Search
- Page Path
- HOME > Search
- Drainage for fluid collections post pancreatic surgery and acute pancreatitis: similar but different?
- Yousuke Nakai, Saburo Matsubara, Tsuyoshi Mukai, Tsuyoshi Hamada, Takashi Sasaki, Hirotoshi Ishiwatari, Susumu Hijioka, Hideyuki Shiomi, Mamoru Takenaka, Takuji Iwashita, Atsuhiro Masuda, Tomotaka Saito, Hiroyuki Isayama, Ichiro Yasuda, for the WONDERFUL study group in Japan
- Received October 3, 2023 Accepted November 1, 2023 Published online May 17, 2024
- DOI: https://doi.org/10.5946/ce.2023.254 [Epub ahead of print]
-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader ePub
ePub - Postoperative pancreatic fistulas (POPFs) are common adverse events that occur after pancreatic surgery. Endoscopic ultrasonography (EUS)-guided drainage (EUS-D) is a first-line treatment, similar to that for pancreatic fluid collection (PFCs) after acute pancreatitis. However, some POPFs do not develop fluid collections depending on the presence or location of the surgical drain, whereas others develop fluid collections, such as postoperative fluid collections (POPFCs). Although POPFCs are similar to PFCs, the strategy and modality for POPF management need to be modified according to the presence of fluid collections, surgical drains, and surgical type. As discussed for PFCs, the indications, timing, and selection of interventions or stents for EUS-D have not been fully elucidated for POPFs. In this review, we discuss the management of POPFs and POPFCs in comparison with PFCs due to acute pancreatitis and summarize the topics that should be addressed in future studies.
- 1,955 View
- 74 Download

- Management of complications related to colorectal endoscopic submucosal dissection
- Tae-Geun Gweon, Dong-Hoon Yang
- Clin Endosc 2023;56(4):423-432. Published online July 27, 2023
- DOI: https://doi.org/10.5946/ce.2023.104

-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader ePub
ePub - Compared to endoscopic mucosal resection (EMR), colonoscopic endoscopic submucosal dissection (C-ESD) has the advantages of higher en bloc resection rates and lower recurrence rates of colorectal neoplasms. Therefore, C-ESD is considered an effective treatment method for laterally spread tumors and early colorectal cancer. However, C-ESD is technically more difficult and requires a longer procedure time than EMR. In addition to therapeutic efficacy and procedural difficulty, safety concerns should always be considered when performing C-ESD in clinical practice. Bleeding and perforation are the main adverse events associated with C-ESD and can occur during C-ESD or after the completion of the procedure. Most bleeding associated with C-ESD can be managed endoscopically, even if it occurs during or after the procedure. More recently, most perforations identified during C-ESD can also be managed endoscopically, unless the mural defect is too large to be sutured with endoscopic devices or the patient is hemodynamically unstable. Delayed perforations are quite rare, but they require surgical treatment more frequently than endoscopically identified intraprocedural perforations or radiologically identified immediate postprocedural perforations. Post-ESD coagulation syndrome is a relatively underestimated adverse event, which can mimic localized peritonitis from perforation. Here, we classify and characterize the complications associated with C-ESD and recommend management options for them.
-
Citations
Citations to this article as recorded by- International Digestive Endoscopy Network consensus on the management of antithrombotic agents in patients undergoing gastrointestinal endoscopy
Seung Joo Kang, Chung Hyun Tae, Chang Seok Bang, Cheol Min Shin, Young-Hoon Jeong, Miyoung Choi, Joo Ha Hwang, Yutaka Saito, Philip Wai Yan Chiu, Rungsun Rerknimitr, Christopher Khor, Vu Van Khien, Kee Don Choi, Ki-Nam Shim, Geun Am Song, Oh Young Lee
Clinical Endoscopy.2024; 57(2): 141. CrossRef - Is there a best choice of equipment for colorectal endoscopic submucosal dissection?
Francesco Cocomazzi, Sonia Carparelli, Nunzia Labarile, Antonio Capogreco, Marco Gentile, Roberta Maselli, Jahnvi Dhar, Jayanta Samanta, Alessandro Repici, Cesare Hassan, Francesco Perri, Antonio Facciorusso
Expert Review of Medical Devices.2024; : 1. CrossRef
- International Digestive Endoscopy Network consensus on the management of antithrombotic agents in patients undergoing gastrointestinal endoscopy
- 1,731 View
- 131 Download
- 4 Web of Science
- 2 Crossref

- Complications of endoscopic resection in the upper gastrointestinal tract
- Takeshi Uozumi, Seiichiro Abe, Mai Ego Makiguchi, Satoru Nonaka, Haruhisa Suzuki, Shigetaka Yoshinaga, Yutaka Saito
- Clin Endosc 2023;56(4):409-422. Published online June 21, 2023
- DOI: https://doi.org/10.5946/ce.2023.024
-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader ePub
ePub - Endoscopic resection (ER) is widely utilized as a minimally invasive treatment for upper gastrointestinal tumors; however, complications could occur during and after the procedure. Post-ER mucosal defect leads to delayed perforation and bleeding; therefore, endoscopic closure methods (endoscopic hand-suturing, the endoloop and endoclip closure method, and over-the-scope clip method) and tissue shielding methods (polyglycolic acid sheets and fibrin glue) are developed to prevent these complications. During duodenal ER, complete closure of the mucosal defect significantly reduces delayed bleeding and should be performed. An extensive mucosal defect that comprises three-quarters of the circumference in the esophagus, gastric antrum, or cardia is a significant risk factor for post-ER stricture. Steroid therapy is considered the first-line option for the prevention of esophageal stricture, but its efficacy for gastric stricture remains unclear. Methods for the prevention and management of ER-related complications in the esophagus, stomach, and duodenum differ according to the organ; therefore, endoscopists should be familiar with ways of preventing and managing organ-specific complications.
