
PubReader ePub
ePub 
- Background
/Aims: This study aims to compare polyp detection performance of “Deep-GI,” a newly developed artificial intelligence (AI) model, to a previously validated AI model computer-aided polyp detection (CADe) using various false positive (FP) thresholds and determining the best threshold for each model.
Methods
Colonoscopy videos were collected prospectively and reviewed by three expert endoscopists (gold standard), trainees, CADe (CAD EYE; Fujifilm Corp.), and Deep-GI. Polyp detection sensitivity (PDS), polyp miss rates (PMR), and false-positive alarm rates (FPR) were compared among the three groups using different FP thresholds for the duration of bounding boxes appearing on the screen.
Results
In total, 170 colonoscopy videos were used in this study. Deep-GI showed the highest PDS (99.4% vs. 85.4% vs. 66.7%, p<0.01) and the lowest PMR (0.6% vs. 14.6% vs. 33.3%, p<0.01) when compared to CADe and trainees, respectively. Compared to CADe, Deep-GI demonstrated lower FPR at FP thresholds of ≥0.5 (12.1 vs. 22.4) and ≥1 second (4.4 vs. 6.8) (both p<0.05). However, when the threshold was raised to ≥1.5 seconds, the FPR became comparable (2 vs. 2.4, p=0.3), while the PMR increased from 2% to 10%.
Conclusions
Compared to CADe, Deep-GI demonstrated a higher PDS with significantly lower FPR at ≥0.5- and ≥1-second thresholds. At the ≥1.5-second threshold, both systems showed comparable FPR with increased PMR.
- 2,072 View
- 136 Download

Reviews
- Computer-aided polyp characterization in colonoscopy: sufficient performance or not?
- Natalie Halvorsen, Yuichi Mori
- Clin Endosc 2024;57(1):18-23. Published online January 5, 2024
- DOI: https://doi.org/10.5946/ce.2023.092
-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader ePub
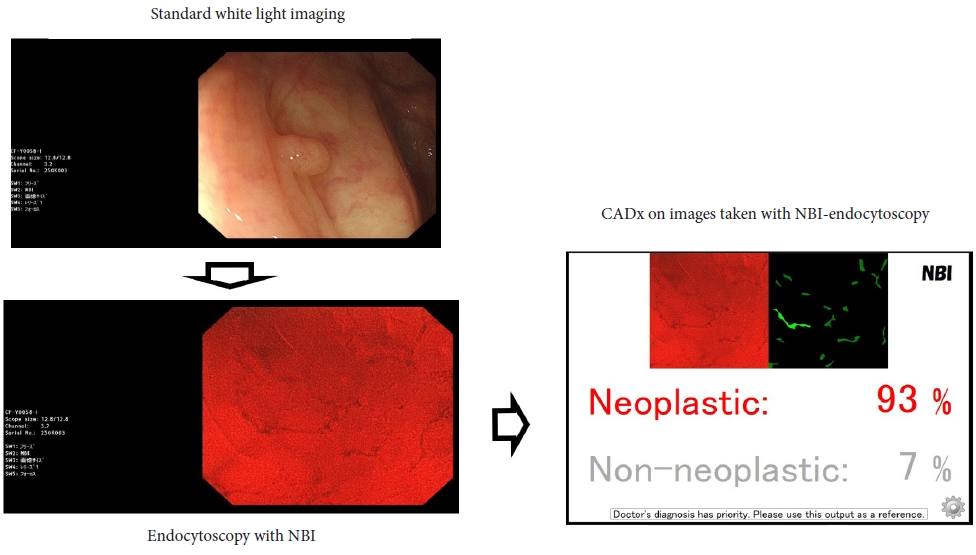
ePub - Computer-assisted polyp characterization (computer-aided diagnosis, CADx) facilitates optical diagnosis during colonoscopy. Several studies have demonstrated high sensitivity and specificity of CADx tools in identifying neoplastic changes in colorectal polyps. To implement CADx tools in colonoscopy, there is a need to confirm whether these tools satisfy the threshold levels that are required to introduce optical diagnosis strategies such as “diagnose-and-leave,” “resect-and-discard” or “DISCARD-lite.” In this article, we review the available data from prospective trials regarding the effect of multiple CADx tools and discuss whether they meet these thresholds.
- 2,106 View
- 159 Download

- Detecting colorectal lesions with image-enhanced endoscopy: an updated review from clinical trials
- Mizuki Nagai, Sho Suzuki, Yohei Minato, Fumiaki Ishibashi, Kentaro Mochida, Ken Ohata, Tetsuo Morishita
- Clin Endosc 2023;56(5):553-562. Published online July 26, 2023
- DOI: https://doi.org/10.5946/ce.2023.055
-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader ePub
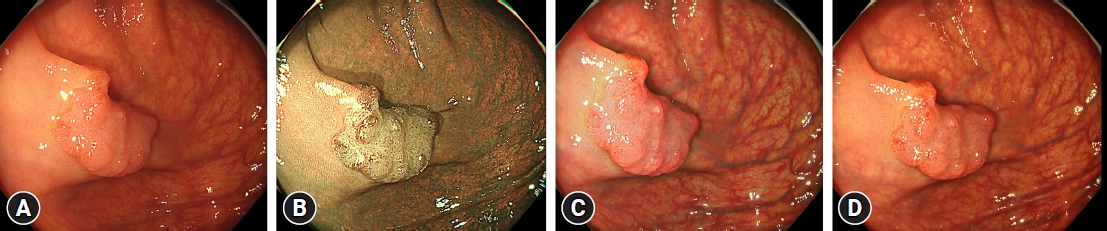
ePub - Colonoscopy plays an important role in reducing the incidence and mortality of colorectal cancer by detecting adenomas and other precancerous lesions. Image-enhanced endoscopy (IEE) increases lesion visibility by enhancing the microstructure, blood vessels, and mucosal surface color, resulting in the detection of colorectal lesions. In recent years, various IEE techniques have been used in clinical practice, each with its unique characteristics. Numerous studies have reported the effectiveness of IEE in the detection of colorectal lesions. IEEs can be divided into two broad categories according to the nature of the image: images constructed using narrowband wavelength light, such as narrowband imaging and blue laser imaging/blue light imaging, or color images based on white light, such as linked color imaging, texture and color enhancement imaging, and i-scan. Conversely, artificial intelligence (AI) systems, such as computer-aided diagnosis systems, have recently been developed to assist endoscopists in detecting colorectal lesions during colonoscopy. To better understand the features of each IEE, this review presents the effectiveness of each type of IEE and their combination with AI for colorectal lesion detection by referencing the latest research data.
-
Citations
Citations to this article as recorded by- Strategy for post-polypectomy colonoscopy surveillance: focus on the revised Korean guidelines
Yong Soo Kwon, Su Young Kim
Journal of the Korean Medical Association.2023; 66(11): 652. CrossRef - AI-powered medical devices for practical clinicians including the diagnosis of colorectal polyps
Donghwan Kim, Eunsun Kim
Journal of the Korean Medical Association.2023; 66(11): 658. CrossRef
- Strategy for post-polypectomy colonoscopy surveillance: focus on the revised Korean guidelines
- 2,442 View
- 204 Download
- 4 Web of Science
- 2 Crossref

Systematic Review and Meta-analysis
- Use of abdominal compression device in colonoscopy: a systematic review and meta-analysis
- Yousaf Zafar, Ahmed Mustafa Rashid, Syed Sarmad Javaid, Ahmed Kamal Siddiqi, Adnan Zafar, Arsalan Zafar Iqbal, Jagpal Singh Klair, Rajesh Krishnamoorthi
- Clin Endosc 2023;56(4):446-452. Published online May 26, 2023
- DOI: https://doi.org/10.5946/ce.2022.304
-
 Graphical Abstract
Graphical Abstract
 Abstract
Abstract
 PDF
PDF Supplementary Material
Supplementary Material PubReader
PubReader ePub
ePub 
- Background
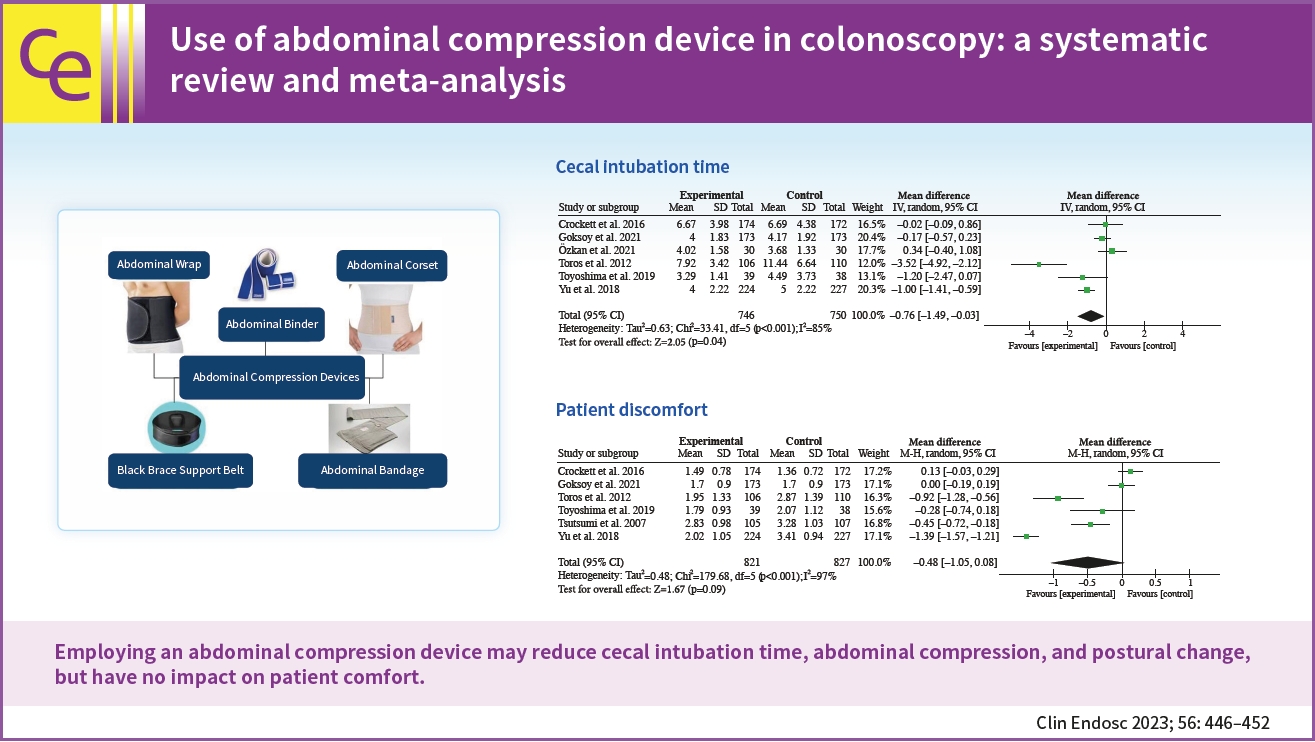
/Aims: Colonoscopy for screening is associated with unpleasant experiences for patients, and abdominal compression devices have been developed to minimize these problems. However, there is a paucity of data supporting the therapeutic benefits of this strategy. This study examined the effects of using an abdominal compression device during colonoscopy on the cecal intubation time (CIT), abdominal compression, patient comfort, and postural changes.
Methods
We searched PubMed and Scopus (from inception to November 2021) for randomized controlled trials that assessed the effects of an abdominal compression device during colonoscopy on CIT, abdominal compression, patient comfort, and postural change. A random-effects meta-analysis was performed. Weighted mean differences (WMDs) and Mantel-Haenszel odds ratios (ORs) were calculated.
Results
Our pooled analysis of seven randomized controlled trials revealed that abdominal compression devices significantly reduced CIT (WMD, –0.76 [–1.49 to –0.03] minutes; p=0.04), abdominal compression (OR, 0.52; 95% confidence interval [CI], 0.28–0.94; p=0.03), and postural changes (OR, 0.46; 95% CI, 0.27–0.78; p=0.004) during colonoscopy. However, our results did not show a significant change in patient comfort (WMD, –0.48; 95% CI, –1.05 to 0.08; p=0.09) when using an abdominal compression device.
Conclusions
Our findings demonstrate that employing an abdominal compression device may reduce CIT, abdominal compression, and postural change but have no impact on patient comfort.
- 2,621 View
- 105 Download

Reviews
- Simulator-based training method in gastrointestinal endoscopy training and currently available simulators
- Yuri Kim, Jeong Hoon Lee, Gin Hyug Lee, Ga Hee Kim, Gunn Huh, Seung Wook Hong, Hwoon-Yong Jung
- Clin Endosc 2023;56(1):1-13. Published online January 6, 2023
- DOI: https://doi.org/10.5946/ce.2022.191
-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader ePub
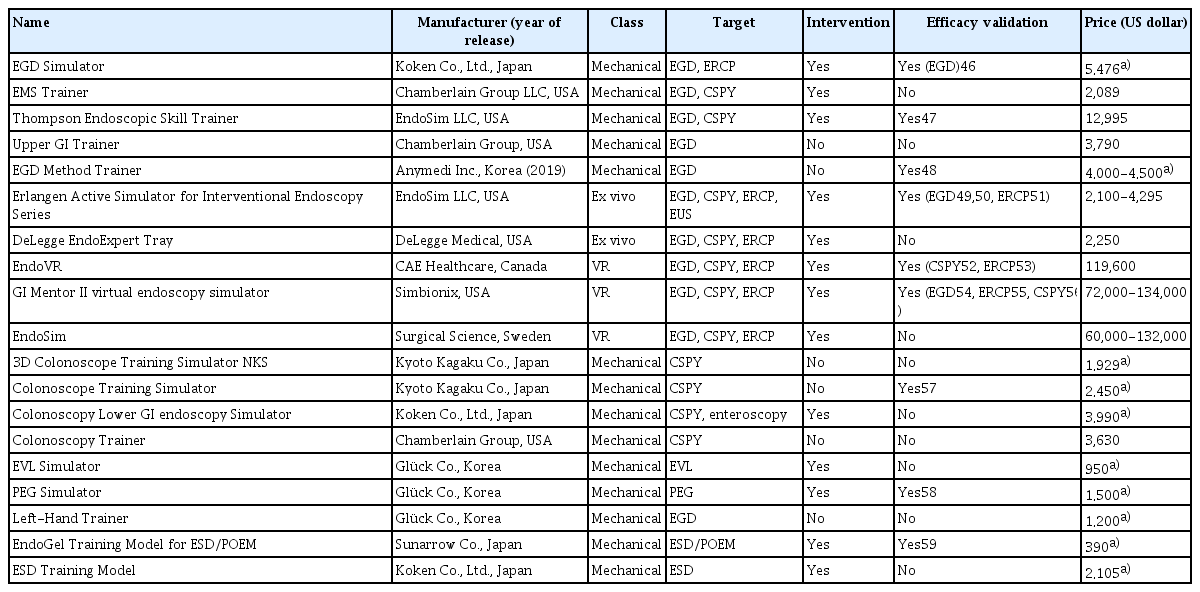
ePub - The apprenticeship-based training method (ABTM) is highly effective for gastrointestinal (GI) endoscopic training. However, the conventional ABTM has significant issues. Although many supplementary training methods (TMs) have been developed and utilized, they cannot entirely replace the ABTM, which remains the major TM strategy. Currently, new TM construction is crucial and necessary due to financial constraints, difficulty of obtaining sufficient training time due to patient safety-related regulations, and catastrophic damage caused by disasters such as the coronavirus disease 2019 pandemic. The simulator-based TM (SBTM) is widely accepted as an alternative to the ABTM, owing to the SBTM’s advantages. Since the 1960s, many GI endoscopy training simulators have been developed and numerous studies have been published on their effectiveness. While previous studies have focused on the simulator’s validity, this review focused on the accessibility of simulators that were introduced by the end of 2021. Although the current SBTM is effective in GI endoscopic education, extensive improvements are needed to replace the ABTM. Incorporating simulator-incorporated TMs into an improved ABTM is an attempt to overcome the incompleteness of the current SBTM. Until a new simulator is developed to replace the ABTM, it is desirable to operate a simulator-integrated and well-coordinated TM that is suitable for each country and institution.
-
Citations
Citations to this article as recorded by- Impact and assessment of training models in interventional endoscopic ultrasound
Bogdan Miutescu, Vinay Dhir
Digestive Endoscopy.2024; 36(1): 59. CrossRef - A Multicenter Survey of Percutaneous Endoscopic Gastrostomy in 2019 at Korean Medical Institutions
Jun Woo Park, Tae Gyun Kim, Kwang Bum Cho, Jeong Seok Kim, Jin Woong Cho, Jung Won Jeon, Sun Gyo Lim, Chan Gyoo Kim, Hong Jun Park, Tae Jun Kim, Eun Sun Kim, Su Jin Jeong, Yong Hwan Kwon
Gut and Liver.2024; 18(1): 77. CrossRef - Exploring Endoscopic Competence in Gastroenterology Training: A Simulation-Based Comparative Analysis of GAGES, DOPS, and ACE Assessment Tools
Faisal Wasim Ismail, Azam Afzal, Rafia Durrani, Rayyan Qureshi, Safia Awan, Michelle R Brown
Advances in Medical Education and Practice.2024; Volume 15: 75. CrossRef - Assemblage of a functional and versatile endoscopy trainer reusing medical waste: Step‐by‐step video tutorial
Riccardo Vasapolli, Jörg Schirra, Christian Schulz
Digestive Endoscopy.2024; 36(5): 634. CrossRef - Navigating the learning landscape: Comprehensive training in third space endoscopy - training, techniques, and practical recommendations
D. Roser, S. Nagl, A. Ebigbo
Best Practice & Research Clinical Gastroenterology.2024; : 101918. CrossRef - EUS and ERCP training in Europe: Time for simulation, optimization, and standardization
Selma J. Lekkerkerker, Rogier P. Voermans
United European Gastroenterology Journal.2023; 11(5): 407. CrossRef - There is no royal road: a shortcut for endoscopic submucosal dissection training
Seong Woo Jeon
Clinical Endoscopy.2023; 56(5): 590. CrossRef - Enhancing the Quality of Upper Gastrointestinal Endoscopy: Current Indicators and Future Trends
Caesar Ferrari, Micheal Tadros
Gastroenterology Insights.2023; 15(1): 1. CrossRef
- Impact and assessment of training models in interventional endoscopic ultrasound
- 2,790 View
- 215 Download
- 7 Web of Science
- 8 Crossref