-
Citations
Citations to this article as recorded by- International Digestive Endoscopy Network consensus on the management of antithrombotic agents in patients undergoing gastrointestinal endoscopy
Seung Joo Kang, Chung Hyun Tae, Chang Seok Bang, Cheol Min Shin, Young-Hoon Jeong, Miyoung Choi, Joo Ha Hwang, Yutaka Saito, Philip Wai Yan Chiu, Rungsun Rerknimitr, Christopher Khor, Vu Van Khien, Kee Don Choi, Ki-Nam Shim, Geun Am Song, Oh Young Lee
Clinical Endoscopy.2024; 57(2): 141. CrossRef - Risk factors for intraoperative and delayed perforation related with gastric endoscopic submucosal dissection
Takuya Mimura, Yoshinobu Yamamoto, Haruhisa Suzuki, Kohei Takizawa, Toshiaki Hirasawa, Yoji Takeuchi, Kenji Ishido, Shu Hoteya, Tomonori Yano, Shinji Tanaka, Norihiko Kudara, Masahiro Nakagawa, Yumi Mashimo, Masahiro Ishigooka, Kazutoshi Fukase, Taichi Sh
Journal of Gastroenterology and Hepatology.2024;[Epub] CrossRef - Endoscopic submucosal dissection for early gastric cancer: It is time to consider the quality of its outcomes
Gwang Ha Kim
World Journal of Gastroenterology.2023; 29(43): 5800. CrossRef
- International Digestive Endoscopy Network consensus on the management of antithrombotic agents in patients undergoing gastrointestinal endoscopy
- 3,262 View
- 165 Download
- 3 Web of Science
- 3 Crossref

- Respiratory complications during recovery from gastrointestinal endoscopies performed by gastroenterologists under moderate sedation
- Inna Eidelman Pozin, Amir Zabida, Moshe Nadler, Guy Zahavi, Dina Orkin, Haim Berkenstadt
- Clin Endosc 2023;56(2):188-193. Published online January 10, 2023
- DOI: https://doi.org/10.5946/ce.2022.033
-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader ePub
ePub - Background
/Aims: Data on the incidence of adverse respiratory events during recovery from gastrointestinal endoscopy are limited. The aim of this study was to investigate the incidence of these complications.
Methods
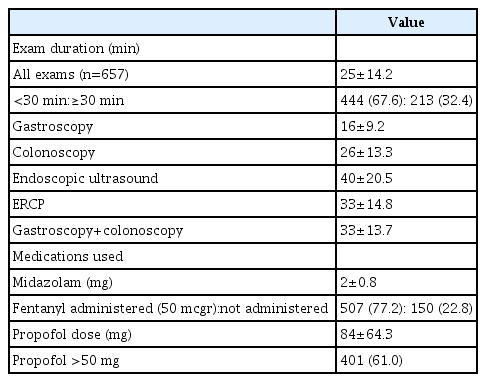
In this retrospective cohort study, data were obtained from the electronic records of 657 consecutive patients, who underwent gastroenterological procedures under sedation.
Results
Pulse oximetry oxygen saturation (SpO2) <90% for <60 seconds occurred in 82 patients (12.5%) and in 11 patients (1.7%), SpO2 of <90% for >60 seconds occurred in 79 patients (12.0%) and in 11 patients (1.7%), and SpO2 <75% occurred in four patients (0.6%) and in no patients during the procedure and recovery period, respectively. No major complications were noted. The occurrence of desaturation during recovery was correlated with desaturation during the procedure (p<0.001). American Society of Anesthesiologists score (odds ratio [OR], 1.867; 95% confidence interval [CI], 1.008–3.458), ischemic heart disease (OR, 1.815; 95% CI, 0.649–5.080), hypertension (OR, 1.289; 95% CI, 0.472–3.516), and diabetes mellitus (OR, 2.406; 95% CI, 0.950–6.095) increased the occurrence of desaturation during recovery.
Conclusions
We found no major complications during recovery after balanced propofol-based sedation administered by a gastroenterologist-nurse team. Patients with the identified risk predictors must be monitored carefully.
- 1,827 View
- 182 Download
- 1 Web of Science

- Clinical Practice Guidelines for the Endoscopic Management of Peripancreatic Fluid Collections
- Chi Hyuk Oh, Jun Kyu Lee, Tae Jun Song, Jin-Seok Park, Jae Min Lee, Jun Hyuk Son, Dong Kee Jang, Miyoung Choi, Jeong-Sik Byeon, In Seok Lee, Soo Teik Lee, Ho Soon Choi, Ho Gak Kim, Hoon Jai Chun, Chan Guk Park, Joo Young Cho
- Clin Endosc 2021;54(4):505-521. Published online July 27, 2021
- DOI: https://doi.org/10.5946/ce.2021.185
-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader ePub
ePub - Endoscopic ultrasonography-guided intervention has gradually become a standard treatment for peripancreatic fluid collections (PFCs). However, it is difficult to popularize the procedure in Korea because of restrictions on insurance claims regarding the use of endoscopic accessories, as well as the lack of standardized Korean clinical practice guidelines. The Korean Society of Gastrointestinal Endoscopy (KSGE) appointed a Task Force to develope medical guidelines by referring to the manual for clinical practice guidelines development prepared by the National Evidence-Based Healthcare Collaborating Agency. Previous studies on PFCs were searched, and certain studies were selected with the help of experts. Then, a set of key questions was selected, and treatment guidelines were systematically reviewed. Answers to these questions and recommendations were selected via peer review. This guideline discusses endoscopic management of PFCs and makes recommendations on Indications for the procedure, pre-procedural preparations, optimal approach for drainage, procedural considerations (e.g., types of stent, advantages and disadvantages of plastic and metal stents, and accessories), adverse events of endoscopic intervention, and procedural quality issues. This guideline was reviewed by external experts and suggests best practices recommended based on the evidence available at the time of preparation. This will be revised as necessary to address advances and changes in technology and evidence obtained in clinical practice and future studies.
-
Citations
Citations to this article as recorded by- Pancreatic Pseudocyst after Fully Covered Self-expandable Metallic Stent Placement: A Case Report
Mitsuhito Koizumi, Sho Ishikawa, Kaori Marui, Masahito Kokubu, Yusuke Okujima, Yuki Numata, Yoshiki Imamura, Teru Kumagi, Yoichi Hiasa
Internal Medicine.2024;[Epub] CrossRef - Neutrophil Gelatinase-Associated Lipocalin for the Differentiation of Mucinous Pancreatic Cystic Lesions
Miruna Patricia Olar, Maria Iacobescu, Sorana D. Bolboacă, Cristina Pojoga, Ofelia Moșteanu, Radu Seicean, Ioana Rusu, Oana Banc, Cristina Adela Iuga, Andrada Seicean
International Journal of Molecular Sciences.2024; 25(6): 3224. CrossRef - Comparative outcome of single versus two double-pigtail stents for endoscopic drainage of pancreatic fluid collections with minimal necrosis: a retrospective analysis
S Giri, S Bhrugumalla, S Gangadhar, S Angadi
Acta Gastro Enterologica Belgica.2024; 87(1): 1. CrossRef - Use of an endoscopic powered debridement device for treatment of post-surgical fatty pancreatic necrosis
Judy Daboul, Shiab Mussad, Anna Cecilia Amaral, Waleed K. Hussain, Peter J. Lee, Samuel Han
Clinical Endoscopy.2024; 57(3): 412. CrossRef - Endoscopic ultrasound-guided drainage for local complications related to pancreatitis
Hyung Ku Chon, Seong-Hun Kim
International Journal of Gastrointestinal Intervention.2023; 12(1): 7. CrossRef - A preferable modality for the differentiation of peripancreatic fluid collections: Endoscopic ultrasound
Ning Xu, Longsong Li, Danqi Zhao, Zixin Wang, Xueting Wang, Runzi Wang, Yanbo Zeng, Lei Zhang, Ning Zhong, Ying Lv, Enqiang Linghu, Ningli Chai
Endoscopic Ultrasound.2022; 11(4): 291. CrossRef - Disconnected pancreatic duct syndrome in acute pancreatitis
A.V. Fedorov, V.N. Ektov, M.A. Khodorkovsky
Khirurgiya. Zhurnal im. N.I. Pirogova.2022; (8): 83. CrossRef - Single balloon enteroscopy-guided endoscopic retrograde pancreatography for the treatment of a symptomatic pancreatic pseudocyst complicated by pancreaticojejunostomy stricture: A case report
Eunae Cho, Chang-Hwan Park, Seo Yeon Cho
Medicine.2022; 101(43): e31293. CrossRef
- Pancreatic Pseudocyst after Fully Covered Self-expandable Metallic Stent Placement: A Case Report
- 4,754 View
- 218 Download
- 7 Web of Science
- 8 Crossref

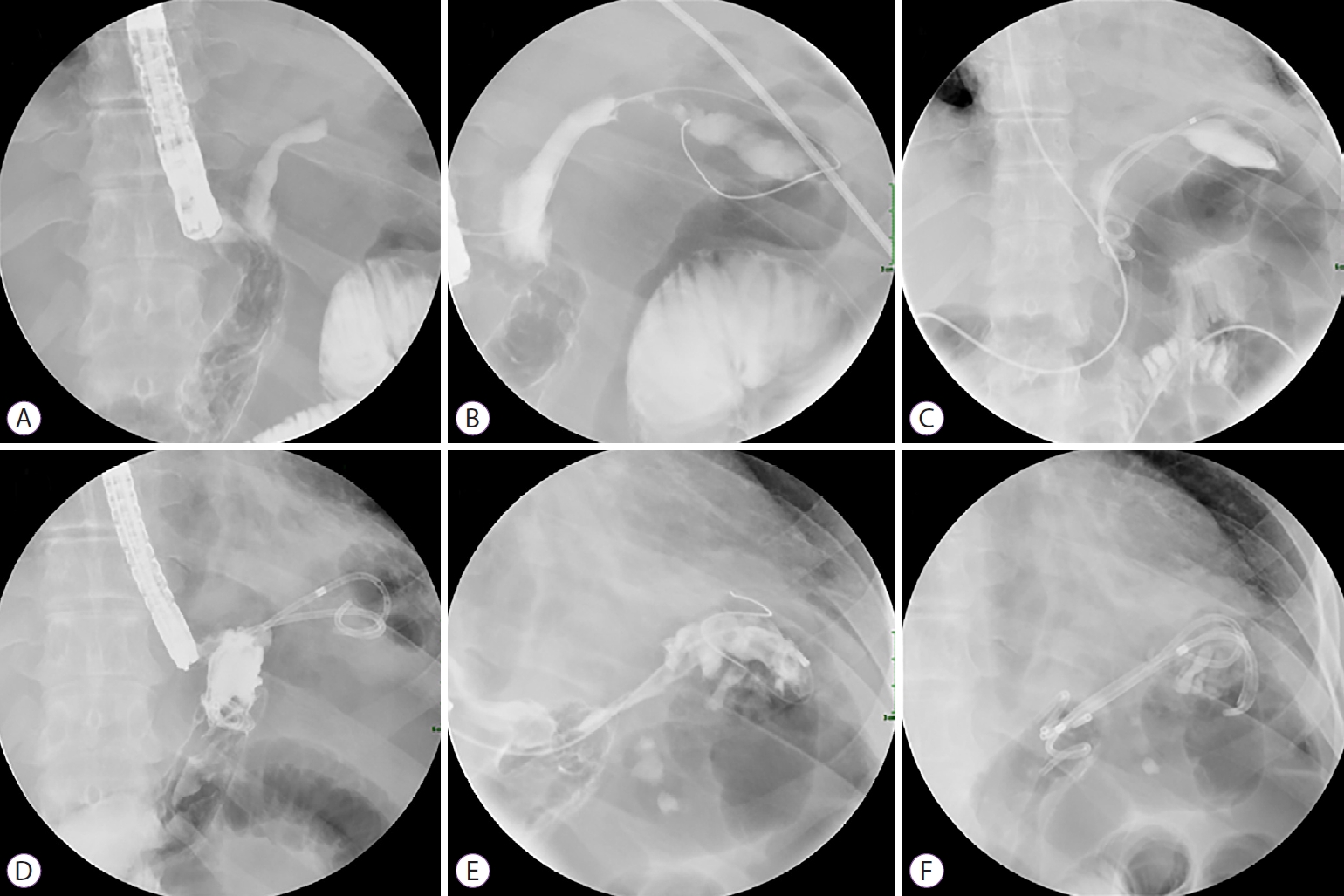
- A Gastrobronchial Fistula Secondary to Endoscopic Internal Drainage of a Post-Sleeve Gastrectomy Fluid Collection
- Paraskevas Gkolfakis, Marc-André Bureau, Marianna Arvanitakis, Jacques Devière, Daniel Blero
- Clin Endosc 2022;55(1):141-145. Published online April 16, 2021