- Korean guidelines for postpolypectomy colonoscopic surveillance: 2022 revised edition
- Su Young Kim, Min Seob Kwak, Soon Man Yoon, Yunho Jung, Jong Wook Kim, Sun-Jin Boo, Eun Hye Oh, Seong Ran Jeon, Seung-Joo Nam, Seon-Young Park, Soo-Kyung Park, Jaeyoung Chun, Dong Hoon Baek, Mi-Young Choi, Suyeon Park, Jeong-Sik Byeon, Hyung Kil Kim, Joo Young Cho, Moon Sung Lee, Oh Young Lee, Korean Society of Gastrointestinal Endoscopy, Korean Society of Gastroenterology, Korean Association for the Study of Intestinal Diseases
- Clin Endosc 2022;55(6):703-725. Published online October 13, 2022
- DOI: https://doi.org/10.5946/ce.2022.136
-
 Abstract
Abstract
 PDF
PDF Supplementary Material
Supplementary Material PubReader
PubReader ePub
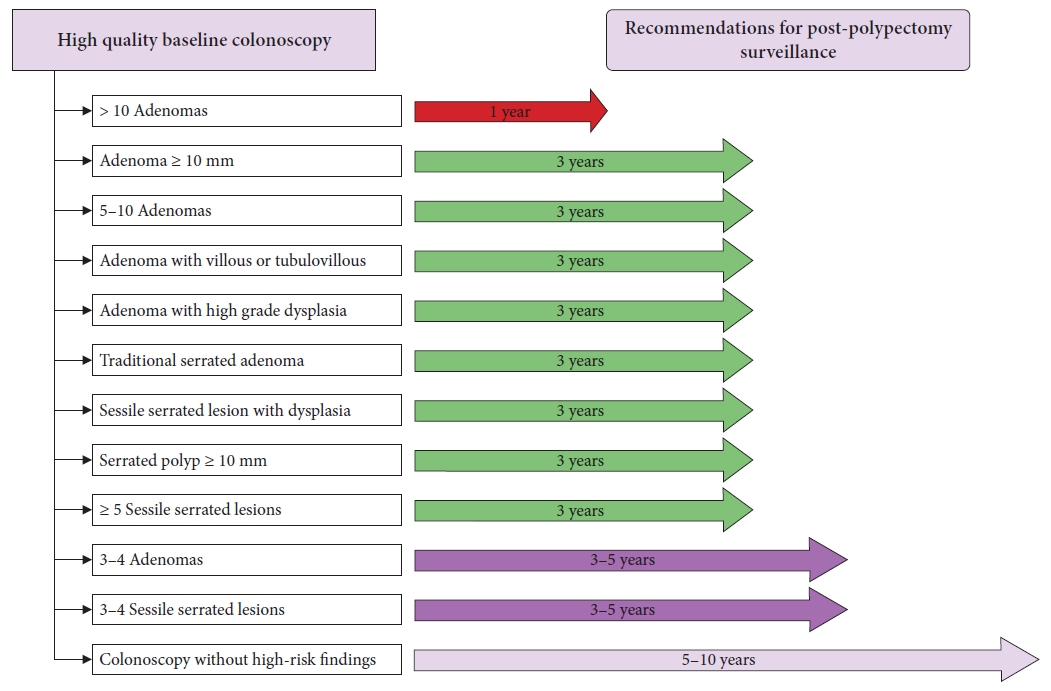
ePub - Colonoscopic polypectomy is effective in decreasing the incidence and mortality of colorectal cancer (CRC). Premalignant polyps discovered during colonoscopy are associated with the risk of metachronous advanced neoplasia. Postpolypectomy surveillance is the most important method for the management of advanced metachronous neoplasia. A more efficient and evidence-based guideline for postpolypectomy surveillance is required because of limited medical resources and concerns regarding colonoscopy complications. In these consensus guidelines, an analytic approach was used to address all reliable evidence to interpret the predictors of CRC or advanced neoplasia during surveillance colonoscopy. The key recommendations state that the high-risk findings for metachronous CRC following polypectomy are as follows: (1) adenoma ≥10 mm in size; (2) 3 to 5 (or more) adenomas; (3) tubulovillous or villous adenoma; (4) adenoma containing high-grade dysplasia; (5) traditional serrated adenoma; (6) sessile serrated lesion (SSL) containing any grade of dysplasia; (7) serrated polyp of at least 10 mm in size; and (8) 3 to 5 (or more) SSLs. More studies are needed to fully comprehend the patients most likely to benefit from surveillance colonoscopy and the ideal surveillance interval to prevent metachronous CRC.
-
Citations
Citations to this article as recorded by- Association between Atherosclerosis and High-Risk Colorectal Adenomas based on Cardio-Ankle Vascular Index and Ankle-Brachial Index
Jung Ho Lee, Hyunseok Cho, Sang Hoon Lee, Sung Joon Lee, Chang Don Kang, Dae Hee Choi, Jin Myung Park, Seung-Joo Nam, Tae Suk Kim, Ji Hyun Kim, Sung Chul Park
The Korean Journal of Gastroenterology.2024; 83(4): 143. CrossRef - A survey of current practices in post-polypectomy surveillance in Korea
Jeongseok Kim, Tae-Geun Gweon, Min Seob Kwak, Su Young Kim, Seong Jung Kim, Hyun Gun Kim, Eun Ran Kim, Sung Noh Hong, Eun Sun Kim, Chang Mo Moon, Dae Seong Myung, Dong Hoon Baek, Shin Ju Oh, Hyun Jung Lee, Ji Young Lee, Yunho Jung, Jaeyoung Chun, Dong-Hoo
Intestinal Research.2024; 22(2): 186. CrossRef - Korean Guidelines for Postpolypectomy Colonoscopic Surveillance: 2022 Revision
Su Young Kim
The Korean Journal of Medicine.2023; 98(3): 102. CrossRef - Detecting colorectal lesions with image-enhanced endoscopy: an updated review from clinical trials
Mizuki Nagai, Sho Suzuki, Yohei Minato, Fumiaki Ishibashi, Kentaro Mochida, Ken Ohata, Tetsuo Morishita
Clinical Endoscopy.2023; 56(5): 553. CrossRef - Understanding colorectal polyps to prevent colorectal cancer
Dong-Hoon Yang
Journal of the Korean Medical Association.2023; 66(11): 626. CrossRef - Classification and endoscopic diagnosis of colorectal polyps
Ji Hyun Kim, Sung Chul Park
Journal of the Korean Medical Association.2023; 66(11): 633. CrossRef - Endoscopic treatment of colorectal polyps and early colorectal cancer
Yunho Jung
Journal of the Korean Medical Association.2023; 66(11): 642. CrossRef - Strategy for post-polypectomy colonoscopy surveillance: focus on the revised Korean guidelines
Yong Soo Kwon, Su Young Kim
Journal of the Korean Medical Association.2023; 66(11): 652. CrossRef
- Association between Atherosclerosis and High-Risk Colorectal Adenomas based on Cardio-Ankle Vascular Index and Ankle-Brachial Index
- 5,241 View
- 515 Download
- 8 Web of Science
- 8 Crossref

Original Article
- Factors influencing endoscopic estimation of colon polyp size in a colon model
- Koen Robert Beukema, Jaimy A. Simmering, Marjolein Brusse-Keizer, Sneha John, Rutger Quispel, Peter B. Mensink
- Clin Endosc 2022;55(4):540-548. Published online July 28, 2022
- DOI: https://doi.org/10.5946/ce.2022.017

-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader ePub
ePub - Background
/Aims: Colorectal polyps are removed to prevent progression to colorectal cancer. Polyp size is an important factor for risk stratification of malignant transformation. Endoscopic size estimation correlates poorly with pathological reports and several factors have been suggested to influence size estimation. We aimed to gain insight into the factors influencing endoscopic polyp size estimation.
Methods
Images of polyps in an artificial model were obtained at 1, 3, and 5 cm from the colonoscope’s tip. Participants were asked to estimate the diameter and volume of each polyp.
Results
Fifteen endoscopists from three large-volume centers participated in this study. With an intraclass correlation coefficient of 0.66 (95% confidence interval [CI], 0.62–0.71) for diameter and 0.56 (95% CI, 0.50–0.62) for volume. Polyp size estimated at 3 cm from the colonoscope’s tip yielded the best results. A lower distance between the tip and the polyp was associated with a larger estimated polyp size.
Conclusions
Correct endoscopic estimation of polyp size remains challenging. This finding can affect size estimation skills and future training programs for endoscopists. -
Citations
Citations to this article as recorded by- Usefulness and Educational Benefit of a Virtual Scale Endoscope in Measuring Colorectal Polyp Size
Yudai Takehara, Ken Yamashita, Shin Morimoto, Fumiaki Tanino, Noriko Yamamoto, Yuki Kamigaichi, Hidenori Tanaka, Hidehiko Takigawa, Ryo Yuge, Yuji Urabe, Shiro Oka
Digestion.2024; 105(2): 73. CrossRef - Expert endoscopist assessment of colorectal polyp size using virtual scale endoscopy, visual or snare-based estimation: a prospective video-based study
Ioana Popescu Crainic, Roupen Djinbachian, Douglas K. Rex, Alan Barkun, Aasma Shaukat, James East, Cesare Hassan, Yuichi Mori, Heiko Pohl, Amit Rastogi, Prateek Sharma, Joseph C. Anderson, Mahsa Taghiakbari, Edgard Medawar, Daniel von Renteln
Scandinavian Journal of Gastroenterology.2024; 59(5): 608. CrossRef
- Usefulness and Educational Benefit of a Virtual Scale Endoscope in Measuring Colorectal Polyp Size
- 3,076 View
- 127 Download
- 1 Web of Science
- 2 Crossref

Review
- Post-polypectomy surveillance: the present and the future
- Masau Sekiguchi, Takahisa Matsuda, Kinichi Hotta, Yutaka Saito
- Clin Endosc 2022;55(4):489-495. Published online July 11, 2022
- DOI: https://doi.org/10.5946/ce.2022.097
-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader ePub
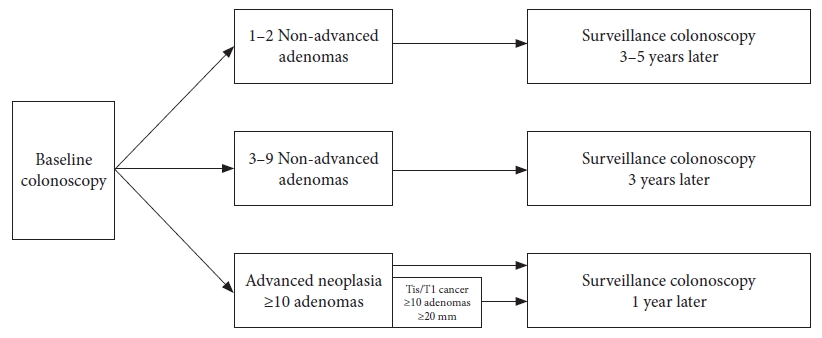
ePub - An appropriate post-polypectomy surveillance program requires the effectiveness of reducing colorectal cancer and safety. In addition, the post-polypectomy surveillance program should consider the burden of limited medical resource capacity, cost-effectiveness, and patient adherence. In this sense, a risk-stratified surveillance program based on baseline colonoscopy results is ideal. Major international guidelines for post-polypectomy surveillance, such as those from the European Union and the United States, have recommended risk-stratified surveillance programs. Both guidelines have recently been updated to better differentiate between high- and low-risk individuals. In both updated guidelines, more individuals have been downgraded to lower-risk groups that require less frequent or no surveillance. Furthermore, increased attention has been paid to the surveillance of patients who undergo serrated polyp removal. Previous guidelines in Japan did not clearly outline the risk stratification in post-polypectomy surveillance. However, the new colonoscopy screening and surveillance guidelines presented by the Japan Gastroenterological Endoscopy Society include a risk-stratified post-polypectomy surveillance program. Further discussion and analysis of unresolved issues in this field, such as the optimal follow-up after the first surveillance, the upper age limit for surveillance, and the ideal method for improving adherence to surveillance guidelines, are warranted.
-
Citations
Citations to this article as recorded by- Protocolo diagnóstico del seguimiento de pólipos colónicos
S. Redondo Evangelista, M. Sierra Morales, I. Bartolomé Oterino, P. García Centeno, A. Santos Rodríguez
Medicine - Programa de Formación Médica Continuada Acreditado.2024; 14(4): 219. CrossRef - Metabolic‐associated fatty liver disease is associated with colorectal adenomas in young and older Korean adults
Jiwon Chang, Yoosoo Chang, Yoosun Cho, Hyun‐Suk Jung, Dong‐Il Park, Soo‐Kyung Park, Soo‐Youn Ham, Sarah H. Wild, Christopher D. Byrne, Seungho Ryu
Liver International.2023; 43(11): 2548. CrossRef - Detecting colorectal lesions with image-enhanced endoscopy: an updated review from clinical trials
Mizuki Nagai, Sho Suzuki, Yohei Minato, Fumiaki Ishibashi, Kentaro Mochida, Ken Ohata, Tetsuo Morishita
Clinical Endoscopy.2023; 56(5): 553. CrossRef - Strategy for post-polypectomy colonoscopy surveillance: focus on the revised Korean guidelines
Yong Soo Kwon, Su Young Kim
Journal of the Korean Medical Association.2023; 66(11): 652. CrossRef - Endoscopic treatment of colorectal polyps and early colorectal cancer
Yunho Jung
Journal of the Korean Medical Association.2023; 66(11): 642. CrossRef - Understanding colorectal polyps to prevent colorectal cancer
Dong-Hoon Yang
Journal of the Korean Medical Association.2023; 66(11): 626. CrossRef
- Protocolo diagnóstico del seguimiento de pólipos colónicos
- 3,556 View
- 249 Download
- 5 Web of Science
- 6 Crossref

Systematic Review and Meta-Analysis
- Does computer-aided diagnostic endoscopy improve the detection of commonly missed polyps? A meta-analysis
- Arun Sivananthan, Scarlet Nazarian, Lakshmana Ayaru, Kinesh Patel, Hutan Ashrafian, Ara Darzi, Nisha Patel
- Clin Endosc 2022;55(3):355-364. Published online May 12, 2022
- DOI: https://doi.org/10.5946/ce.2021.228
-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader ePub
ePub - Background
/Aims: Colonoscopy is the gold standard diagnostic method for colorectal neoplasia, allowing detection and resection of adenomatous polyps; however, significant proportions of adenomas are missed. Computer-aided detection (CADe) systems in endoscopy are currently available to help identify lesions. Diminutive (≤5 mm) and nonpedunculated polyps are most commonly missed. This meta-analysis aimed to assess whether CADe systems can improve the real-time detection of these commonly missed lesions.
Methods
A comprehensive literature search was performed. Randomized controlled trials evaluating CADe systems categorized by morphology and lesion size were included. The mean number of polyps and adenomas per patient was derived. Independent proportions and their differences were calculated using DerSimonian and Laird random-effects modeling.
Results
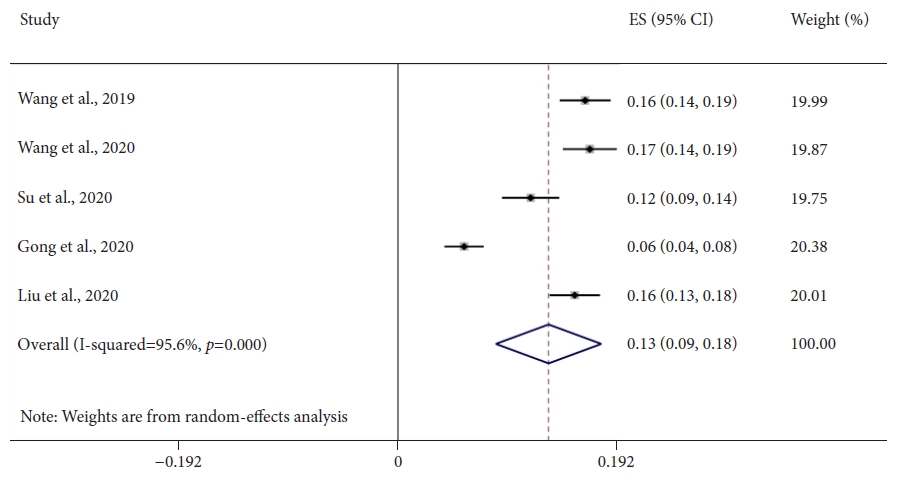
Seven studies, including 2,595 CADe-assisted colonoscopies and 2,622 conventional colonoscopies, were analyzed. CADe-assisted colonoscopy demonstrated an 80% increase in the mean number of diminutive adenomas detected per patient compared with conventional colonoscopy (0.31 vs. 0.17; effect size, 0.13; 95% confidence interval [CI], 0.09–0.18); it also demonstrated a 91.7% increase in the mean number of nonpedunculated adenomas detected per patient (0.32 vs. 0.19; effect size, 0.05; 95% CI, 0.02–0.07).
Conclusions
CADe-assisted endoscopy significantly improved the detection of most commonly missed adenomas. Although this method is a potentially exciting technology, limitations still apply to current data, prompting the need for further real-time studies. -
Citations
Citations to this article as recorded by- Use of artificial intelligence in the management of T1 colorectal cancer: a new tool in the arsenal or is deep learning out of its depth?
James Weiquan Li, Lai Mun Wang, Katsuro Ichimasa, Kenneth Weicong Lin, James Chi-Yong Ngu, Tiing Leong Ang
Clinical Endoscopy.2024; 57(1): 24. CrossRef - As how artificial intelligence is revolutionizing endoscopy
Jean-Francois Rey
Clinical Endoscopy.2024; 57(3): 302. CrossRef - Eye tracking technology in endoscopy: Looking to the future
Arun Sivananthan, Jabed Ahmed, Alexandros Kogkas, George Mylonas, Ara Darzi, Nisha Patel
Digestive Endoscopy.2023; 35(3): 314. CrossRef - Artificial intelligence and the push for small adenomas: all we need?
Katharina Zimmermann-Fraedrich, Thomas Rösch
Endoscopy.2023; 55(04): 320. CrossRef - Recent advances in devices and technologies that might prove revolutionary for colonoscopy procedures
Jonathan S. Galati, Kevin Lin, Seth A. Gross
Expert Review of Medical Devices.2023; 20(12): 1087. CrossRef - Detecting colorectal lesions with image-enhanced endoscopy: an updated review from clinical trials
Mizuki Nagai, Sho Suzuki, Yohei Minato, Fumiaki Ishibashi, Kentaro Mochida, Ken Ohata, Tetsuo Morishita
Clinical Endoscopy.2023; 56(5): 553. CrossRef - KI-Werkzeuge als smarte Helfer in Klinik und Forschung
Zeitschrift für Gastroenterologie.2023; 61(11): 1544. CrossRef - AI-powered medical devices for practical clinicians including the diagnosis of colorectal polyps
Donghwan Kim, Eunsun Kim
Journal of the Korean Medical Association.2023; 66(11): 658. CrossRef - The Role of Artificial Intelligence in Colorectal Cancer Screening: Lesion Detection and Lesion Characterization
Edward Young, Louisa Edwards, Rajvinder Singh
Cancers.2023; 15(21): 5126. CrossRef - Artificial intelligence for colorectal neoplasia detection during colonoscopy: a systematic review and meta-analysis of randomized clinical trials
Shenghan Lou, Fenqi Du, Wenjie Song, Yixiu Xia, Xinyu Yue, Da Yang, Binbin Cui, Yanlong Liu, Peng Han
eClinicalMedicine.2023; 66: 102341. CrossRef - Pouring some water into the wine—Poor performance of endoscopists in artificial intelligence studies
Jochen Weigt
United European Gastroenterology Journal.2022; 10(8): 793. CrossRef
- Use of artificial intelligence in the management of T1 colorectal cancer: a new tool in the arsenal or is deep learning out of its depth?
- 3,032 View
- 155 Download
- 11 Web of Science
- 11 Crossref