- DOI: https://doi.org/10.5946/ce.2021.033
-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader ePub
ePub - A 44-year-old woman underwent sleeve gastrectomy, which was complicated by a leak. She was treated with two sessions of endoscopic internal drainage using plastic double-pigtail stents. Her clinical evolution was favorable, but four months after the initial stent placement, she became symptomatic, and a gastrobronchial fistula with the proximal end of the stents invading the diaphragm was diagnosed. She was treated with antibiotics, plastic stents were removed, and a partially covered metallic esophageal stent was placed. Eleven weeks later, the esophageal stent was removed with no evidence of fistula. Inappropriate stent size, position, stenting duration, and persistence of low-grade inflammation could explain the patient’s symptoms and provide a mechanism for gradual muscle rupture and fistula formation. Although endoscopic internal drainage is usually safe and effective for the management of post-laparoscopic sleeve gastrectomy leaks, close clinical and radiological follow-up is mandatory.
-
Citations
Citations to this article as recorded by- Management of Leakage and Fistulas after Bariatric Surgery
Stephen A. Firkins, Roberto Simons-Linares
Best Practice & Research Clinical Gastroenterology.2024; : 101926. CrossRef - Role of Endoscopic Internal Drainage in Treating Gastro-Bronchial and Gastro-Colic Fistula After Sleeve Gastrectomy
Alessandra D’Alessandro, Giovanni Galasso, Francesco Paolo Zito, Cristiano Giardiello, Fabrizio Cereatti, Roberto Arienzo, Filippo Pacini, Jean-Marc Chevallier, Gianfranco Donatelli
Obesity Surgery.2022; 32(2): 342. CrossRef
- Management of Leakage and Fistulas after Bariatric Surgery
- 3,672 View
- 144 Download
- 2 Crossref

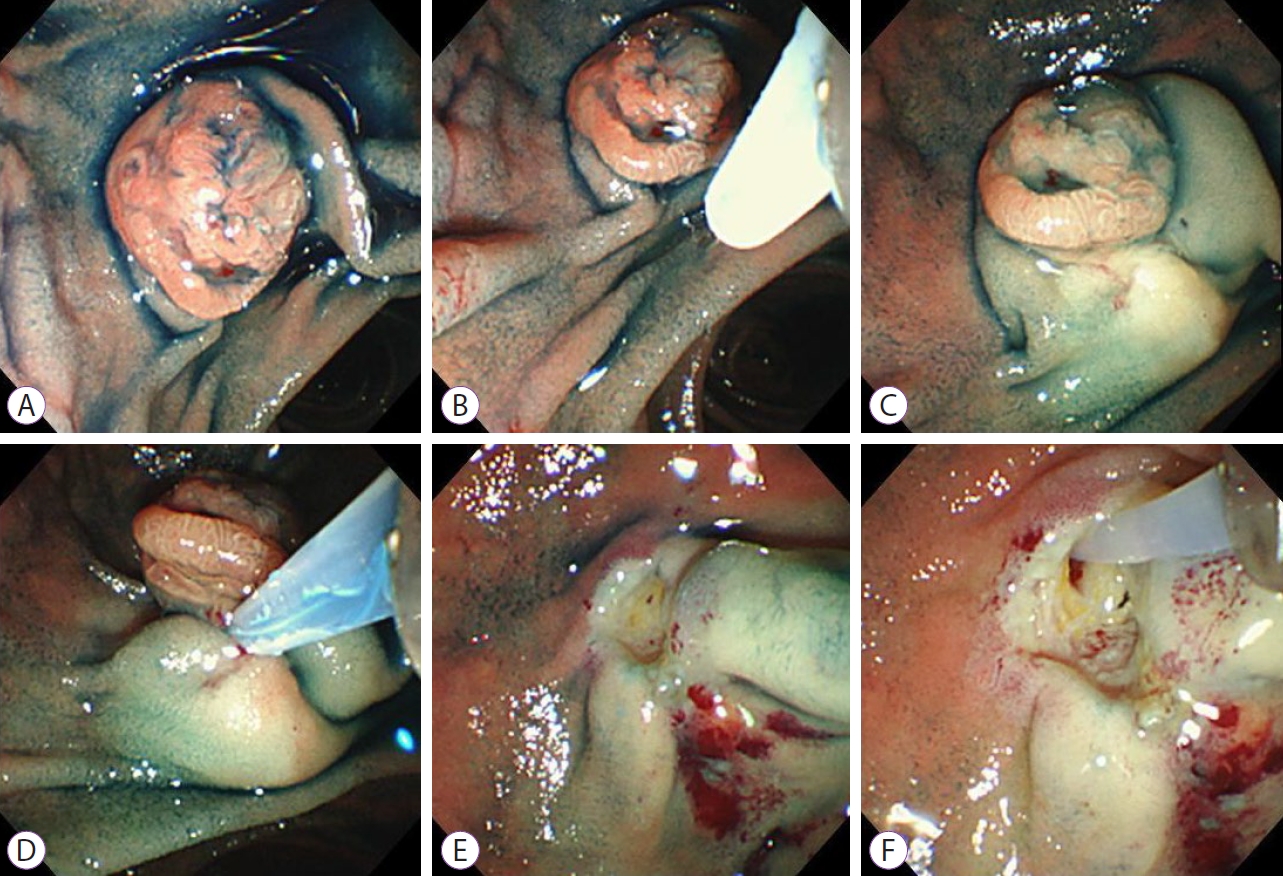
- Efficacy of Hypertonic Saline-Epinephrine Local Injection Around the Anal Side before Endoscopic Papillectomy for Ampullary Tumors
- Naoki Okano, Yoshinori Igarashi, Ken Ito, Saori Mizutani, Hiroki Nakagawa, Kouji Watanabe, Yuuto Yamada, Kensuke Yoshimoto, Yuusuke Kimura, Susumu Iwasaki, Kensuke Takuma, Seiichi Hara, Yuui Kishimoto
- Clin Endosc 2021;54(5):706-712. Published online March 10, 2021
- DOI: https://doi.org/10.5946/ce.2020.208
-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader ePub
ePub - Background
/Aims: Bleeding is a complication of endoscopic snare papillectomy for ampullary tumors. This study aimed to investigate the clinical efficacy of hypertonic saline-epinephrine (HSE) local injection before endoscopic papillectomy for prevention of bleeding.
Methods
We retrospectively reviewed the data of 107 consecutive patients with ampullary tumors who underwent endoscopic papillectomy. The rates of en bloc resection, pathological resection margins, and prevention of immediate or delayed bleeding in the simple snaring resection group (Group A) and the HSE injection group (Group B) were compared.
Results
A total of 44 and 63 patients were enrolled in Groups A and B, respectively. The total complete resection rate was 89.7% (96/107); the clinical complete resection rates in Group A and Group B were 86.3% (38/44) and 92.1% (58/63), respectively (p=0.354). Post-papillectomy bleeding occurred in 22 patients. In Groups A and B, the immediate bleeding rates were 20.5% (9/44) and 4.8% (3/63), respectively (p=0.0255), while the delayed bleeding rates were 7% (3/44) and 11% (7/63), respectively (p=0.52). The rates of positive horizontal and vertical pathological margin in both groups were 27% and 16%, respectively.
Conclusions
HSE local injection was effective in preventing immediate bleeding and was useful for safely performing endoscopic papillectomy for ampullary tumors. -
Citations
Citations to this article as recorded by- Endoscopic papillectomy for ampullary lesions of minor papilla
Kien Vu Trung, Christian Heise, Einas Abou-Ali, Francesco Auriemma, Elias Karam, Sophia E. van der Wiel, Marco J. Bruno, Fabrice Caillol, Marc Giovannini, Viliam Masaryk, Uwe Will, Andrea Anderloni, Enrique Pérez-Cuadrado-Robles, Ana Dugic, Benjamin Meier
Gastrointestinal Endoscopy.2024; 99(4): 587. CrossRef - Endoscopic papillectomy for ampullary lesions in patients with familial adenomatous polyposis compared with sporadic lesions: a propensity score-matched cohort
Kien Vu Trung, Einas Abou-Ali, Fabrice Caillol, Woo H. Paik, Bertrand Napoleon, Viliam Masaryk, Sophia E. van der Wiel, Enrique Pérez-Cuadrado-Robles, Nicolas Musquer, Asif Halimi, Kevin Soares, Francois R. Souche, Steffen Seyfried, Maria C. Petrone, Stef
Endoscopy.2023; 55(08): 709. CrossRef - Updates in endoscopic management of ampullary and duodenal adenomas
Pravallika Chadalavada, Tilak Upendra Shah
Current Opinion in Gastroenterology.2023; 39(6): 496. CrossRef - Submucosal Epinephrine Injection Before Endoscopic Papillectomy: Less is More?
Roy L.J. van Wanrooij, Jeanin E. van Hooft
Clinical Endoscopy.2021; 54(5): 627. CrossRef
- Endoscopic papillectomy for ampullary lesions of minor papilla
- 3,712 View
- 92 Download
- 4 Web of Science
- 4 Crossref

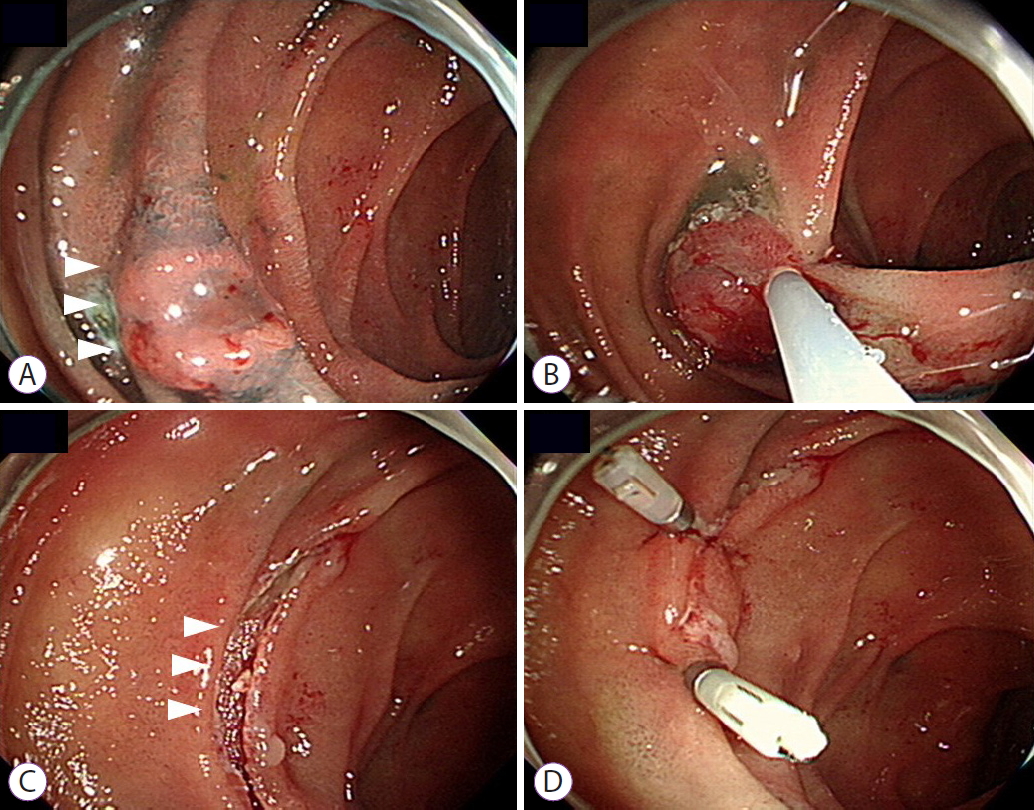
- The Use of Endoscopic Clipping in Preventing Delayed Complications after Endoscopic Resection for Superficial Non-Ampullary Duodenal Tumors
- Jee Young An, Byung-Wook Kim, Joon Sung Kim, Jae-Myung Park, Tae Ho Kim, Jaesin Lee
- Clin Endosc 2021;54(4):563-569. Published online November 24, 2020
- DOI: https://doi.org/10.5946/ce.2020.109
-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader ePub
ePub - Background
/Aims: Endoscopic resection (ER) has recently been accepted as the standard treatment modality for superficial nonampullary duodenal tumors (SNADTs). However, the procedure can cause adverse events such as perforation and bleeding. This study aimed to investigate the efficacy of prophylactic clipping in the prevention of delayed complications.
Methods
A retrospective review of the medical records of patients who underwent ER for SNADT from 3 centers was performed. Patients were divided into 2 groups: the immediate clipping group (ICG) and the no clipping group (NCG). Various baseline characteristics and factors associated with the appearance of delayed complications, such as size of the lesion, tumor location, histologic type, and co-morbidities, were compared between the two groups.