Reviews
- Quality indicators in colonoscopy: the chasm between ideal and reality
- Su Bee Park, Jae Myung Cha
- Clin Endosc 2022;55(3):332-338. Published online April 4, 2022
- DOI: https://doi.org/10.5946/ce.2022.037
-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader ePub
ePub - Continuous measurement of quality indicators (QIs) should be a routine part of colonoscopy, as a wide variation still exists in the performance and quality levels of colonoscopy in Korea. Among the many QIs of colonoscopy, the adenoma detection rate, average withdrawal time, bowel preparation adequacy, and cecal intubation rate should be monitored in daily clinical practice to improve the quality of the procedure. The adenoma detection rate is the best indicator of the quality of colonoscopy; however, it has many limitations for universal use in daily practice. With the development of natural language processing, the adenoma detection rate is expected to become more effective and useful. It is important that colonoscopists do not strictly and mechanically maintain an average withdrawal time of 6 minutes but instead perform careful colonoscopy to maximally expose the colonic mucosa with a withdrawal time of at least 6 minutes. To achieve adequate bowel preparation, documentation of bowel preparation with the Boston Bowel Preparation Scale (BBPS) should be a routine part of colonoscopy. When colonoscopists routinely followed the bowel preparation protocols, ≥85% of outpatient screening colonoscopies had a BBPS score of ≥6. In addition, the cecal intubation rate should be ≥95% of all screening colonoscopies. The first step in improving colonoscopy quality in Korea is to apply these key performance measurements in clinical practice.
-
Citations
Citations to this article as recorded by- What are the priority quality indicators for colonoscopy in real‐world clinical practice?
Kasenee Tiankanon, Satimai Aniwan
Digestive Endoscopy.2024; 36(1): 30. CrossRef - A Systematic Review of Exercise Therapy for Bowel Preparation
Yuan-Yuan Zhang, Ramoo Vimala, Ping Lei Chui, Ida Normiha Hilmi
Gastroenterology Nursing.2023; 46(5): 393. CrossRef - Detecting colorectal lesions with image-enhanced endoscopy: an updated review from clinical trials
Mizuki Nagai, Sho Suzuki, Yohei Minato, Fumiaki Ishibashi, Kentaro Mochida, Ken Ohata, Tetsuo Morishita
Clinical Endoscopy.2023; 56(5): 553. CrossRef - AI-powered medical devices for practical clinicians including the diagnosis of colorectal polyps
Donghwan Kim, Eunsun Kim
Journal of the Korean Medical Association.2023; 66(11): 658. CrossRef
- What are the priority quality indicators for colonoscopy in real‐world clinical practice?
- 3,125 View
- 219 Download
- 5 Web of Science
- 4 Crossref

- Can Computed Tomography Colonography Replace Optical Colonoscopy in Detecting Colorectal Lesions?: State of the Art
- Alessia Chini, Michele Manigrasso, Grazia Cantore, Rosa Maione, Marco Milone, Francesco Maione, Giovanni Domenico De Palma
- Clin Endosc 2022;55(2):183-190. Published online February 24, 2022
- DOI: https://doi.org/10.5946/ce.2021.254
-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader ePub
ePub - Colorectal cancer is an important cause of morbidity and mortality worldwide. Optical colonoscopy (OC) is widely accepted as the reference standard for the screening of colorectal polyps and cancers, and computed tomography colonography (CTC) is a valid alternative to OC. The purpose of this review was to assess the diagnostic accuracy of OC and CTC for colorectal lesions. A literature search was performed in PubMed, Embase, and Cochrane Library, and 18 articles were included. CTC has emerged in recent years as a potential screening examination with high accuracy for the detection of colorectal lesions. However, the clinical application of CTC as a screening technique is limited because it is highly dependent on the size of the lesions and has poor performance in detecting individual lesions <5 mm or flat lesions, which, although rarely, can have a malignant potential.
-
Citations
Citations to this article as recorded by- Multi-view orientational attention network combining point-based affinity for polyp segmentation
Yan Liu, Yan Yang, Yongquan Jiang, Zhuyang Xie
Expert Systems with Applications.2024; 249: 123663. CrossRef - The Influence of Mechanical Bowel Preparation on Volatile Organic Compounds for the Detection of Gastrointestinal Disease—A Systematic Review
Ashwin Krishnamoorthy, Subashini Chandrapalan, Sofie Bosch, Ayman Bannaga, Nanne K.H. De Boer, Tim G.J. De Meij, Marcis Leja, George B. Hanna, Nicoletta De Vietro, Donato Altomare, Ramesh P. Arasaradnam
Sensors.2023; 23(3): 1377. CrossRef - The Detection of Colorectal Cancer through Machine Learning-Based Breath Sensor Analysis
Inese Poļaka, Linda Mežmale, Linda Anarkulova, Elīna Kononova, Ilona Vilkoite, Viktors Veliks, Anna Marija Ļeščinska, Ilmārs Stonāns, Andrejs Pčolkins, Ivars Tolmanis, Gidi Shani, Hossam Haick, Jan Mitrovics, Johannes Glöckler, Boris Mizaikoff, Mārcis Lej
Diagnostics.2023; 13(21): 3355. CrossRef
- Multi-view orientational attention network combining point-based affinity for polyp segmentation
- 3,800 View
- 233 Download
- 4 Web of Science
- 3 Crossref

Original Article
- Comparison of conventional and new endoscopic band ligation devices for colonic diverticular bleeding
- Ayaka Takasu, Takashi Ikeya, Yasutoshi Shiratori
- Clin Endosc 2022;55(3):408-416. Published online February 18, 2022
- DOI: https://doi.org/10.5946/ce.2021.200

-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader ePub
ePub - Background
/Aims: Endoscopic band ligation (EBL) is used to treat colonic diverticular bleeding (CDB). An endoscopic variceal ligation device for esophageal varices is used as a conventional EBL device (C-EBL). A new EBL device (N-EBL) was developed by Sumitomo Bakelite Co. in August 2018. We aimed to evaluate the clinical outcomes of N-EBL compared with those of C-EBL.
Methods
Seventy-nine patients who underwent EBL for CDB at St. Luke’s International Hospital, Japan, between 2017 and 2020 were included in this retrospective study. Patients were divided into the C-EBL and N-EBL groups. Their clinical outcomes, including achieving initial hemostasis, early rebleeding, procedure time, and EBL-associated adverse events, were evaluated.
Results
Of the 79 patients, 36 (45.6%) were in the C-EBL group and 43 (54.4%) were in the N-EBL group. The rate of achieving initial hemostasis was 100% in the C-EBL group and 93.0% in the N-EBL group. No significant difference was noted in the early rebleeding rate between the groups (p=0.24). The N-EBL group achieved a shorter median EBL procedure time than the C-EBL group (18.2 minutes vs. 14.2 minutes, p=0.02). No adverse events were observed in either group.
Conclusions
The N-EBL device is safe and useful and may reduce EBL procedure time. -
Citations
Citations to this article as recorded by- Advances in endoscopic management of colonic diverticular bleeding
Yasutoshi Shiratori, Syed Matthew Kodilinye, Ahmed E. Salem
Current Opinion in Gastroenterology.2024;[Epub] CrossRef - Management of Patients With Acute Lower Gastrointestinal Bleeding: An Updated ACG Guideline
Neil Sengupta, Joseph D. Feuerstein, Vipul Jairath, Amandeep K. Shergill, Lisa L. Strate, Robert J. Wong, David Wan
American Journal of Gastroenterology.2023; 118(2): 208. CrossRef - Effective endoscopic band ligation for diverticular perforation with a refractory pelvic abscess
Koichi Soga, Atsushi Majima
Clinical Endoscopy.2023; 56(2): 252. CrossRef - A new band ligation device to treat colonic diverticular bleeding
Yunho Jung
Clinical Endoscopy.2022; 55(3): 367. CrossRef
- Advances in endoscopic management of colonic diverticular bleeding
- 3,842 View
- 237 Download
- 2 Web of Science
- 4 Crossref

Special Article: Celebrating the 10th Anniversary of Clinical Endoscopy
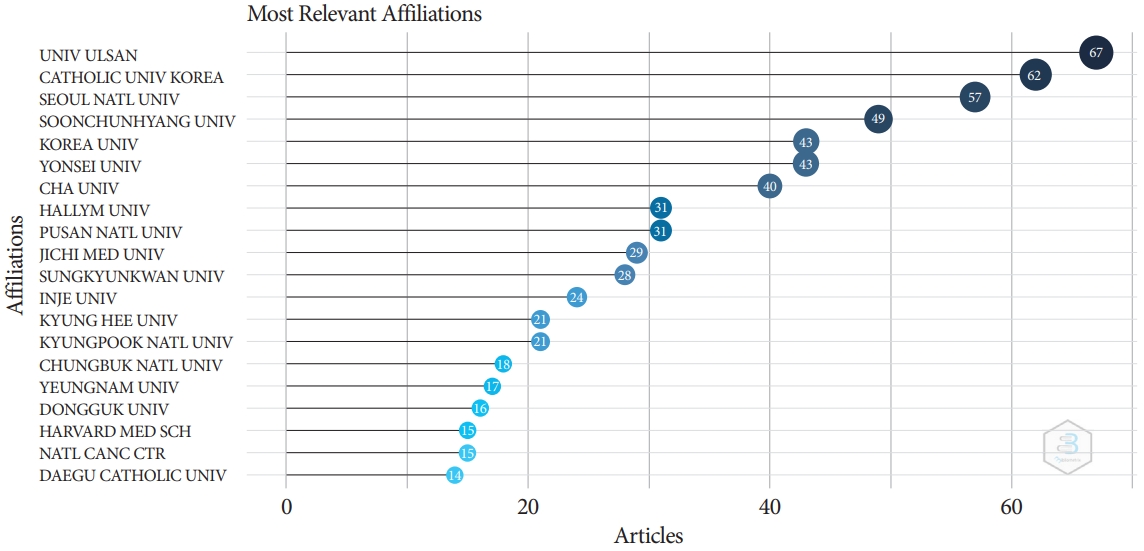
- Document Network and Conceptual and Social Structures of Clinical Endoscopy from 2015 to July 2021 Based on the Web of Science Core Collection: A Bibliometric Study
- Sun Huh
- Clin Endosc 2021;54(5):641-650. Published online September 30, 2021
- DOI: https://doi.org/10.5946/ce.2021.207
-
 Abstract
Abstract
 PDF
PDF Supplementary Material
Supplementary Material PubReader
PubReader ePub
ePub - Background
/Aims: The present study investigated the relevance and network of institutions, keywords, and authors’ countries of the articles in Clinical Endoscopy published from 2015 to May 2021 based on the Web of Science Core Collection.
Methods
The Web of Science Core Collection was searched with the term Clinical Endoscopy as the publication title on July 12, 2021. All 776 citations published from 2015 to May 2021 and 2,964 articles citing those 776 articles were analyzed using Biblioshiny.
Results
The corresponding authors were from 73 countries. Document coupling showed that the colorectal cancer-colonoscopyrandomized controlled trial cluster had the most significant impact and highest centrality. There were 442 articles with corresponding authors from Korea (57.0%). The number of collaborative works by Korean authors with the authors of other countries was 33 (7.5%). The articles were cited 2,964 times by corresponding authors from 37 countries.
Conclusions
The above results show that Clinical Endoscopy has published several studies on gastrointestinal endoscopy. A large proportion of citations (84.7 %) were from outside Korea, indicating that the journal content is useful for global physicians. Collaborative work between authors from Korea and other countries should be encouraged to promote the journal. -
Citations
Citations to this article as recorded by- Research Progress in Land Consolidation and Rural Revitalization: Current Status, Characteristics, Regional Differences, and Evolution Laws
Shuchang Li, Wei Song
Land.2023; 12(1): 210. CrossRef - Journal metrics, document network, and conceptual and social structures of the Korean Journal of Anesthesiology from 2017 to July 2022: a bibliometric study
Sun Huh
Korean Journal of Anesthesiology.2023; 76(1): 3. CrossRef - Promotion to Top-Tier Journal and Development Strategy of the Annals of Laboratory Medicine for Strengthening its Leadership in the Medical Laboratory Technology Category: A Bibliometric Study
Sun Huh
Annals of Laboratory Medicine.2022; 42(3): 321. CrossRef - Research trends on endoscopic mucosal resection: A bibliometric analysis from 1991 to 2021
Yihan Yang, Xuan Xu, Menghui Wang, Yang Zhang, Pinglang Zhou, Sifan Yang, Xu Shu, Chuan Xie
Frontiers in Surgery.2022;[Epub] CrossRef - Riesgo de sangrado gastrointestinal por uso de anticoagulantes directos orales: ¿cuál es más seguro?
Ivan David Lozada Martinez, Luis Carlos Solano Díaz, Marcela Barbosa Pérez, Víctor Andrés Rueda Oviedo, Brainerd Lenin Caicedo Moncada, Gustavo Andrés Diaz Cruz, Adriana cristina Ceballos Espitia, David Esteban Diaz Gómez, Daiana Andrea Rojas Ramí
Revista Cuarzo.2022; 28(2): 31. CrossRef
- Research Progress in Land Consolidation and Rural Revitalization: Current Status, Characteristics, Regional Differences, and Evolution Laws
- 3,433 View
- 61 Download
- 4 Web of Science
- 5 Crossref

Original Article
- Colorectal Cancer Screening with Computed Tomography Colonography: Single Region Experience in Kazakhstan
- Jandos Amankulov, Dilyara Kaidarova, Zhamilya Zholdybay, Marianna Zagurovskaya, Nurlan Baltabekov, Madina Gabdullina, Akmaral Ainakulova, Dias Toleshbayev, Alexandra Panina, Elvira Satbayeva, Zhansaya Kalieva
- Clin Endosc 2022;55(1):101-112. Published online July 15, 2021
- DOI: https://doi.org/10.5946/ce.2021.066
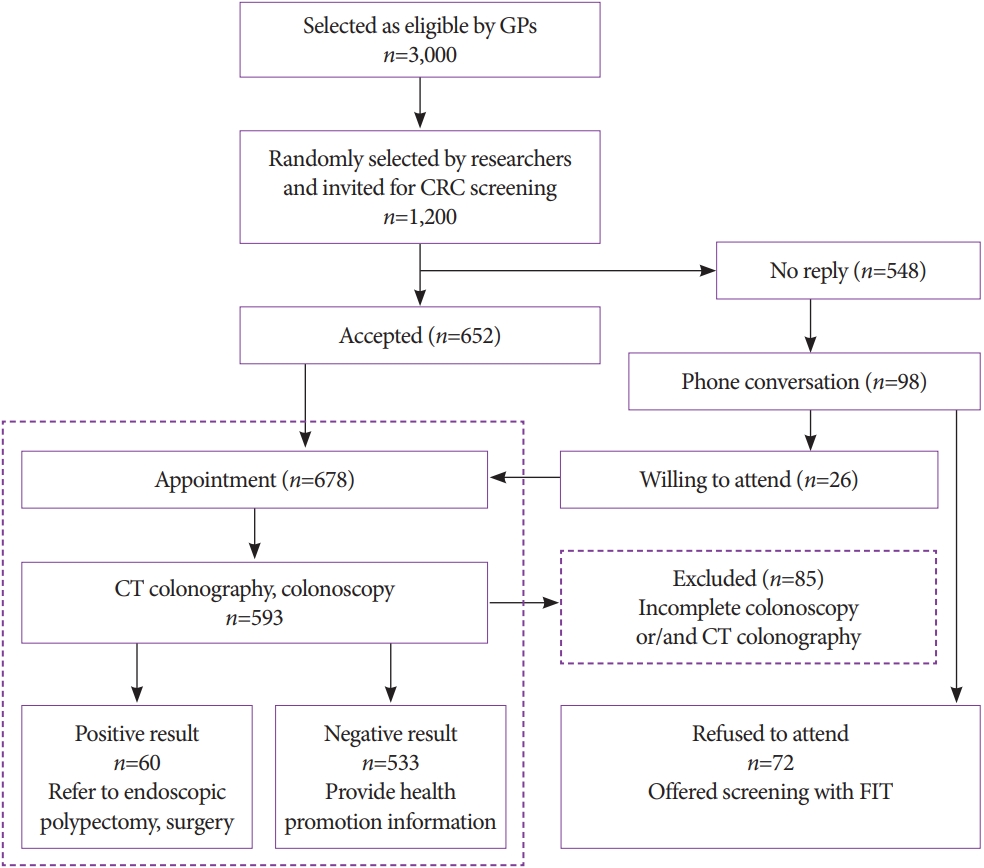
-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader ePub
ePub - Background
/Aims: The aim of our study was to determine the efficacy of computed tomography colonography (CTC) in screening for colorectal cancer (CRC).
Methods
A total of 612 females and 588 males aged 45 to 75 years were enrolled in CTC screening. CTC was performed following standard bowel preparation and colonic insufflation with carbon dioxide. The main outcomes were the detection rate of CRC and advanced adenoma (AA), prevalence of colorectal lesions in relation to socio-demographic and health factors, and overall diagnostic performance of CTC.
Results
Overall, 56.5% of the 1,200 invited subjects underwent CTC screening. The sensitivity for CRC and AA was 0.89 and 0.97, respectively, while the specificity was 0.71 and 0.99, respectively. The prevalence of CRC and AA was 3.0% (18/593) and 7.1% (42/593), respectively, with the highest CRC prevalence in the 66-75 age group (≥12 times; odds ratio [OR], 12.11; 95% confidence interval [CI], 4.45-32.92). CRC and AA prevalence were inversely correlated with Asian descent, physical activity, and negative fecal immunochemical test results (OR=0.43; 95% CI, 0.22-0.83; OR=0.16; 95% CI, 0.04-0.68; OR=0.5; 95% CI, 0.07-3.85, respectively).
Conclusions
Our study revealed high accuracy of CTC in diagnosing colonic neoplasms, good compliance with CTC screening, and high detection rate of CRC.
- 5,700 View
- 205 Download
- 1 Web of Science