Results
A total of 99 lesions from 99 patients were included in this study. Fifty-two patients were allocated into ICG and 47 patients were allocated into NCG. Delayed bleeding occurred in 1 patient from ICG and in 8 patients from NCG. Delayed perforation occurred in 1 patient from ICG and in 3 patients from NCG. There were no procedure-related deaths in both groups.
Conclusions
Although the use of endoscopic clipping seemed to reduce the risk of developing delayed complications, further studies using a prospective design is required. -
Citations
Citations to this article as recorded by- The Effect of Tegoprazan on the Treatment of Endoscopic Resection-Induced Artificial Ulcers: A Multicenter, Randomized, Active-Controlled Study
Byung-Wook Kim, Jong Jae Park, Hee Seok Moon, Wan Sik Lee, Ki-Nam Shim, Gwang Ho Baik, Yun Jeong Lim, Hang Lak Lee, Young Hoon Youn, Jun Chul Park, In-Kyung Sung, Hyunsoo Chung, Jeong Seop Moon, Gwang Ha Kim, Su Jin Hong, Hyuk Soon Choi
Gut and Liver.2024; 18(2): 257. CrossRef - Endoscopic diagnosis and treatment of superficial non-ampullary duodenal epithelial tumors: A review
Zheng Zhao, Yue Jiao, Shuyue Yang, Anni Zhou, Guiping Zhao, Shuilong Guo, Peng Li, Shutian Zhang
Journal of Translational Internal Medicine.2023; 11(3): 206. CrossRef - Long-term outcomes of endoscopic resection for duodenal neuroendocrine tumors
Kiyoun Yi, Gwang Ha Kim, Su Jin Kim, Cheol Woong Choi, Moon Won Lee, Bong Eun Lee, Geun Am Song
Scientific Reports.2023;[Epub] CrossRef - Endoscopic clipping in non-variceal upper gastrointestinal bleeding treatment
Giuseppe Galloro, Angelo Zullo, Gaetano Luglio, Alessia Chini, Donato Alessandro Telesca, Rosa Maione, Matteo Pollastro, Giovanni Domenico De Palma, Raffaele Manta
Clinical Endoscopy.2022; 55(3): 339. CrossRef - Endoscopic Closure After Endoscopic Resection for Superficial Non-Ampullary Duodenal Tumors
Satoshi Tanabe, Takuya Wada
Clinical Endoscopy.2021; 54(4): 453. CrossRef
- The Effect of Tegoprazan on the Treatment of Endoscopic Resection-Induced Artificial Ulcers: A Multicenter, Randomized, Active-Controlled Study
- 3,333 View
- 73 Download
- 4 Web of Science
- 5 Crossref

- Endoscopic Yield, Appropriateness, and Complications of Pediatric Upper Gastrointestinal Endoscopy in an Adult Suite: A Retrospective Study of 822 Children
- Manzoor Ahmad Wani, Showkat Ali Zargar, Ghulam Nabi Yatoo, Inaamul Haq, Altaf Shah, Jaswinder Singh Sodhi, Ghulam Mohammad Gulzar, Mushtaq Khan
- Clin Endosc 2020;53(4):436-442. Published online April 7, 2020
- DOI: https://doi.org/10.5946/ce.2019.118

-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader ePub
ePub - Background
/Aims: This study aimed to study the endoscopic yield, appropriateness, and complications of pediatric endoscopy performed by adult gastroenterologists in an adult endoscopic suite.
Methods
This a retrospective study in which records of all the patients less than 18 years of age who underwent endoscopy in the last 5 years were studied. The indications of endoscopy in children were categorized as appropriate or inappropriate per the latest guidelines by American Society for Gastrointestinal Endoscopy and North American Society for Pediatric Gastroenterology, Hepatology and Nutrition. Positive endoscopic yield was defined as the presence of any abnormality on endoscopy.
Results
Among the total of 822 children (age <18 years), the most common indications were variceal surveillance/eradication in 157 (19.1%), followed by dyspepsia in 143 (17.4%), upper gastrointestinal (UGI) bleeding in 136 (16.5%), recurrent abdominal pain in 94 (11.4%), unexplained anemia in 74 (9%), recurrent vomiting in 50 (6.08%), chronic refractory gastroesophageal reflux disease in 34 (4.1%) and others; 780 out of 822 endoscopic procedures (94.9%) done in children were appropriate as per the guidelines. The endoscopic yield was 45.8%, highest in patients with UGI bleeding (71.3%), followed by variceal surveillance (54.8%), recurrent vomiting (38%), dyspepsia (37.8%), and recurrent abdominal pain (36%). Minor adverse events occurred in 7.3% of children.
Conclusions
Pediatric endoscopy performed by an experienced adult gastroenterologist may be acceptable if done in cooperation with a pediatrician. -
Citations
Citations to this article as recorded by- Which Alarm Symptoms Are Associated With Abnormal Gastrointestinal Endoscopy Among Thai Children?
Anundorn Wongteerasut
Pediatric Gastroenterology, Hepatology & Nutrition.2024; 27(2): 113. CrossRef - Paediatric gastrointestinal endoscopy in the Asian-Pacific region: Recent advances in diagnostic and therapeutic techniques
James Guoxian Huang, Pornthep Tanpowpong
World Journal of Gastroenterology.2023; 29(18): 2717. CrossRef - Gastrointestinal Bleeding in Children: The Role of Endoscopy and the Sheffield Scoring System in a Resource-Limited Setting
Oluwafunmilayo Funke Adeniyi, Olufunmilayo Adenike Lesi, Emuobor Aghoghor Odeghe, Ganiyat Oyeleke, Nicholas Croft
JPGN Reports.2023; 4(4): e369. CrossRef - Pediatric esophagogastroduodenoscopy in china: indications, diagnostic yield, and factors associated with findings
Shengnan Wang, Xiaoxia Qiu, Jingfang Chen, Hong Mei, Haiyan Yan, Jieyu You, Ying Huang
BMC Pediatrics.2022;[Epub] CrossRef - Safety and Competency are the Main Priorities in Pediatric Endoscopy
Byung-Ho Choe
Clinical Endoscopy.2020; 53(4): 379. CrossRef
- Which Alarm Symptoms Are Associated With Abnormal Gastrointestinal Endoscopy Among Thai Children?
- 4,423 View
- 152 Download
- 6 Web of Science
- 5 Crossref

- Endoscopic Management of Post-Polypectomy Bleeding
- Aditya Gutta, Mark A. Gromski
- Clin Endosc 2020;53(3):302-310. Published online September 17, 2019
- DOI: https://doi.org/10.5946/ce.2019.062

-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader ePub
ePub - Post-polypectomy bleeding (PPB) is one of the most common complications of endoscopic polypectomy. There are multiple risk factors related to patient and polyp characteristics that should be considered. In most cases, immediate PPB can be effectively managed endoscopically when recognized and managed promptly. Delayed PPB can manifest in a myriad of ways. In severe delayed PPB, resuscitation for hemodynamic stabilization should be prioritized, followed by endoscopic evaluation and therapy once the patient is stabilized. Future areas of research in PPB include the risks of direct oral anticoagulants and of specific electrosurgical settings for hot-snare polypectomy vs. cold-snare polypectomy, benefits of closure of post-polypectomy mucosal defects using through-the-scope clips, and prospective comparative evaluation of newer hemostasis agents such as hemostatic spray powder and over-the-scope clips.
-
Citations
Citations to this article as recorded by- Updates on the Prevention and Management of Post-Polypectomy Bleeding in the Colon
Hisham Wehbe, Aditya Gutta, Mark A. Gromski
Gastrointestinal Endoscopy Clinics of North America.2024; 34(2): 363. CrossRef - Colonoscopic polypectomy of juvenile polyps in children: Experience from a tertiary centre of Bangladesh
Salahuddin Mahmud, Mashud Parvez, Madhabi Baidya, Farhana Tasneem, Ahmed Rashidul Hasan, Tanzila Farhana, Md Jahangir Alam, Syed Shafi Ahmed
Gastroenterology & Endoscopy.2024; 2(1): 1. CrossRef - Review article: Advances in the management of lower gastrointestinal bleeding
Ali A. Alali, Majid A. Almadi, Alan N. Barkun
Alimentary Pharmacology & Therapeutics.2024; 59(5): 632. CrossRef - Endoscopic management of intraprocedural bleeding during endoscopic interventions
Ali A. Alali, Asma A. Alkandari
Best Practice & Research Clinical Gastroenterology.2024; 69: 101912. CrossRef - Effect of Cold Versus Hot Snare Polypectomy on Colon Postpolypectomy Bleeding in Patients with End-Stage Renal Disease: A Retrospective Cohort Study
Hsueh-Chien Chiang, Chien-Ming Chiang, Xi-Zhang Lin, Po-Jun Chen
Digestive Diseases and Sciences.2024;[Epub] CrossRef - Isolated ischaemic appendicitis as a rare complication of selective angioembolization for lower gastrointestinal bleed