Focused Review Series: Image-Enhanced Endoscopy: Update on Clinical Practice
- Clinical Applications of Linked Color Imaging and Blue Laser/Light Imaging in the Screening, Diagnosis, and Treatment of Superficial Colorectal Tumors
- Taku Sakamoto, Hourin Cho, Yutaka Saito
- Clin Endosc 2021;54(4):488-493. Published online July 14, 2021
- DOI: https://doi.org/10.5946/ce.2021.157
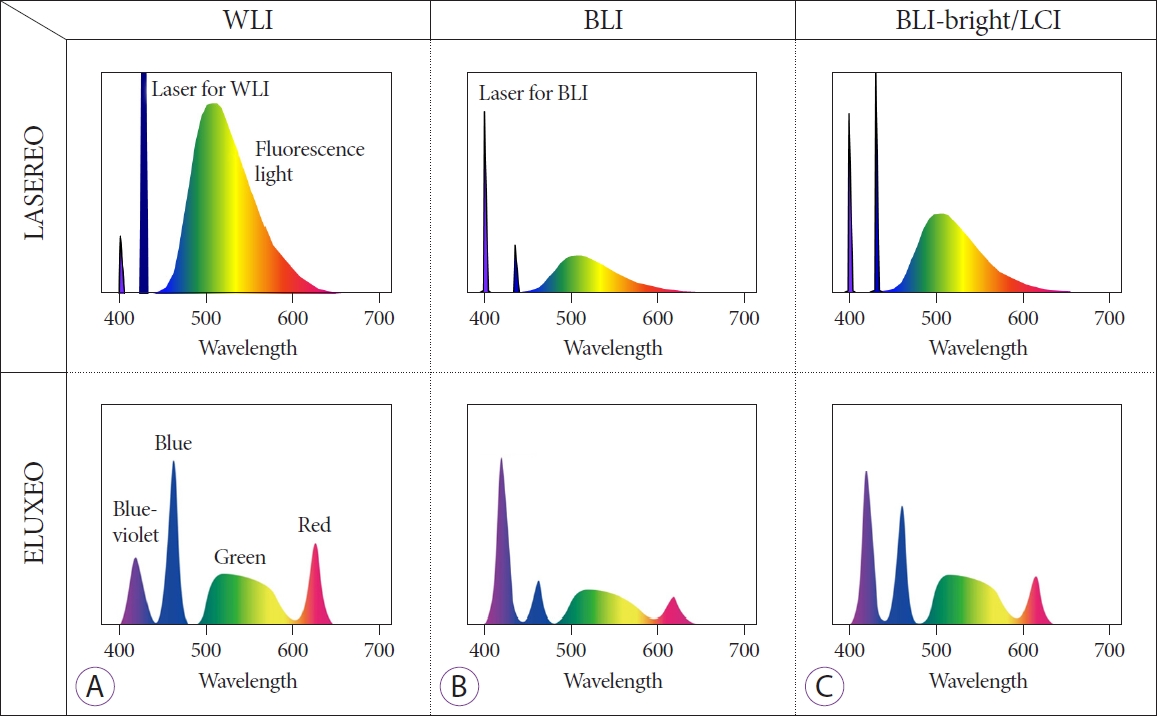
-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader ePub
ePub - Considering its contribution to reducing colorectal cancer morbidity and mortality, the most important task of colonoscopy is to find all existing polyps. Moreover, the accurate detection of existing polyps determines the risk of colorectal cancer morbidity and is an important factor in deciding the appropriate surveillance program for patients. Image-enhanced endoscopy is an easy-to-use modality with improved lesion detection. Linked color imaging (LCI) and blue laser/light imaging (BLI) are useful modalities for improving colonoscopy quality. Each mode has unique optical features; therefore, their intended use differs. LCI contributes to improved polyp detection due to its brightness and high color contrast between the lesion and normal mucosa, while BLI contributes to the characterization of detected polyps by evaluating the vessel and surface patterns of detected lesions. The proper use of these observation modes allows for more efficient endoscopic diagnosis. Moreover, recent developments in artificial intelligence will soon change the clinical practice of colonoscopy and this system will provide an efficient education modality for novice endoscopists.
-
Citations
Citations to this article as recorded by- Endoscopic features with associated histological and molecular alterations in serrated polyps with dysplasia: Retrospective analysis of a tertiary case series
Antonello Trecca, Raffaele Borghini, Daniela Medicina, Rachele Del Sordo, Giulio Mandelli, Antonino Bella, Giuseppe Galloro, Kuang-I Fu, Vincenzo Villanacci
Digestive and Liver Disease.2024; 56(4): 687. CrossRef - Linked-color imaging with or without artificial intelligence for adenoma detection: a randomized trial
Kazuya Miyaguchi, Yoshikazu Tsuzuki, Nobutaka Hirooka, Hisashi Matsumoto, Hideki Ohgo, Hidetomo Nakamoto, Hiroyuki Imaeda
Endoscopy.2024; 56(05): 376. CrossRef - The Diagnostic Performance of Linked Color Imaging Compared to White Light Imaging in Endoscopic Diagnosis of Helicobacter pylori Infection: A Systematic Review and Meta-Analysis
Jae Gon Lee, In Kyung Yoo, Abdullah Ozgur Yeniova, Sang Pyo Lee
Gut and Liver.2024; 18(3): 444. CrossRef - Linked color imaging improves the diagnostic accuracy of eosinophilic esophagitis
Yasuhiko Abe, Yu Sasaki, Makoto Yagi, Naoko Mizumoto, Yusuke Onozato, Takashi Kon, Masakuni Shoji, Kazuhiro Sakuta, Takayuki Sakai, Matsuki Umehara, Minami Ito, Shuhei Nakamura, Hidemoto Tsuchida, Yoshiyuki Ueno
DEN Open.2023;[Epub] CrossRef - Comparison of LED and LASER Colonoscopy About Linked Color Imaging and Blue Laser/Light Imaging of Colorectal Tumors in a Multinational Study
Naohisa Yoshida, Peter V. Draganov, Sneha John, Helmut Neumann, Rafiz Abdul Rani, Wen-Hsin Hsu, Nilesh Fernandopulle, Kewin Tien Ho Siah, Ricardo Morgenstern, Yuri Tomita, Ken Inoue, Osamu Dohi, Ryohei Hirose, Yoshito Itoh, Takaaki Murakami, Yoshikazu Ina
Digestive Diseases and Sciences.2023; 68(10): 3943. CrossRef - Classification and endoscopic diagnosis of colorectal polyps
Ji Hyun Kim, Sung Chul Park
Journal of the Korean Medical Association.2023; 66(11): 633. CrossRef - Role of linked color imaging for upper gastrointestinal disease: present and future
Sang Pyo Lee
Clinical Endoscopy.2023; 56(5): 546. CrossRef - Detecting colorectal lesions with image-enhanced endoscopy: an updated review from clinical trials
Mizuki Nagai, Sho Suzuki, Yohei Minato, Fumiaki Ishibashi, Kentaro Mochida, Ken Ohata, Tetsuo Morishita
Clinical Endoscopy.2023; 56(5): 553. CrossRef - Images of laser and light‐emitting diode colonoscopy for comparing large colorectal lesion visibility with linked color imaging and white‐light imaging
Naohisa Yoshida, Yoshikazu Hayashi, Hiroshi Kashida, Yuri Tomita, Osamu Dohi, Ken Inoue, Ryohei Hirose, Yoshito Itoh, Masahiro Okada, Shiori Yoshimoto, Toshihiro Fujinuma, Hirotsugu Sakamoto, Keijiro Sunada, Yoriaki Komeda, Ikue Sekai, Natsuki Okai, Hiron
Digestive Endoscopy.2022; 34(7): 1413. CrossRef
- Endoscopic features with associated histological and molecular alterations in serrated polyps with dysplasia: Retrospective analysis of a tertiary case series
- 3,978 View
- 147 Download
- 9 Web of Science
- 9 Crossref

Case Report
- Endoscopic Treatment of Iatrogenic Perforation of Sigmoid Diverticulum: A Case Report of Multidisciplinary Management
- Giacomo Emanuele Maria Rizzo, Giuseppina Ferro, Giovanna Rizzo, Giovanni Di Carlo, Alessandro Cantone, Gaetano Giuseppe Di Vita, Carmelo Sciumè
- Clin Endosc 2022;55(2):292-296. Published online June 7, 2021
- DOI: https://doi.org/10.5946/ce.2021.005
-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader ePub
ePub - Iatrogenic perforations are severe complications of gastrointestinal endoscopy; therefore, their management should be adequately planned. A 77-year-old man with a history of diverticulosis underwent a colonoscopy for anemia. During the procedure, an iatrogenic perforation occurred suddenly in the sigmoid colon, near a severe angle among the numerous diverticula. Through-the-scope clips were immediately applied to treat it and close mucosal edges. Laboratory tests showed increased levels of inflammation and infection, and although there were no complaints of abdominal pain, the patient had an extremely distended abdomen. A multidisciplinary board began management based on a conservative approach. Pneumoperitoneum was treated with computed tomography-assisted drainage. After 72 hours, his intestinal canalization and laboratory tests were normal. Though this adverse event is rare, a multidisciplinary board should be promptly gathered upon occurrence, even if the patient appears clinically stable, to consider a conservative approach and avoid surgical treatment.
- 3,550 View
- 161 Download

Original Article
- Insufflation of Carbon Dioxide versus Air During Colonoscopy Among Pediatric Patients: A Systematic Review and Meta-Analysis of Randomized Controlled Trials
- John Alexander Lata Guacho, Diogo Turiani Hourneaux de Moura, Igor Braga Ribeiro, Bruna Furia Buzetti Hourneaux de Moura, Megui Marilia Mansilla Gallegos, Thomas McCarty, Ricardo Katsuya Toma, Eduardo Guimarães Hourneaux de Moura
- Clin Endosc 2021;54(2):242-249. Published online March 25, 2021
- DOI: https://doi.org/10.5946/ce.2020.275
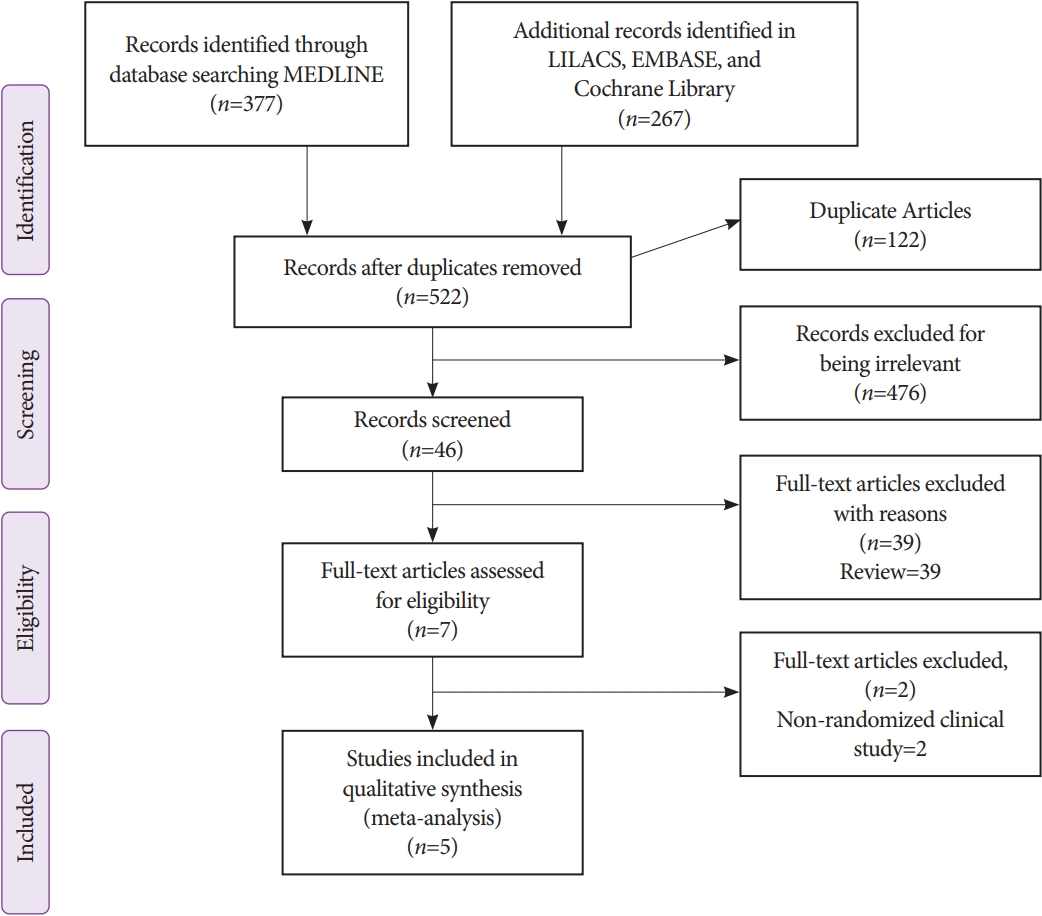
-
 Abstract
Abstract
 PDF
PDF Supplementary Material
Supplementary Material PubReader
PubReader ePub
ePub - Background
/Aims: Carbon dioxide is increasingly used in insufflation during colonoscopy in adult patients; however, air insufflation remains the primary practice among pediatric gastroenterologists. This systematic review and meta-analysis aims to evaluate insufflation using CO2 versus air in colonoscopies in pediatric patients.
Methods
Individualized search strategies were performed using MEDLINE, Cochrane Library, EMBASE, and LILACS databases following Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines and Cochrane working methodology. Randomized control trials (RCTs) were selected for the present meta-analysis. Pooled proportions were calculated for outcomes including procedure time and abdominal pain immediately and 24 hours post-procedure.
Results
The initial search yielded 644 records, of which five RCTs with a total of 358 patients (CO2: n=178 versus air: n=180) were included in the final analysis. The procedure time was not different between the CO2 and air insufflation groups (mean difference, 10.84; 95% confidence interval [CI], -2.55 to 24.22; p=0.11). Abdominal pain immediately post-procedure was significantly lower in the CO2 group (risk difference, -0.15; 95% CI; -0.26 to -0.03; p=0.01) while abdominal pain at 24 hours post-procedure was similar (risk difference, -0.05; 95% CI; -0.11 to 0.01; p=0.11).
Conclusions
Based on this systematic review and meta-analysis of RCT data, CO2 insufflation reduced abdominal pain immediately following the procedure, while pain was similar at 24 hours post-procedure. These results suggest that CO2 is a preferred insufflation technique when performing colonoscopy in pediatric patients. -
Citations
Citations to this article as recorded by- Elevations in End-Tidal CO2 With CO2 Use During Pediatric Endoscopy With Airway Protection: Is This Physiologically Significant?
Chinenye R. Dike, Andrew Huang Pacheco, Elizabeth Lyden, David Freestone, Ojasvini Choudhry, Warren P. Bishop, Mohanad Shukry
Journal of Pediatric Gastroenterology & Nutrition.2023; 76(5): 660. CrossRef
- Elevations in End-Tidal CO2 With CO2 Use During Pediatric Endoscopy With Airway Protection: Is This Physiologically Significant?
- 5,244 View
- 150 Download
- 1 Web of Science
- 1 Crossref

Reviews
- Current Status of Colorectal Cancer and Its Public Health Burden in Thailand
- Kasenee Tiankanon, Satimai Aniwan, Rungsun Rerknimitr
- Clin Endosc 2021;54(4):499-504. Published online March 15, 2021
- DOI: https://doi.org/10.5946/ce.2020.245-IDEN

-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader ePub
ePub - Colorectal cancer (CRC) accounts for approximately 10.3% of new cancer cases in Thailand and is currently the 3rd most prevalent cancer found among the Thai population. Starting in 2017, the Thai government announced the national CRC screening program as a response to this important issue. Among the 70 million people currently residing in Thailand, 14 million require screening, while there are approximately a total of 1,000 endoscopists available to perform colonoscopy. Due to the limited resources and shortage of endoscopists in Thailand, applying a population-based one-step colonoscopy program as a primary screening method is not feasible. To reduce colonoscopy workload, with the help of others, including village health volunteers, institution-based health personnel, reimbursement coders, pathologists, and patients due for CRC screening, a two-step approach of one-time fecal immunochemical test (FIT), which prioritizes and filters out subjects for colonoscopy, is chosen. Moreover, additional adjustments to the optimal FIT cutoff value and the modified Asia-Pacific Colorectal Screening risk score, including body weight, were proposed to stratify the priority of colonoscopy schedule. This article aims to give an overview of the past and current policy developmental strategies and the current status of the Thailand CRC screening program.
-
Citations
Citations to this article as recorded by- Integrated Care Model by the Village Health Volunteers to Prevent and Slow down Progression of Chronic Kidney Disease in a Rural Community, Thailand
Ampornpan Theeranut, Nonglak Methakanjanasak, Sunee Lertsinudom, Pattama Surit, Nichanun Panyaek, Saisamon Leeladapattarakul, Peangtikumporn Nilpetch, Pattapong Kessomboon, Chalongchai Chalermwat, Watcharapong Rintara, Wudipong Khongtong, Pawich Paktipat,
Journal of Primary Care & Community Health.2024;[Epub] CrossRef - Dynamics of colorectal cancer screening in low and middle-income countries: A modeling analysis from Thailand
Peeradon Wongseree, Zeynep Hasgul, Borwornsom Leerapan, Cherdsak Iramaneerat, Pochamana Phisalprapa, Mohammad S. Jalali
Preventive Medicine.2023; 175: 107694. CrossRef - An Assessment of Physicians’ Recommendations for Colorectal Cancer Screening and International Guidelines Awareness and Adherence: Results From a Thai National Survey
Nonthalee Pausawasdi, Pongkamon Tongpong, Tanawat Geeratragool, Phunchai Charatcharoenwitthaya
Frontiers in Medicine.2022;[Epub] CrossRef - Cytotoxic effect of metformin on butyrate-resistant PMF-K014 colorectal cancer spheroid cells
Kesara Nittayaboon, Kittinun Leetanaporn, Surasak Sangkhathat, Sittirak Roytrakul, Raphatphorn Navakanitworakul
Biomedicine & Pharmacotherapy.2022; 151: 113214. CrossRef
- Integrated Care Model by the Village Health Volunteers to Prevent and Slow down Progression of Chronic Kidney Disease in a Rural Community, Thailand
- 5,164 View
- 168 Download
- 5 Web of Science
- 4 Crossref

- Artificial Intelligence in Lower Gastrointestinal Endoscopy: The Current Status and Future Perspective
- Sebastian Manuel Milluzzo, Paola Cesaro, Leonardo Minelli Grazioli, Nicola Olivari, Cristiano Spada
- Clin Endosc 2021;54(3):329-339. Published online January 13, 2021
- DOI: https://doi.org/10.5946/ce.2020.082
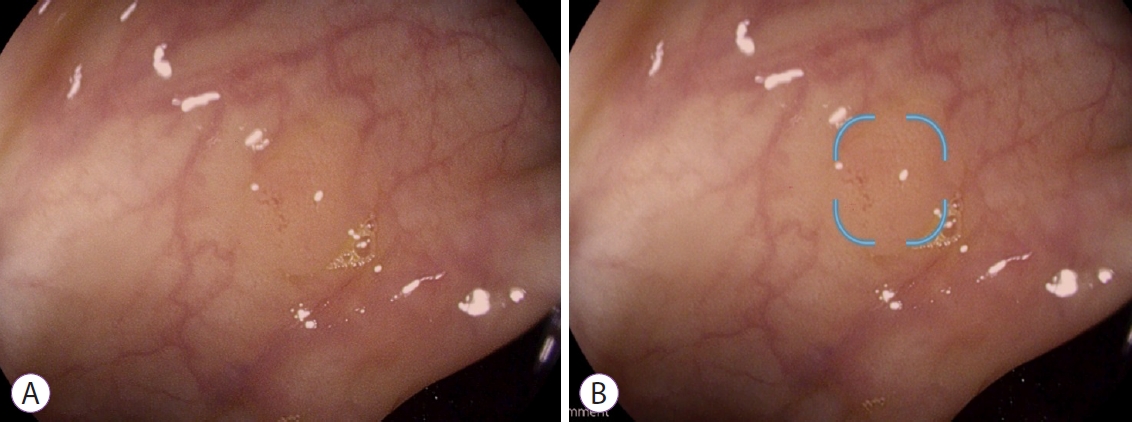
-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader ePub
ePub - The present manuscript aims to review the history, recent advances, evidence, and challenges of artificial intelligence (AI) in colonoscopy. Although it is mainly focused on polyp detection and characterization, it also considers other potential applications (i.e., inflammatory bowel disease) and future perspectives. Some of the most recent algorithms show promising results that are similar to human expert performance. The integration of AI in routine clinical practice will be challenging, with significant issues to overcome (i.e., regulatory, reimbursement). Medico-legal issues will also need to be addressed. With the exception of an AI system that is already available in selected countries (GI Genius; Medtronic, Minneapolis, MN, USA), the majority of the technology is still in its infancy and has not yet been proven to reach a sufficient diagnostic performance to be adopted in the clinical practice. However, larger players will enter the arena of AI in the next few months.
-
Citations
Citations to this article as recorded by- “AI for the new GI”: What role does artificial intelligence have in early colonoscopy training?
Lawrence Hookey
Gastrointestinal Endoscopy.2024; 99(1): 100. CrossRef - Colonoscopy Quality, Innovation, and the Assessment of New Technology
Sanjay R.V. Gadi, Sriya S. Muralidharan, Jeremy R. Glissen Brown
Techniques and Innovations in Gastrointestinal Endoscopy.2024; 26(2): 177. CrossRef - GI genius endoscopy module: a clinical profile
Alberto Savino, Emanuele Rondonotti, Simone Rocchetto, Alessandra Piagnani, Niccolò Bina, Pasquale Di Domenico, Francesco Segatta, Franco Radaelli
Expert Review of Medical Devices.2024; 21(5): 359. CrossRef - Real-time artificial intelligence (AI)-aided endoscopy improves adenoma detection rates even in experienced endoscopists: a cohort study in Singapore
Frederick H. Koh, Jasmine Ladlad, Fung-Joon Foo, Winson J. Tan, Sharmini S. Sivarajah, Leonard M. L. Ho, Jia-Lin Ng, Frederick H. Koh, Cheryl Chong, Darius Aw, Juinn-Haur Kam, Alvin Y. H. Tan, Choon-Chieh Tan, Baldwin P. M. Yeung, Wai-Keong Wong, Bin-Chet
Surgical Endoscopy.2023; 37(1): 165. CrossRef - Systematic meta-analysis of computer-aided detection to detect early esophageal cancer using hyperspectral imaging
Wei-Chih Liao, Arvind Mukundan, Cleorita Sadiaza, Yu-Ming Tsao, Chien-Wei Huang, Hsiang-Chen Wang
Biomedical Optics Express.2023; 14(8): 4383. CrossRef - Detecting colorectal lesions with image-enhanced endoscopy: an updated review from clinical trials
Mizuki Nagai, Sho Suzuki, Yohei Minato, Fumiaki Ishibashi, Kentaro Mochida, Ken Ohata, Tetsuo Morishita
Clinical Endoscopy.2023; 56(5): 553. CrossRef - Accuracy of polyp characterization by artificial intelligence and endoscopists: a prospective, non-randomized study in a tertiary endoscopy center
Sebastian Baumer, Kilian Streicher, Saleh A. Alqahtani, Dominic Brookman-Amissah, Monika Brunner, Christoph Federle, Klaus Muehlenberg, Lukas Pfeifer, Andrea Salzberger, Wolfgang Schorr, Jozef Zustin, Oliver Pech
Endoscopy International Open.2023; 11(09): E818. CrossRef - AI-powered medical devices for practical clinicians including the diagnosis of colorectal polyps
Donghwan Kim, Eunsun Kim
Journal of the Korean Medical Association.2023; 66(11): 658. CrossRef - Artificial Intelligence in Colonoscopic Polyp Detection and Characterization: Merging Computer Technology and Endoscopic Skill for Better Patient Care
Uday C. Ghoshal, Saikat Chakrabarti, Mahesh K. Goenka
Journal of Digestive Endoscopy.2023; 14(04): 239. CrossRef - Diagnostic accuracy of a novel artificial intelligence system for adenoma detection in daily practice: a prospective nonrandomized comparative study
Carolin Zippelius, Saleh A. Alqahtani, Jörg Schedel, Dominic Brookman-Amissah, Klaus Muehlenberg, Christoph Federle, Andrea Salzberger, Wolfgang Schorr, Oliver Pech
Endoscopy.2022; 54(05): 465. CrossRef - Development and validation of a deep learning-based algorithm for colonoscopy quality assessment
Yuan-Yen Chang, Pai-Chi Li, Ruey-Feng Chang, Yu-Yao Chang, Siou-Ping Huang, Yang-Yuan Chen, Wen-Yen Chang, Hsu-Heng Yen
Surgical Endoscopy.2022; 36(9): 6446. CrossRef - Innovation in Gastroenterology—Can We Do Better?
Eyal Klang, Shelly Soffer, Abraham Tsur, Eyal Shachar, Adi Lahat
Biomimetics.2022; 7(1): 33. CrossRef - Hybrid and Deep Learning Approach for Early Diagnosis of Lower Gastrointestinal Diseases
Suliman Mohamed Fati, Ebrahim Mohammed Senan, Ahmad Taher Azar
Sensors.2022; 22(11): 4079. CrossRef - No-Code Platform-Based Deep-Learning Models for Prediction of Colorectal Polyp Histology from White-Light Endoscopy Images: Development and Performance Verification
Eun Jeong Gong, Chang Seok Bang, Jae Jun Lee, Seung In Seo, Young Joo Yang, Gwang Ho Baik, Jong Wook Kim
Journal of Personalized Medicine.2022; 12(6): 963. CrossRef - Impact of the Volume and Distribution of Training Datasets in the Development of Deep-Learning Models for the Diagnosis of Colorectal Polyps in Endoscopy Images
Eun Jeong Gong, Chang Seok Bang, Jae Jun Lee, Young Joo Yang, Gwang Ho Baik
Journal of Personalized Medicine.2022; 12(9): 1361. CrossRef - Preparation of image databases for artificial intelligence algorithm development in gastrointestinal endoscopy
Chang Bong Yang, Sang Hoon Kim, Yun Jeong Lim
Clinical Endoscopy.2022; 55(5): 594. CrossRef - Artificial Intelligence-Based Colorectal Polyp Histology Prediction: High Accuracy in Larger Polyps
Naoki Muguruma, Tetsuji Takayama
Clinical Endoscopy.2022; 55(1): 45. CrossRef - Artificial Intelligence and Advanced Melanoma: Treatment Management Implications
Antonino Guerrisi, Italia Falcone, Fabio Valenti, Marco Rao, Enzo Gallo, Sara Ungania, Maria Teresa Maccallini, Maurizio Fanciulli, Pasquale Frascione, Aldo Morrone, Mauro Caterino
Cells.2022; 11(24): 3965. CrossRef - Clinical feasibility of panintestinal (or panenteric) capsule endoscopy: a systematic review
Pablo Cortegoso Valdivia, Alfonso Elosua, Charles Houdeville, Marco Pennazio, Ignacio Fernández-Urién, Xavier Dray, Ervin Toth, Rami Eliakim, Anastasios Koulaouzidis
European Journal of Gastroenterology & Hepatology.2021; 33(7): 949. CrossRef - Predictors of Positive Video Capsule Endoscopy Findings for Chronic Unexplained Abdominal Pain: Single-Center Retrospective Study and Meta-Analysis
Wonshik Kim, Beomjae Lee, Ahyoung Yoo, Seunghan Kim, Moonkyung Joo, Jong-Jae Park
Diagnostics.2021; 11(11): 2123. CrossRef
- “AI for the new GI”: What role does artificial intelligence have in early colonoscopy training?
- 5,182 View
- 254 Download
- 20 Web of Science
- 20 Crossref

Original Article
- Comparative Study of Narrow-Band Imaging and i-scan for Predicting the Histology of Intermediate-to-Large Colorectal Polyps: A Prospective, Randomized Pilot Study
- Joon Seop Lee, Seong Woo Jeon, Yong Hwan Kwon
- Clin Endosc 2021;54(6):881-887. Published online January 6, 2021
- DOI: https://doi.org/10.5946/ce.2020.257
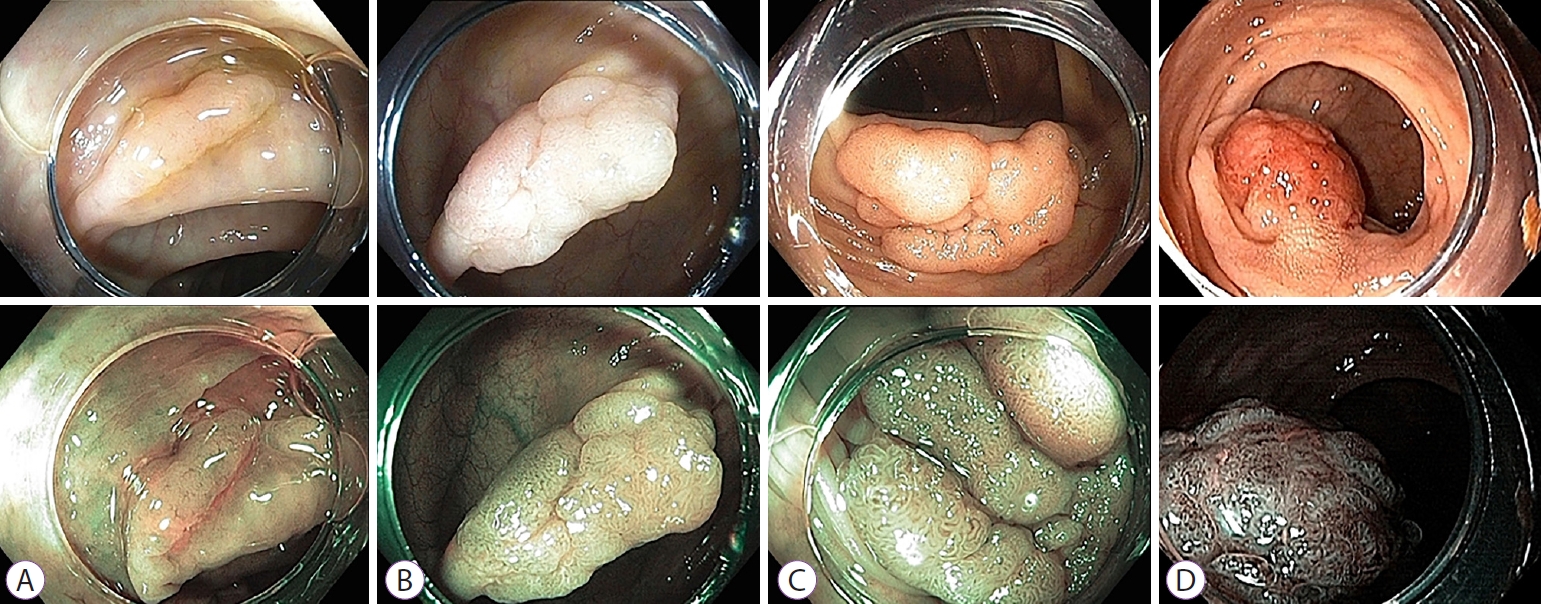
-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader ePub
ePub - Background
/Aims: To date, no reports have compared the diagnostic efficacy of narrow-band imaging (NBI) and i-scan for the histologic prediction of intermediate-to-large colorectal polyps. We aimed to compare the diagnostic accuracy of NBI and i-scan in predicting histology, and their inter-/intra-observer agreement.
Methods
We performed a prospective, randomized study that included 66 patients (NBI, n=33 vs. i-scan, n=33) with colorectal polyps (size >10 mm but <50 mm) who underwent colonoscopic resection. During the procedure, three endoscopists documented their prediction using the Japan NBI Expert Team (JNET) classification. Two months after study completion, the endoscopists reviewed still images and video clips for analysis.
Results
The overall diagnostic accuracies in the NBI and i-scan groups were 73.7% (73/99) and 75.8% (75/99), respectively, and there was no statistical significance between the two groups (p=0.744). The JNET classification as applied to NBI and i-scan showed substantial inter-observer agreement (NBI κ-value 0.612, p=0.001 vs. i-scan κ-value 0.662, p=0.002). Additionally, the κ-values of intra-observer agreement were in the range of 0.385–0.660 with NBI and 0.364–0.741 with i-scan.
Conclusions
NBI and i-scan have similar diagnostic accuracies for the histologic prediction of intermediate-to-large colorectal polyps. Furthermore, the inter-/intra-observer agreement was acceptable for both modalities when the JNET classification was applied. -
Citations
Citations to this article as recorded by- Ultra-minimally invasive endoscopic techniques and colorectal diseases: Current status and its future
Nalini Kanta Ghosh, Ashok Kumar
Artificial Intelligence in Gastrointestinal Endoscopy.2024;[Epub] CrossRef - The Utility of Narrow-Band Imaging International Colorectal Endoscopic Classification in Predicting the Histologies of Diminutive Colorectal Polyps Using I-Scan Optical Enhancement: A Prospective Study
Yeo Wool Kang, Jong Hoon Lee, Jong Yoon Lee
Diagnostics.2023; 13(16): 2720. CrossRef - Detecting colorectal lesions with image-enhanced endoscopy: an updated review from clinical trials
Mizuki Nagai, Sho Suzuki, Yohei Minato, Fumiaki Ishibashi, Kentaro Mochida, Ken Ohata, Tetsuo Morishita
Clinical Endoscopy.2023; 56(5): 553. CrossRef - Classification and endoscopic diagnosis of colorectal polyps
Ji Hyun Kim, Sung Chul Park
Journal of the Korean Medical Association.2023; 66(11): 633. CrossRef - Usefulness of optical enhancement endoscopy combined with magnification to improve detection of intestinal metaplasia in the stomach
Sergio Sobrino-Cossío, Oscar Teramoto-Matsubara, Fabian Emura, Raúl Araya, Vítor Arantes, Elymir S. Galvis-García, Marisi Meza-Caballero, Blanca Sinahi García-Aguilar, Arturo Reding-Bernal, Noriya Uedo
Endoscopy International Open.2022; 10(04): E441. CrossRef - Interventions to improve adenoma detection rates for colonoscopy
Aasma Shaukat, Anne Tuskey, Vijaya L. Rao, Jason A. Dominitz, M. Hassan Murad, Rajesh N. Keswani, Fateh Bazerbachi, Lukejohn W. Day
Gastrointestinal Endoscopy.2022; 96(2): 171. CrossRef - A modified fujinon intelligent color enhancement (FICE) in the diagnostics of superficial epithelial neoplasms of the colon
V. A. Duvanskiy, A. V. Belkov
Experimental and Clinical Gastroenterology.2022; (5): 154. CrossRef - Mucosal imaging in colon polyps: New advances and what the future may hold
Edward John Young, Arvinf Rajandran, Hamish Lachlan Philpott, Dharshan Sathananthan, Sophie Fenella Hoile, Rajvinder Singh
World Journal of Gastroenterology.2022; 28(47): 6632. CrossRef - Commentary on “Comparative Study of Narrow-Band Imaging and i-scan for Predicting the Histology of Intermediate-to-Large Colorectal Polyps: A Prospective, Randomized Pilot Study”
Yunho Jung, Masayuki Kato
Clinical Endoscopy.2021; 54(6): 781. CrossRef
- Ultra-minimally invasive endoscopic techniques and colorectal diseases: Current status and its future
- 4,427 View
- 145 Download
- 8 Web of Science
- 9 Crossref

Case Report
- Fatal Necrotizing Fasciitis Following Uncomplicated Colonoscopic Polypectomy: A Case Report
- Min Kyu Chae, Sang Youn Shin, Min Seob Kwak, Jin Young Yoon, Ha Il Kim, Jae Myung Cha
- Clin Endosc 2021;54(2):280-284. Published online December 11, 2020
- DOI: https://doi.org/10.5946/ce.2020.117

-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader ePub
ePub - Necrotizing fasciitis (NF) is a life-threatening infection that can be caused by various procedures or surgery and may develop in healthy elderly patients. Here, we report a case of a 66-year-old man with diabetes mellitus who underwent colonoscopic polypectomy, without complications. However, he visited the emergency department 24 hours after the procedure complaining of abdominal pain. Abdominopelvic computed tomography revealed multiple air bubbles in the right lateral abdominal muscles. After a diagnosis of NF was made, immediate surgical debridement was performed. However, despite three sessions of extensive surgical debridement and best supportive care at the intensive care unit, the patient died because of sepsis and NF-associated multiple-organ failure. In conclusion, physicians should pay special attention to the possibility of NF if a patient with risk factors for NF develops sepsis after colonoscopic polypectomy.
- 3,314 View
- 112 Download

Original Articles
- Changes in Policy and Endoscopic Procedures during the 2019 Coronavirus Disease Outbreak: A Single Center Experience
- Adi Lahat, Avidan Benjamin
- Clin Endosc 2021;54(1):48-54. Published online November 6, 2020
- DOI: https://doi.org/10.5946/ce.2020.132

-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader ePub
ePub - Background
/Aims: The coronavirus disease-19 (COVID-19) pandemic forced endoscopy units to enact major changes on daily practice and policy. The Chaim Sheba Medical Center is a tertiary referral center located in the center of Israel, and serves cities with high infection rates. Our aim was to review the policies enacted during this outbreak and study their influence on the performance of endoscopic procedures.
Methods
Following the revision of work protocols, personnel were divided into two permanent and physically separate working groups and screening procedures were rescheduled. Relevant data including the number of endoscopic examinations, type of procedure performed, and patient referrals and indications were taken from a computerized database and evaluated. The study included data for January–March 2018–2020, and a comparison among the data from each year was performed.
Results
As of March 2020, the total number of endoscopic examinations performed reduced by 44% (p<0.0001) as compared to previous years, gastroscopy examinations reduced by 39% (p=0.02), and lower endoscopy procedures reduced by 57% (p<0.0001). Meanwhile, the number of advanced endoscopic procedures performed remained consistent with previous years. The indications for performance of gastroscopy and lower endoscopy were different in March 2020, while these remained unchanged for advanced endoscopic procedures.
Conclusions
The current policy appears to serve both our initial goals: protecting personnel and patients’ safety and minimizing potential damage from delayed endoscopic procedures. A longer term follow-up study is needed in order to fully analyze our results. -
Citations
Citations to this article as recorded by- Association of COVID-19 Pandemic with Colorectal Cancer Screening: Impact of Race/Ethnicity and Social Vulnerability
Muhammad Muntazir Mehdi Khan, Muhammad Musaab Munir, Selamawit Woldesenbet, Yutaka Endo, Mujtaba Khalil, Diamantis Tsilimigras, Alan Harzman, Emily Huang, Matthew Kalady, Timothy M. Pawlik
Annals of Surgical Oncology.2024; 31(5): 3222. CrossRef - Setting up a three‐stage pre‐endoscopy triage during the coronavirus disease 2019 pandemic: A multicenter observational study
Tao‐Chieh Liu, Chen‐Ling Peng, Fang‐Yu Hsu, Li‐Chun Chang, Hsiu‐Po Wang, Wei‐Kuo Chang
DEN Open.2023;[Epub] CrossRef - Impact of the COVID-19 Pandemic on Colorectal Cancer Screening: a Systematic Review
Afrooz Mazidimoradi, Azita Tiznobaik, Hamid Salehiniya
Journal of Gastrointestinal Cancer.2022; 53(3): 730. CrossRef - The impact of the COVID-19 pandemic on colorectal and gastric cancer diagnosis, disease stage and mortality
Naim Abu-Freha, Reut Hizkiya, Muhammad Abu-Abed, Tal Michael, Binil Mathew Jacob, Keren Rouvinov, Doron Schwartz, Avraham Reshef, Uri Netz, Ilia Pinsk, Ohad Etzion
Frontiers in Medicine.2022;[Epub] CrossRef - The Dramatic Change in Endoscopic Activities Following the Coronavirus Disease 2019 Outbreak. Is It Evolution?
Kook Hyun Kim
Clinical Endoscopy.2021; 54(3): 445. CrossRef - Protecting Your Endoscopy Unit during the COVID-19 Pandemic
Hyeong Ho Jo, Eun Young Kim
The Korean Journal of Helicobacter and Upper Gastrointestinal Research.2021; 21(3): 239. CrossRef - Capsule Endoscopy in Crohn’s Disease—From a Relative Contraindication to Habitual Monitoring Tool
Adi Lahat, Ido Veisman
Diagnostics.2021; 11(10): 1737. CrossRef
- Association of COVID-19 Pandemic with Colorectal Cancer Screening: Impact of Race/Ethnicity and Social Vulnerability
- 4,968 View
- 123 Download
- 6 Web of Science
- 7 Crossref

- Comparison of Fentanyl versus Meperidine in Combination with Midazolam for Sedative Colonoscopy in Korea
- Gwan Woo Hong, Jun Kyu Lee, Jung Hyeon Lee, Ji Hun Bong, Sung Hun Choi, Hyeki Cho, Ji Hyung Nam, Dong Kee Jang, Hyoun Woo Kang, Jae Hak Kim, Yun Jeong Lim, Moon Soo Koh, Jin Ho Lee
- Clin Endosc 2020;53(5):562-567. Published online July 3, 2020
- DOI: https://doi.org/10.5946/ce.2020.022
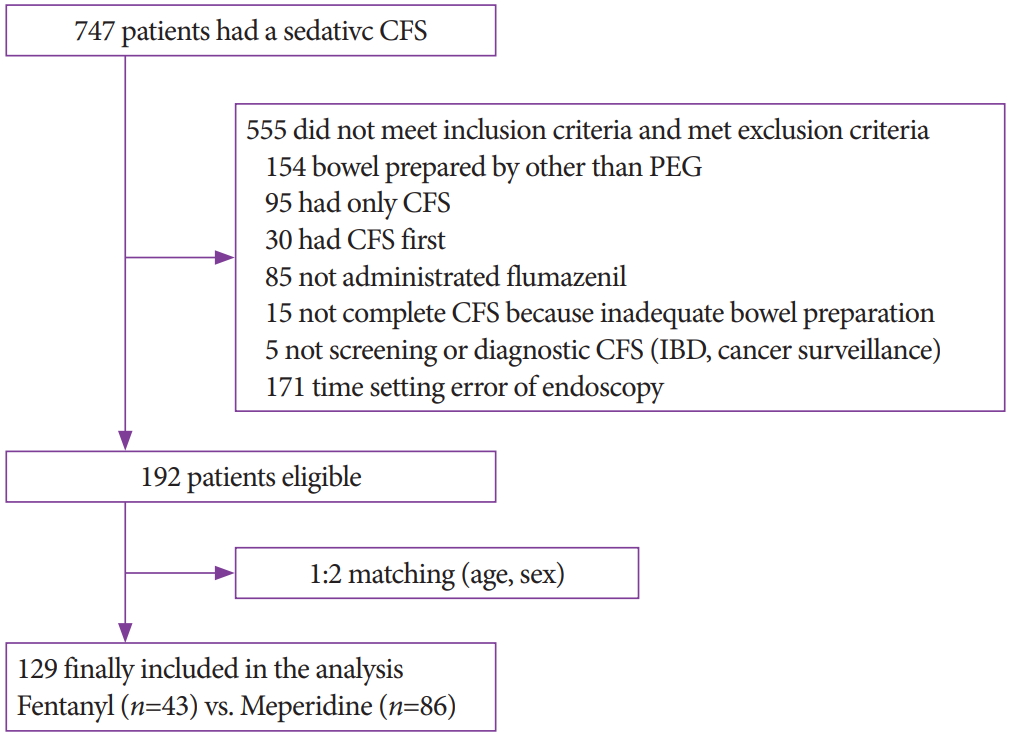
-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader ePub
ePub - Background
/Aims: Combination of midazolam and opioids is used widely for endoscopic sedation. Compared with meperidine, fentanyl is reportedly associated with rapid recovery, turnover rate of endoscopy room, and quality of endoscopy. We compared fentanyl with meperidine when combined with midazolam for sedative colonoscopy.
Methods
A retrospective, cross-sectional, 1:2 matching study was conducted. Induction and recovery time were compared as the primary outcomes. Moreover, cecal intubation time, withdrawal time, total procedure time of colonoscopy, paradoxical reaction, adenoma detection rate, and adverse effect of midazolam or opioids were assessed as the secondary outcomes.
Results
A total of 129 subjects (43 fentanyl vs. 86 meperidine) were included in the analysis. The fentanyl group showed significantly more rapid induction time (4.5±2.7 min vs. 7.5±4.7 min, p<0.001), but longer recovery time (59.5±25.6 min vs. 50.3±10.9 min, p=0.030) than the meperidine group. In multivariate analysis, the induction time of the fentanyl group was 3.40 min faster (p<0.001), but the recovery time was 6.38 min longer (p=0.046) than that of the meperidine group. There was no difference in withdrawal time and adenoma detection rate between the two groups.
Conclusions
The fentanyl group had more rapid sedation induction time but longer recovery time than the meperidine group. -
Citations
Citations to this article as recorded by- Efficacy and safety of EBUS‐TBNA under conscious sedation with meperidine and midazolam
Roberto Piro, Eleonora Casalini, Matteo Fontana, Carla Galeone, Patrizia Ruggiero, Sofia Taddei, Giulia Ghidoni, Giulia Patricelli, Nicola Facciolongo
Thoracic Cancer.2022; 13(4): 533. CrossRef - Propofol Alone versus Propofol in Combination with Midazolam for Sedative Endoscopy in Patients with Paradoxical Reactions to Midazolam
Ji Hyung Nam, Dong Kee Jang, Jun Kyu Lee, Hyoun Woo Kang, Byung-Wook Kim, Byung Ik Jang
Clinical Endoscopy.2022; 55(2): 234. CrossRef - Efficacy of Analgesic Propofol/Esketamine and Propofol/Fentanyl for Painless Induced Abortion: A Randomized Clinical Trial
Naixing Xin, Wei Yan, Shuangfen Jin, Min Tang
BioMed Research International.2022; 2022: 1. CrossRef - Endoscopist-Driven Sedation Practices in South Korea: Re-evaluation Considering the Nationwide Survey in 2019
Seon-Young Park, Jun Kyu Lee, Chang-Hwan Park, Byung-Wook Kim, Chang Kyun Lee, Hong Jun Park, Byung Ik Jang, Dong Uk Kim, Jin Myung Park, Jae Min Lee, Young Sin Cho, Hyung Ku Chon, Seung Young Seo, Woo Hyun Paik
Gut and Liver.2022; 16(6): 899. CrossRef - Drugs used for sedation in gastrointestinal endoscopy
Jun Kyu Lee
Journal of the Korean Medical Association.2022; 65(11): 735. CrossRef - Risk Factors for Prolonged Hospital Stay after Endoscopy
Toshihiro Nishizawa, Shuntaro Yoshida, Osamu Toyoshima, Tatsuya Matsuno, Masataka Irokawa, Toru Arano, Hirotoshi Ebinuma, Hidekazu Suzuki, Takanori Kanai, Kazuhiko Koike
Clinical Endoscopy.2021; 54(6): 851. CrossRef
- Efficacy and safety of EBUS‐TBNA under conscious sedation with meperidine and midazolam
- 7,731 View
- 127 Download
- 6 Web of Science
- 6 Crossref

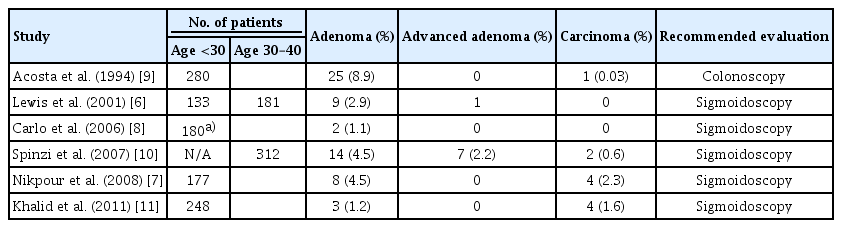
- Endoscopic Findings in Patients Under the Age of 40 Years with Hematochezia in Singapore
- Man Hon Tang, Fung Joon Foo, Chee Yung Ng
- Clin Endosc 2020;53(4):466-470. Published online June 18, 2020
- DOI: https://doi.org/10.5946/ce.2019.029
-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader ePub
ePub - Background
/Aims: Sigmoidoscopy is performed in most medical centers to evaluate the distal colons of young adults presenting with hematochezia who are at risk of developing proximal lesions. Colonoscopies offer more complete evaluations but are associated with a higher incidence of complications and possible low yield.
Methods
An analysis was conducted on colonoscopies performed in our center on patients 40 years of age or younger. The study population was sub-divided into 2 age groups for analysis: <30 years of age and 30–39 years of age.
Results
We recruited 453 patients for the study. Patients were 115 and 338 individuals that were <30 and 30–39 years of age, respectively. Hemorrhoids was identified as the cause of bleeding in the majority of cases. The overall incidence of polyps was 6.5%; this was significantly higher in the 30–39 age group (7.4% vs. 1.7%, p=0.026). There were two cases of advanced/malignant polyps. While the majority of the polyps were in the distal colon, 28% of the polyps in the older age group were found in the proximal colon. There was one case of colonic perforation.
Conclusions
Colonic polyps are more prevalent in patients aged 30–39. Colonoscopies should be considered for patients over the age of 30 with rectal bleeding. -
Citations
Citations to this article as recorded by- Comparing efficacy and factors of postoperative bleeding in endoscopic mucosal resection vs coagulation for intestinal polyps
Zhiang Li, Fei Yu, Chaoqian Wang, Zhang Du
Medicine.2023; 102(37): e34941. CrossRef - The role of colonoscopy in young patients with rectal bleeding: a systematic review and meta-analysis
Tuane Colles, Patrícia K. Ziegelmann, Daniel C. Damin
International Journal of Colorectal Disease.2023;[Epub] CrossRef - Usefulness of Colonoscopy in Patients with Hematochezia Aged under 40 Years
Hee Chan Yang, Sang Wook Kim
Clinical Endoscopy.2020; 53(4): 385. CrossRef
- Comparing efficacy and factors of postoperative bleeding in endoscopic mucosal resection vs coagulation for intestinal polyps
- 4,133 View
- 82 Download
- 2 Web of Science
- 3 Crossref

Review
- Trends of Colorectal Cancer Prevalence in Kazakhstan Related to Screening
- Alma Zhylkaidarova, Dilyara Kaidarova, Kanat Batyrbekov, Oxana Shatkovskaya, Dinara Begimbetova
- Clin Endosc 2021;54(1):32-37. Published online May 25, 2020
- DOI: https://doi.org/10.5946/ce.2019.198
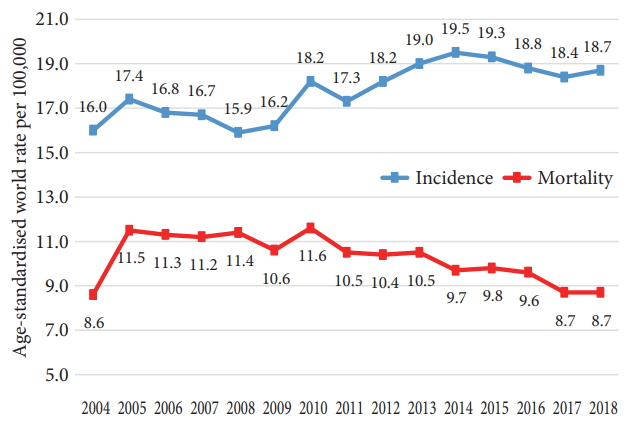
-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader ePub
ePub - We carried out an analysis of the total incidence of colon cancer throughout Kazakhstan. Retrospectively, according to the regional reports on endoscopic screening, the study showed an increase in the age-related incidence of colorectal cancer (CRC) cases from 2004–2008 to 2009–2014. The peak of morbidity in both periods was noted in the age category of >70 years. The indicators of the territorial distribution of CRC incidence make it possible to divide the regions into areas with low or high rates of CRC. Specific indicators showed newly diagnosed cases of CRC stages I, II, III, and IV in 2004–2018. The incidence rates of stages I and II showed a two-fold increase (35%–67.4%) and the incidence of stage IV showed a decline from 19.3% to 13.1% and of stage III from 45.7% to 19.5% from 2004 to 2018, respectively. An analysis of CRC incidence throughout Kazakhstan showed an increase in the overall incidence. Since population-based CRC screening was introduced in 2011, the morbidity was found to increase for stages I and II.
-
Citations
Citations to this article as recorded by- Colorectal cancer’s burden attributable to a diet high in processed meat in the Belt and Road Initiative countries
Gu Liu, Chang-Min Li, Fei Xie, Qi-Lai Li, Liang-Yan Liao, Wen-Jun Jiang, Xiao-Pan Li, Guan-Ming Lu
World Journal of Gastrointestinal Oncology.2024; 16(1): 182. CrossRef - Serum Interleukins 8, 17, and 33 as Potential Biomarkers of Colon Cancer
Constantin-Dan Tâlvan, Liviuța Budișan, Elena-Teodora Tâlvan, Valentin Grecu, Oana Zănoagă, Cosmin Mihalache, Victor Cristea, Ioana Berindan-Neagoe, Călin Ilie Mohor
Cancers.2024; 16(4): 745. CrossRef - Kazakh version of the beck depression inventory: Validation study in female cancer patients
Indira Karibayeva, Botagoz Turdaliyeva, Nor Zuraida Zainal, Fatima Bagiyarova, Dinara Kussainova
Heliyon.2023; 9(7): e18146. CrossRef - Colorectal Cancer Screening with Computed Tomography Colonography: Single Region Experience in Kazakhstan
Jandos Amankulov, Dilyara Kaidarova, Zhamilya Zholdybay, Marianna Zagurovskaya, Nurlan Baltabekov, Madina Gabdullina, Akmaral Ainakulova, Dias Toleshbayev, Alexandra Panina, Elvira Satbayeva, Zhansaya Kalieva
Clinical Endoscopy.2022; 55(1): 101. CrossRef - Association of four genetic variants with colorectal cancer in Kazakhstan population
Yevgeniya Kolesnikova, Dmitriy Babenko, Irina Kadyrova, Svetlana Kolesnichenko, Lyudmila Akhmaltdinova, Ilya Korshukov, Naylya Kabildina, Valentina Sirota, Vera Zhumaliyeva, Dana Taizhanova, Dmitriy Vazenmiller, Anar Turmukhambetova
Oncotarget.2021; 12(21): 2215. CrossRef
- Colorectal cancer’s burden attributable to a diet high in processed meat in the Belt and Road Initiative countries
- 6,135 View
- 164 Download
- 4 Web of Science
- 5 Crossref

Original Article
- Comparison of Oral Sulfate Solution and Polyethylene Glycol Plus Ascorbic Acid on the Efficacy of Bowel Preparation
- Ji Hyung Nam, Seok Bo Hong, Yun Jeong Lim, Seongju Lee, Hyoun Woo Kang, Jae Hak Kim, Jin Ho Lee
- Clin Endosc 2020;53(5):568-574. Published online April 24, 2020
- DOI: https://doi.org/10.5946/ce.2019.209
-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader ePub
ePub - Background
/Aims: The quality of bowel preparation is one of the quality indicators for colonoscopy. The aim of this study was to compare the efficacy of oral sulfate solution (OSS) and polyethylene glycol plus ascorbic acid (PEG-AA) for bowel preparation.
Methods
The study involved 167 patients who underwent diagnostic colonoscopies. Inadequate bowel preparation was defined as any score of ≤1 in each colon section based on the Boston Bowel Preparation Scale. Multivariate logistic regression was used to compare the efficacy of OSS and PEG-AA. Subgroup analyses were performed based on patient characteristics.
Results
Overall, 106 (63.5%) patients received OSS, and 61 (36.5%) patients received PEG-AA. The rate of inadequate bowel preparation was 12.3% in patients receiving OSS and 32.8% in patients receiving PEG-AA (p=0.001). OSS (odds ratio [OR] = 0.26; p=0.003) and morning examination (OR=0.11; p=0.038) were significantly associated with efficient bowel preparation. The efficacy of OSS compared with PEG-AA was only significant in patients ≥50 years of age vs. <50 years of age (OR=0.13; p=0.001 vs. OR=0.96; p=0.959) and female vs. male patients (OR=0.06; p=0.002 vs. OR=0.58; p=0.339).
Conclusions
OSS was significantly more efficient for bowel preparation than PEG-AA, especially in patients ≥50 years of age and female patients. Morning examination led to a good quality of bowel preparation, irrespective of the preparation regimen. -
Citations
Citations to this article as recorded by- Observation of the application effect of low-volume polyethylene glycol electrolyte lavage solution (PEG-ELS) combined with ascorbic acid tablets in bowel preparation for colonoscopy in hospitalized patients
Le-Can Wu, En-Dian Zheng, Hao-Yue Sun, Xi-Zhou Lin, Ju-Yi Pan, Xiao-Xiao Lin
Frontiers in Oncology.2023;[Epub] CrossRef - Comparison of the efficacy and safety of an oral sulfate solution and 3-L polyethylene glycol on bowel preparation before colonoscopy: a phase III multicenter randomized controlled trial
Peng Pan, Shengbing Zhao, Shuling Wang, Yihang Song, Lun Gu, Youxiang Chen, Jiangrong Zhao, Lungen Lu, Xiuling Li, Hongzhi Xu, Gaifang Liu, Yanqing Li, Le Xu, Jiangbin Wang, Zhaoshen Li, Yu Bai
Gastrointestinal Endoscopy.2023; 98(6): 977. CrossRef - Randomized trial of oral sulfate solution versus polyethylene glycol–ascorbic acid for bowel cleansing in elderly people
Seung‐Joo Nam, Sung Chul Park, Sung Joon Lee, Sang Hoon Lee, Ji Hyun Kim, Chang Seok Bang, Hyun Il Seo
Journal of Gastroenterology and Hepatology.2022; 37(2): 319. CrossRef - Comparison of 2 L Polyethylene Glycol Plus Ascorbic Acid and 4 L Polyethylene Glycol in Elderly Patients Aged 60–79: A Prospective Randomized Study
Sung Hoon Jung, Chul-Hyun Lim, Tae-Geun Gweon, Jinsu Kim, Jung Hwan Oh, Kyu-Tae Yoon, Jee Young An, Jeong‑Seon Ji, Hwang Choi
Digestive Diseases and Sciences.2022; 67(10): 4841. CrossRef - Oral sulfate solution benefits polyp and adenoma detection during colonoscopy: Meta‐analysis of randomized controlled trials
Cheng Chen, Mengyang Shi, Zhongli Liao, Weiqing Chen, Yongzhong Wu, Xu Tian
Digestive Endoscopy.2022; 34(6): 1121. CrossRef - Efficacy, safety and tolerability of oral sulphate tablet for bowel preparation in patients with inflammatory bowel disease: A multicentre randomized controlled study
Kyeong Ok Kim, Eun Young Kim, Yoo Jin Lee, Hyun Seok Lee, Eun Soo Kim, Yun Jin Chung, Byung Ik Jang, Sung Kook Kim, Chang Heon Yang
Journal of Crohn's and Colitis.2022; 16(11): 1706. CrossRef - How to Choose the Optimal Bowel Preparation Regimen for Colonoscopy
Ji Eun Na, Eun Ran Kim
The Ewha Medical Journal.2021; 44(4): 122. CrossRef - Oral Sulfate Solution is as Effective as 2 L Polyethylene Glycol Plus Ascorbic Acid
Sung Hyun Shin, Kwang An Kwon
Clinical Endoscopy.2020; 53(5): 503. CrossRef
- Observation of the application effect of low-volume polyethylene glycol electrolyte lavage solution (PEG-ELS) combined with ascorbic acid tablets in bowel preparation for colonoscopy in hospitalized patients
- 4,835 View
- 175 Download
- 9 Web of Science
- 8 Crossref

Focused Review Series: Application of Artificial Intelligence in GI Endoscopy
- Artificial Intelligence in Gastrointestinal Endoscopy
- Alexander P. Abadir, Mohammed Fahad Ali, William Karnes, Jason B. Samarasena
- Clin Endosc 2020;53(2):132-141. Published online March 30, 2020
- DOI: https://doi.org/10.5946/ce.2020.038
-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader ePub
ePub - Artificial intelligence (AI) is rapidly integrating into modern technology and clinical practice. Although in its nascency, AI has become a hot topic of investigation for applications in clinical practice. Multiple fields of medicine have embraced the possibility of a future with AI assisting in diagnosis and pathology applications.
In the field of gastroenterology, AI has been studied as a tool to assist in risk stratification, diagnosis, and pathologic identification. Specifically, AI has become of great interest in endoscopy as a technology with substantial potential to revolutionize the practice of a modern gastroenterologist. From cancer screening to automated report generation, AI has touched upon all aspects of modern endoscopy.
Here, we review landmark AI developments in endoscopy. Starting with broad definitions to develop understanding, we will summarize the current state of AI research and its potential applications. With innovation developing rapidly, this article touches upon the remarkable advances in AI-assisted endoscopy since its initial evaluation at the turn of the millennium, and the potential impact these AI models may have on the modern clinical practice. As with any discussion of new technology, its limitations must also be understood to apply clinical AI tools successfully. -
Citations
Citations to this article as recorded by- Artificial intelligence‐aided diagnostic imaging: A state‐of‐the‐art technique in precancerous screening
Yang‐Bor Lu, Si‐Cun Lu, Fu‐Dong Li, Puo‐Hsien Le, Kai‐Hua Zhang, Zi‐Zheng Sun, Yung‐Ning Huang, Yu‐Chieh Weng, Wei‐Ting Chen, Yi‐Wei Fu, Jun‐Bo Qian, Bin Hu, Hong Xu, Cheng‐Tang Chiu, Qin‐Wei Xu, Wei Gong
Journal of Gastroenterology and Hepatology.2024; 39(3): 544. CrossRef - Development of convolutional neural network models that recognize normal anatomic structures during real-time radial-array and linear-array EUS (with videos)
Carlos Robles-Medranda, Jorge Baquerizo-Burgos, Miguel Puga-Tejada, Raquel Del Valle, Juan C. Mendez, Maria Egas-Izquierdo, Martha Arevalo-Mora, Domenica Cunto, Juan Alcívar-Vasquez, Hannah Pitanga-Lukashok, Daniela Tabacelia
Gastrointestinal Endoscopy.2024; 99(2): 271. CrossRef - Repurposing of Drug Aspirin in Colon Cancer: Therapeutic Approach
Vrushali Neve, Abhijeet Kamble, Pawan Karwa
Clinical Cancer Investigation Journal.2024; 13(1): 23. CrossRef - Consensus statements on the current landscape of artificial intelligence applications in endoscopy, addressing roadblocks, and advancing artificial intelligence in gastroenterology
Sravanthi Parasa, Tyler Berzin, Cadman Leggett, Seth Gross, Alessandro Repici, Omer F. Ahmad, Austin Chiang, Nayantara Coelho-Prabhu, Jonathan Cohen, Evelien Dekker, Rajesh N. Keswani, Charles E. Kahn, Cesare Hassan, Nicholas Petrick, Peter Mountney, Jona
Gastrointestinal Endoscopy.2024;[Epub] CrossRef - Colorectal Polyp Detection Model by Using Super-Resolution Reconstruction and YOLO
Shaofang Wang, Jun Xie, Yanrong Cui, Zhongju Chen
Electronics.2024; 13(12): 2298. CrossRef - Artificial intelligence in pancreatic cancer: diagnosis, limitations, and the future prospects—a narrative review
Maanya Rajasree Katta, Pavan Kumar Reddy Kalluru, Divyaraj Amber Bavishi, Maha Hameed, Sai Sudha Valisekka
Journal of Cancer Research and Clinical Oncology.2023; 149(9): 6743. CrossRef - Deep learning-based clinical decision support system for gastric neoplasms in real-time endoscopy: development and validation study
Eun Jeong Gong, Chang Seok Bang, Jae Jun Lee, Gwang Ho Baik, Hyun Lim, Jae Hoon Jeong, Sung Won Choi, Joonhee Cho, Deok Yeol Kim, Kang Bin Lee, Seung-Il Shin, Dick Sigmund, Byeong In Moon, Sung Chul Park, Sang Hoon Lee, Ki Bae Bang, Dae-Soon Son
Endoscopy.2023; 55(08): 701. CrossRef - Update zur Navigation im OP-Saal
Philipp Anthony Wise, Alexander Studier-Fischer, Thilo Hackert, Felix Nickel
Zentralblatt für Chirurgie - Zeitschrift für Allgemeine, Viszeral-, Thorax- und Gefäßchirurgie.2023;[Epub] CrossRef - ДІАГНОСТИКА ДОБРОЯКІСНИХ ПУХЛИН ТОНКОГО КИШЕЧНИКА: СУЧАСНИЙ СТАН ПРОБЛЕМИ
В. Ю. ІЛЬЇНА-СТОГНІЄНКО, О. М. ЧАЙКА
Шпитальна хірургія. Журнал імені Л. Я. Ковальчука.2023; (1): 96. CrossRef - Future Directions in EndoHepatology
Ahmad Najdat Bazarbashi, Lolwa Al-Obaid, Marvin Ryou
Techniques and Innovations in Gastrointestinal Endoscopy.2022; 24(1): 98. CrossRef - El papel emergente de la inteligencia artificial en la endoscopia gastrointestinal: una revisión de la literatura
María José Aguilera-Chuchuca, Sergio A. Sánchez-Luna, Begoña González Suárez, Kenneth Ernest-Suárez, Andres Gelrud, Tyler M. Berzin
Gastroenterología y Hepatología.2022; 45(6): 492. CrossRef - Artificial Intelligence-Based Colorectal Polyp Histology Prediction: High Accuracy in Larger Polyps
Naoki Muguruma, Tetsuji Takayama
Clinical Endoscopy.2022; 55(1): 45. CrossRef - Assessment of the Risk of Severe Dengue Using Intrahost Viral Population in Dengue Virus Serotype 2 Patients via Machine Learning
Su-Jhen Hung, Huey-Pin Tsai, Ya-Fang Wang, Wen-Chien Ko, Jen-Ren Wang, Sheng-Wen Huang
Frontiers in Cellular and Infection Microbiology.2022;[Epub] CrossRef - Artificial intelligence in colorectal cancer screening in patients with inflammatory bowel disease
Kêmily Fuentes Marques, Alana Fuentes Marques, Marina Amorim Lopes, Rodrigo Fedatto Beraldo, Talles Bazeia Lima, Ligia Yukie Sassaki
Artificial Intelligence in Gastrointestinal Endoscopy.2022; 3(1): 1. CrossRef - Weakly supervised end-to-end artificial intelligence in gastrointestinal endoscopy
Lukas Buendgens, Didem Cifci, Narmin Ghaffari Laleh, Marko van Treeck, Maria T. Koenen, Henning W. Zimmermann, Till Herbold, Thomas Joachim Lux, Alexander Hann, Christian Trautwein, Jakob Nikolas Kather
Scientific Reports.2022;[Epub] CrossRef - Automated Detection of Bowel Preparation Scoring and Adequacy With Deep Convolutional Neural Networks
Daniel J Low, Zhuoqiao Hong, Sechiv Jugnundan, Anjishnu Mukherjee, Samir C Grover
Journal of the Canadian Association of Gastroenterology.2022; 5(6): 256. CrossRef - The emerging role of artificial intelligence in gastrointestinal endoscopy: a review
María José Aguilera-Chuchuca, Sergio A. Sánchez-Luna, Begoña González Suárez, Kenneth Ernest-Suárez, Andres Gelrud, Tyler M. Berzin
Gastroenterología y Hepatología (English Edition).2022; 45(6): 492. CrossRef - Artificial Intelligence for Colonoscopy: Past, Present, and Future
Wallapak Tavanapong, JungHwan Oh, Michael A. Riegler, Mohammed Khaleel, Bhuvan Mittal, Piet C. de Groen
IEEE Journal of Biomedical and Health Informatics.2022; 26(8): 3950. CrossRef - Künstliche Intelligenz in der Viszeralmedizin – „brave new world“ oder digitaler Horror?
R. Jakobs, M. Fried, J. Hampe
Der Gastroenterologe.2021; 16(1): 1. CrossRef - Challenges in Crohn’s Disease Management after Gastrointestinal Cancer Diagnosis
Claudio Fiorillo, Carlo Alberto Schena, Giuseppe Quero, Vito Laterza, Daniela Pugliese, Giuseppe Privitera, Fausto Rosa, Tommaso Schepis, Lisa Salvatore, Brunella Di Stefano, Luigi Larosa, Laura Maria Minordi, Luigi Natale, Giampaolo Tortora, Alessandro A
Cancers.2021; 13(3): 574. CrossRef - Robotics and Artificial Intelligence in Gastrointestinal Endoscopy: Updated Review of the Literature and State of the Art
Ivo Boškoski, Beatrice Orlandini, Luigi Giovanni Papparella, Maria Valeria Matteo, Martina De Siena, Valerio Pontecorvi, Guido Costamagna
Current Robotics Reports.2021; 2(1): 43. CrossRef - Use of Endoscopic Images in the Prediction of Submucosal Invasion of Gastric Neoplasms: Automated Deep Learning Model Development and Usability Study
Chang Seok Bang, Hyun Lim, Hae Min Jeong, Sung Hyeon Hwang
Journal of Medical Internet Research.2021; 23(4): e25167. CrossRef - Artificial intelligence in brachytherapy: a summary of recent developments
Susovan Banerjee, Shikha Goyal, Saumyaranjan Mishra, Deepak Gupta, Shyam Singh Bisht, Venketesan K, Kushal Narang, Tejinder Kataria
The British Journal of Radiology.2021;[Epub] CrossRef - Artificial Intelligence in Colorectal Cancer Screening, Diagnosis and Treatment. A New Era
Athanasia Mitsala, Christos Tsalikidis, Michail Pitiakoudis, Constantinos Simopoulos, Alexandra K. Tsaroucha
Current Oncology.2021; 28(3): 1581. CrossRef - Predicting Colorectal Cancer Occurrence in IBD
Mehmet Yalchin, Ann-Marie Baker, Trevor A. Graham, Ailsa Hart
Cancers.2021; 13(12): 2908. CrossRef - Usefulness of artificial intelligence in gastric neoplasms
Ji Hyun Kim, Seung-Joo Nam, Sung Chul Park
World Journal of Gastroenterology.2021; 27(24): 3543. CrossRef - Deep neural network approaches for detecting gastric polyps in endoscopic images
Serdar Durak, Bülent Bayram, Tolga Bakırman, Murat Erkut, Metehan Doğan, Mert Gürtürk, Burak Akpınar
Medical & Biological Engineering & Computing.2021; 59(7-8): 1563. CrossRef - Use of artificial intelligence in endoscopic ultrasound evaluation of pancreatic pathologies
Ravinder Mankoo, Ahmad H Ali, Ghassan M Hammoud
Artificial Intelligence in Gastrointestinal Endoscopy.2021; 2(3): 89. CrossRef - Role of Artificial Intelligence in Video Capsule Endoscopy
Ioannis Tziortziotis, Faidon-Marios Laskaratos, Sergio Coda
Diagnostics.2021; 11(7): 1192. CrossRef - Use of Artificial Intelligence to Improve the Quality Control of Gastrointestinal Endoscopy
Ya-qi Song, Xin-li Mao, Xian-bin Zhou, Sai-qin He, Ya-hong Chen, Li-hui Zhang, Shi-wen Xu, Ling-ling Yan, Shen-ping Tang, Li-ping Ye, Shao-wei Li
Frontiers in Medicine.2021;[Epub] CrossRef - Use of artificial intelligence in endoscopic ultrasound evaluation of pancreatic pathologies
Ravinder Mankoo, Ahmad H Ali, Ghassan M Hammoud
Artificial Intelligence in Gastrointestinal Endoscopy.2021; 2(3): 88. CrossRef - Endoscopic diagnosis and treatment of gastric dysplasia and early cancer: Current evidence and what the future may hold
Edward Young, Hamish Philpott, Rajvinder Singh
World Journal of Gastroenterology.2021; 27(31): 5126. CrossRef - Computer-Aided Diagnosis of Diminutive Colorectal Polyps in Endoscopic Images: Systematic Review and Meta-analysis of Diagnostic Test Accuracy
Chang Seok Bang, Jae Jun Lee, Gwang Ho Baik
Journal of Medical Internet Research.2021; 23(8): e29682. CrossRef - Applications of Artificial Intelligence for the Diagnosis of Gastrointestinal Diseases
Silvia Pecere, Sebastian Manuel Milluzzo, Gianluca Esposito, Emanuele Dilaghi, Andrea Telese, Leonardo Henry Eusebi
Diagnostics.2021; 11(9): 1575. CrossRef - Editors' Choice of Noteworthy Clinical Endoscopy Publications in the First Decade
Gwang Ha Kim, Kwang An Kwon, Do Hyun Park, Jimin Han
Clinical Endoscopy.2021; 54(5): 633. CrossRef - Artificial Intelligence in Capsule Endoscopy: A Practical Guide to Its Past and Future Challenges
Sang Hoon Kim, Yun Jeong Lim
Diagnostics.2021; 11(9): 1722. CrossRef - Digital surgery for gastroenterological diseases
Niall Philip Hardy, Ronan Ambrose Cahill
World Journal of Gastroenterology.2021; 27(42): 7240. CrossRef - Choledochoscopy: An update
Tsinrong Lee, Thomas Zheng Jie Teng, Vishal G Shelat
World Journal of Gastrointestinal Endoscopy.2021; 13(12): 571. CrossRef - Prediction of Submucosal Invasion for Gastric Neoplasms in Endoscopic Images Using Deep-Learning
Bum-Joo Cho, Chang Seok Bang, Jae Jun Lee, Chang Won Seo, Ju Han Kim
Journal of Clinical Medicine.2020; 9(6): 1858. CrossRef - The Impact of Artificial Intelligence in the Endoscopic Assessment of Premalignant and Malignant Esophageal Lesions: Present and Future
Daniela Cornelia Lazăr, Mihaela Flavia Avram, Alexandra Corina Faur, Adrian Goldiş, Ioan Romoşan, Sorina Tăban, Mărioara Cornianu
Medicina.2020; 56(7): 364. CrossRef - Techniques to integrate artificial intelligence systems with medical information in gastroenterology
Hong-Yu Jin, Man Zhang, Bing Hu
Artificial Intelligence in Gastrointestinal Endoscopy.2020; 1(1): 19. CrossRef - Assessing the risk of dengue severity using demographic information and laboratory test results with machine learning
Sheng-Wen Huang, Huey-Pin Tsai, Su-Jhen Hung, Wen-Chien Ko, Jen-Ren Wang, Michael R. Holbrook
PLOS Neglected Tropical Diseases.2020; 14(12): e0008960. CrossRef - Emerging use of artificial intelligence in inflammatory bowel disease
Arushi Kohli, Erik A Holzwanger, Alexander N Levy
World Journal of Gastroenterology.2020; 26(44): 6923. CrossRef
- Artificial intelligence‐aided diagnostic imaging: A state‐of‐the‐art technique in precancerous screening
- 8,278 View
- 283 Download
- 32 Web of Science
- 43 Crossref

Case Report
- Acute Pancreatitis: A Rare Post-Colonoscopy Sequela
- Sujit P. Nair, Prasanta Debnath, Suhas Udgirkar, Parmeshwar Junare, Sanjay Chandnani, Shubham Jain, Vinay B. Pawar, Pravin M. Rathi
- Clin Endosc 2020;53(5):611-614. Published online February 12, 2020
- DOI: https://doi.org/10.5946/ce.2019.151
-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader ePub
ePub - Abdominal pain is a common but benign symptom after colonoscopy. We report a case of acute pancreatitis that occurred just after an elective screening colonoscopy; this is a rare event with very few reported cases. A healthy, asymptomatic male underwent screening colonoscopy at our center and developed abdominal pain and emesis after the procedure. An abdominal X-ray ruled out perforation but laboratory tests revealed elevated levels of amylase and lipase. The patient had no etiological risk factors for pancreatitis. The presumed mechanism of pancreatitis in this case is mechanical and pressure trauma from excessive insufflation, external abdominal pressure, and repeated withdrawal of the colonoscope due to tight angulation of the splenic flexure, a structure that is in close proximity to the pancreatic tail. Acute pancreatitis should be considered in the differential diagnosis of patients who present with abdominal pain after colonoscopy once more common etiologies have been excluded.
-
Citations
Citations to this article as recorded by- Aggravated pancreatitis after performing a colonoscopy
Han‐Lin Liao, Tyng‐Yuan Jang
Advances in Digestive Medicine.2024;[Epub] CrossRef - Acute Pancreatitis: A Rare Complication of Colonoscopy
Saima H Shawl, Usama Bilal, Chandra Essar Mal, Veera Durga Vaishnavi Kurra, Romil Singh
Cureus.2022;[Epub] CrossRef - CT imaging findings of complications of optical colonoscopy
Abhishek Keraliya, Hei Shun Yu, Jennifer W. Uyeda
Emergency Radiology.2022; 29(5): 915. CrossRef
- Aggravated pancreatitis after performing a colonoscopy
- 5,866 View
- 108 Download
- 2 Web of Science
- 3 Crossref

Original Article
- Endocuff-Assisted versus Cap-Assisted Colonoscopy Performed by Trainees: A Retrospective Study
- Yutaka Okagawa, Tetsuya Sumiyoshi, Yusuke Tomita, Shutaro Oiwa, Fumihiro Ogata, Takashi Jin, Masahiro Yoshida, Ryoji Fujii, Takeyoshi Minagawa, Kohtaro Morita, Hideyuki Ihara, Michiaki Hirayama, Hitoshi Kondo
- Clin Endosc 2020;53(3):339-345. Published online January 10, 2020
- DOI: https://doi.org/10.5946/ce.2019.124
-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader ePub
ePub - Background
/Aims: The adenoma detection rate (ADR) of screening colonoscopies performed by trainees is often lower than that of colonoscopies performed by experts. The effcacy of cap-assisted colonoscopy (CAC) in adenoma detection is well documented, especially that of CACs performed by trainees. Endocuff, a new endoscopic cap, is reportedly useful for adenoma detection; however, no trials have compared the effcacy of Endocuff-assisted colonoscopy (EAC) and CAC conducted by trainees. Therefore, the present study retrospectively compared the effcacy between EAC and CAC in trainees.
Methods
This was a single-center, retrospective study involving 305 patients who underwent either EAC or CAC performed by three trainees between January and December 2018. We evaluated the ADR, mean number of adenomas detected per patient (MAP), cecal intubation rate, cecal intubation time, and occurrence of complications between the EAC and CAC groups.
Results
The ADR was significantly higher in the EAC group than in the CAC group (54.3% vs. 37.3%, p=0.019), as was the MAP (1.36 vs. 0.74, p=0.003). No significant differences were found between the groups with respect to the cecal intubation rate or cecal intubation time. No major complications occurred in either group.
Conclusions
Our results suggest that EAC exhibits increased ADR and MAP compared to CAC when performed by trainees. -
Citations
Citations to this article as recorded by- The impact of EndoCuff-assisted colonoscopy on the polyp detection rate: A cross-over randomized back-to-back study
Mohammed Sherif Naguib, Ahmed Khairy, Hany Shehab, Hazem Abosheaishaa, Abdel Meguid Kassem
Arab Journal of Gastroenterology.2024; 25(2): 102. CrossRef - Comparative Efficacy of Endoscopic Assist Devices on Colonic Adenoma Detection
Simcha Weissman, Tej I. Mehta, Daniel J. Stein, Kartikeya Tripathi, Nathan Rosenwald, Sindhura Kolli, Muhammad Aziz, Joseph D. Feuerstein
Journal of Clinical Gastroenterology.2022; 56(10): 889. CrossRef - Accessories to Enhance Adenoma Detection Rates: Is the Endocuff Better than the Conventional Cap for Trainees?
Tae-Geun Gweon, Sang-Bum Kang
Clinical Endoscopy.2020; 53(3): 251. CrossRef - Endocuff‐assisted colonoscopy versus cap‐assisted colonoscopy for adenoma detection rate: A meta‐analysis of randomized controlled trials
Ai Li, Jing‐Ze Yang, Xiao‐Xiao Yang, Bing‐Cheng Feng, Ming‐Ming Zhang, Jun‐Yan Qu, Ru‐Chen Zhou, Peng Wang, Li‐Xiang Li, Xiu‐Li Zuo, Yan‐Qing Li
Journal of Gastroenterology and Hepatology.2020; 35(12): 2066. CrossRef
- The impact of EndoCuff-assisted colonoscopy on the polyp detection rate: A cross-over randomized back-to-back study
- 4,664 View
- 121 Download
- 3 Web of Science
- 4 Crossref

Review
- Endoscopic Management of Post-Polypectomy Bleeding
- Aditya Gutta, Mark A. Gromski
- Clin Endosc 2020;53(3):302-310. Published online September 17, 2019
- DOI: https://doi.org/10.5946/ce.2019.062

-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader ePub
ePub - Post-polypectomy bleeding (PPB) is one of the most common complications of endoscopic polypectomy. There are multiple risk factors related to patient and polyp characteristics that should be considered. In most cases, immediate PPB can be effectively managed endoscopically when recognized and managed promptly. Delayed PPB can manifest in a myriad of ways. In severe delayed PPB, resuscitation for hemodynamic stabilization should be prioritized, followed by endoscopic evaluation and therapy once the patient is stabilized. Future areas of research in PPB include the risks of direct oral anticoagulants and of specific electrosurgical settings for hot-snare polypectomy vs. cold-snare polypectomy, benefits of closure of post-polypectomy mucosal defects using through-the-scope clips, and prospective comparative evaluation of newer hemostasis agents such as hemostatic spray powder and over-the-scope clips.
-
Citations
Citations to this article as recorded by- Updates on the Prevention and Management of Post-Polypectomy Bleeding in the Colon
Hisham Wehbe, Aditya Gutta, Mark A. Gromski
Gastrointestinal Endoscopy Clinics of North America.2024; 34(2): 363. CrossRef - Colonoscopic polypectomy of juvenile polyps in children: Experience from a tertiary centre of Bangladesh
Salahuddin Mahmud, Mashud Parvez, Madhabi Baidya, Farhana Tasneem, Ahmed Rashidul Hasan, Tanzila Farhana, Md Jahangir Alam, Syed Shafi Ahmed
Gastroenterology & Endoscopy.2024; 2(1): 1. CrossRef - Review article: Advances in the management of lower gastrointestinal bleeding
Ali A. Alali, Majid A. Almadi, Alan N. Barkun
Alimentary Pharmacology & Therapeutics.2024; 59(5): 632. CrossRef - Endoscopic management of intraprocedural bleeding during endoscopic interventions
Ali A. Alali, Asma A. Alkandari
Best Practice & Research Clinical Gastroenterology.2024; 69: 101912. CrossRef - Effect of Cold Versus Hot Snare Polypectomy on Colon Postpolypectomy Bleeding in Patients with End-Stage Renal Disease: A Retrospective Cohort Study
Hsueh-Chien Chiang, Chien-Ming Chiang, Xi-Zhang Lin, Po-Jun Chen
Digestive Diseases and Sciences.2024;[Epub] CrossRef - Isolated ischaemic appendicitis as a rare complication of selective angioembolization for lower gastrointestinal bleed
Luke S. Crawford, Nadim S. Jafri, Dingle Foote, Melissa M. Felinski, Peter A. Walker, Kulvinder S. Bajwa, Shinil K. Shah
ANZ Journal of Surgery.2023; 93(1-2): 404. CrossRef - JAG consensus statements for training and certification in colonoscopy
Keith Siau, Stavroula Pelitari, Susi Green, Brian McKaig, Arun Rajendran, Mark Feeney, Mo Thoufeeq, John Anderson, Vathsan Ravindran, Paul Hagan, Neil Cripps, Ian L P Beales, Karen Church, Nicholas I Church, Elizabeth Ratcliffe, Said Din, Rupert D Pullan,
Frontline Gastroenterology.2023; 14(3): 201. CrossRef - JAG consensus statements for training and certification in flexible sigmoidoscopy
Keith Siau, Stavroula Pelitari, Susi Green, Brian McKaig, Arun Rajendran, Mark Feeney, Mo Thoufeeq, John Anderson, Vathsan Ravindran, Paul Hagan, Neil Cripps, Ian L P Beales, Karen Church, Nicholas I Church, Elizabeth Ratcliffe, Said Din, Rupert D Pullan,
Frontline Gastroenterology.2023; 14(3): 181. CrossRef - Management of Patients With Acute Lower Gastrointestinal Bleeding: An Updated ACG Guideline
Neil Sengupta, Joseph D. Feuerstein, Vipul Jairath, Amandeep K. Shergill, Lisa L. Strate, Robert J. Wong, David Wan
American Journal of Gastroenterology.2023; 118(2): 208. CrossRef - Korean Guidelines for Postpolypectomy Colonoscopic Surveillance: 2022 revised edition
Su Young Kim, Min Seob Kwak, Soon Man Yoon, Yunho Jung, Jong Wook Kim, Sun-Jin Boo, Eun Hye Oh, Seong Ran Jeon, Seung-Joo Nam, Seon-Young Park, Soo-Kyung Park, Jaeyoung Chun, Dong Hoon Baek, Mi-Young Choi, Suyeon Park, Jeong-Sik Byeon, Hyung Kil Kim, Joo
Intestinal Research.2023; 21(1): 20. CrossRef - A Review of Colonoscopy in Intestinal Diseases
Seung Min Hong, Dong Hoon Baek
Diagnostics.2023; 13(7): 1262. CrossRef - Bleeding After Endoscopic Resection of Colonic Adenomatous Polyps Sized 4-10 mm
Violeta Hristova Janik
PRILOZI.2023; 44(2): 157. CrossRef - Endoscopic management of delayed bleeding after polypectomy of small colorectal polyps: two or more clips may be safe
Xue-Feng Guo, Xiang-An Yu, Jian-Cong Hu, De-Zheng Lin, Jia-Xin Deng, Ming-Li Su, Juan Li, Wei Liu, Jia-Wei Zhang, Qing-Hua Zhong
Gastroenterology Report.2022;[Epub] CrossRef - Clinical progress note: Diagnostic approach to lower gastrointestinal bleeding
Daniel J. Stein, Hyder Said, Joseph D. Feuerstein
Journal of Hospital Medicine.2022; 17(7): 547. CrossRef - Safety of Cold Snare Polypectomy for Small Colorectal Polyps in Patients Receiving Antithrombotic Therapy
Dai Nakamatsu, Tsutomu Nishida, Yoshifumi Fujii, Sho Yamaoka, Naoto Osugi, Aya Sugimoto, Kaori Mukai, Kengo Matsumoto, Masashi Yamamoto, Shiro Hayashi, Sachiko Nakajima
Techniques and Innovations in Gastrointestinal Endoscopy.2022; 24(3): 246. CrossRef - Postcolonoscopy Complications
Jetsen A. Rodriguez-Silva, Justin A. Maykel
Diseases of the Colon & Rectum.2022; 65(5): 622. CrossRef - Expanding rather than closing the wound can rescue the endoscopic procedure when massive bleeding occurs during endoscopic submucosal dissection
Ming-Ching Yuan, Ching-Tai Lee, Kun-Feng Tsai, Chao-Wen Hsu, Chu-Kuang Chou
Endoscopy.2022; 54(S 02): E1036. CrossRef - Korean Guidelines for Postpolypectomy Colonoscopic Surveillance: 2022 Revised Edition
Su Young Kim, Min Seob Kwak, Soon Man Yoon, Yunho Jung, Jong Wook Kim, Sun-Jin Boo, Eun Hye Oh, Seong Ran Jeon, Seung-Joo Nam, Seon-Young Park, Soo-Kyung Park, Jaeyoung Chun, Dong Hoon Baek, Mi-Young Choi, Suyeon Park, Jeong-Sik Byeon, Hyung Kil Kim, Joo
The Korean Journal of Gastroenterology.2022; 80(3): 115. CrossRef - Korean guidelines for postpolypectomy colonoscopic surveillance: 2022 revised edition
Su Young Kim, Min Seob Kwak, Soon Man Yoon, Yunho Jung, Jong Wook Kim, Sun-Jin Boo, Eun Hye Oh, Seong Ran Jeon, Seung-Joo Nam, Seon-Young Park, Soo-Kyung Park, Jaeyoung Chun, Dong Hoon Baek, Mi-Young Choi, Suyeon Park, Jeong-Sik Byeon, Hyung Kil Kim, Joo
Clinical Endoscopy.2022; 55(6): 703. CrossRef - Can prophylactic argon plasma coagulation reduce delayed post‐papillectomy bleeding? A prospective multicenter trial
Jae Kook Yang, Jong Jin Hyun, Tae Hoon Lee, Jun‐Ho Choi, Yun Nah Lee, Jung Wan Choe, Jin‐Seok Park, Chang‐Il Kwon, Seok Jeong, Hong Ja Kim, Jong Ho Moon, Sang‐Heum Park
Journal of Gastroenterology and Hepatology.2021; 36(2): 467. CrossRef - Diagnosis and management of acute lower gastrointestinal bleeding: European Society of Gastrointestinal Endoscopy (ESGE) Guideline
Konstantinos Triantafyllou, Paraskevas Gkolfakis, Ian M. Gralnek, Kathryn Oakland, Gianpiero Manes, Franco Radaelli, Halim Awadie, Marine Camus Duboc, Dimitrios Christodoulou, Evgeny Fedorov, Richard J. Guy, Marcus Hollenbach, Mostafa Ibrahim, Ziv Neeman,
Endoscopy.2021; 53(08): 850. CrossRef - Estimation and influence of blood loss under endoscope for percutaneous endoscopic lumbar discectomy (PELD): a clinical observational study combined with in vitro experiment
Dong Dong Sun, Dan Lv, Wei Zhou Wu, He Fei Ren, Bu He Bao, Qun Liu, Ming Lin Sun
Journal of Orthopaedic Surgery and Research.2020;[Epub] CrossRef
- Updates on the Prevention and Management of Post-Polypectomy Bleeding in the Colon
- 14,676 View
- 589 Download
- 18 Web of Science
- 22 Crossref


 KSGE
KSGE


 First
First Prev
Prev