Luke S. Crawford, Nadim S. Jafri, Dingle Foote, Melissa M. Felinski, Peter A. Walker, Kulvinder S. Bajwa, Shinil K. Shah
ANZ Journal of Surgery.2023; 93(1-2): 404. CrossRef - JAG consensus statements for training and certification in colonoscopy
Keith Siau, Stavroula Pelitari, Susi Green, Brian McKaig, Arun Rajendran, Mark Feeney, Mo Thoufeeq, John Anderson, Vathsan Ravindran, Paul Hagan, Neil Cripps, Ian L P Beales, Karen Church, Nicholas I Church, Elizabeth Ratcliffe, Said Din, Rupert D Pullan,
Frontline Gastroenterology.2023; 14(3): 201. CrossRef - JAG consensus statements for training and certification in flexible sigmoidoscopy
Keith Siau, Stavroula Pelitari, Susi Green, Brian McKaig, Arun Rajendran, Mark Feeney, Mo Thoufeeq, John Anderson, Vathsan Ravindran, Paul Hagan, Neil Cripps, Ian L P Beales, Karen Church, Nicholas I Church, Elizabeth Ratcliffe, Said Din, Rupert D Pullan,
Frontline Gastroenterology.2023; 14(3): 181. CrossRef - Management of Patients With Acute Lower Gastrointestinal Bleeding: An Updated ACG Guideline
Neil Sengupta, Joseph D. Feuerstein, Vipul Jairath, Amandeep K. Shergill, Lisa L. Strate, Robert J. Wong, David Wan
American Journal of Gastroenterology.2023; 118(2): 208. CrossRef - Korean Guidelines for Postpolypectomy Colonoscopic Surveillance: 2022 revised edition
Su Young Kim, Min Seob Kwak, Soon Man Yoon, Yunho Jung, Jong Wook Kim, Sun-Jin Boo, Eun Hye Oh, Seong Ran Jeon, Seung-Joo Nam, Seon-Young Park, Soo-Kyung Park, Jaeyoung Chun, Dong Hoon Baek, Mi-Young Choi, Suyeon Park, Jeong-Sik Byeon, Hyung Kil Kim, Joo
Intestinal Research.2023; 21(1): 20. CrossRef - A Review of Colonoscopy in Intestinal Diseases
Seung Min Hong, Dong Hoon Baek
Diagnostics.2023; 13(7): 1262. CrossRef - Bleeding After Endoscopic Resection of Colonic Adenomatous Polyps Sized 4-10 mm
Violeta Hristova Janik
PRILOZI.2023; 44(2): 157. CrossRef - Endoscopic management of delayed bleeding after polypectomy of small colorectal polyps: two or more clips may be safe
Xue-Feng Guo, Xiang-An Yu, Jian-Cong Hu, De-Zheng Lin, Jia-Xin Deng, Ming-Li Su, Juan Li, Wei Liu, Jia-Wei Zhang, Qing-Hua Zhong
Gastroenterology Report.2022;[Epub] CrossRef - Clinical progress note: Diagnostic approach to lower gastrointestinal bleeding
Daniel J. Stein, Hyder Said, Joseph D. Feuerstein
Journal of Hospital Medicine.2022; 17(7): 547. CrossRef - Safety of Cold Snare Polypectomy for Small Colorectal Polyps in Patients Receiving Antithrombotic Therapy
Dai Nakamatsu, Tsutomu Nishida, Yoshifumi Fujii, Sho Yamaoka, Naoto Osugi, Aya Sugimoto, Kaori Mukai, Kengo Matsumoto, Masashi Yamamoto, Shiro Hayashi, Sachiko Nakajima
Techniques and Innovations in Gastrointestinal Endoscopy.2022; 24(3): 246. CrossRef - Postcolonoscopy Complications
Jetsen A. Rodriguez-Silva, Justin A. Maykel
Diseases of the Colon & Rectum.2022; 65(5): 622. CrossRef - Expanding rather than closing the wound can rescue the endoscopic procedure when massive bleeding occurs during endoscopic submucosal dissection
Ming-Ching Yuan, Ching-Tai Lee, Kun-Feng Tsai, Chao-Wen Hsu, Chu-Kuang Chou
Endoscopy.2022; 54(S 02): E1036. CrossRef - Korean Guidelines for Postpolypectomy Colonoscopic Surveillance: 2022 Revised Edition
Su Young Kim, Min Seob Kwak, Soon Man Yoon, Yunho Jung, Jong Wook Kim, Sun-Jin Boo, Eun Hye Oh, Seong Ran Jeon, Seung-Joo Nam, Seon-Young Park, Soo-Kyung Park, Jaeyoung Chun, Dong Hoon Baek, Mi-Young Choi, Suyeon Park, Jeong-Sik Byeon, Hyung Kil Kim, Joo
The Korean Journal of Gastroenterology.2022; 80(3): 115. CrossRef - Korean guidelines for postpolypectomy colonoscopic surveillance: 2022 revised edition
Su Young Kim, Min Seob Kwak, Soon Man Yoon, Yunho Jung, Jong Wook Kim, Sun-Jin Boo, Eun Hye Oh, Seong Ran Jeon, Seung-Joo Nam, Seon-Young Park, Soo-Kyung Park, Jaeyoung Chun, Dong Hoon Baek, Mi-Young Choi, Suyeon Park, Jeong-Sik Byeon, Hyung Kil Kim, Joo
Clinical Endoscopy.2022; 55(6): 703. CrossRef - Can prophylactic argon plasma coagulation reduce delayed post‐papillectomy bleeding? A prospective multicenter trial
Jae Kook Yang, Jong Jin Hyun, Tae Hoon Lee, Jun‐Ho Choi, Yun Nah Lee, Jung Wan Choe, Jin‐Seok Park, Chang‐Il Kwon, Seok Jeong, Hong Ja Kim, Jong Ho Moon, Sang‐Heum Park
Journal of Gastroenterology and Hepatology.2021; 36(2): 467. CrossRef - Diagnosis and management of acute lower gastrointestinal bleeding: European Society of Gastrointestinal Endoscopy (ESGE) Guideline
Konstantinos Triantafyllou, Paraskevas Gkolfakis, Ian M. Gralnek, Kathryn Oakland, Gianpiero Manes, Franco Radaelli, Halim Awadie, Marine Camus Duboc, Dimitrios Christodoulou, Evgeny Fedorov, Richard J. Guy, Marcus Hollenbach, Mostafa Ibrahim, Ziv Neeman,
Endoscopy.2021; 53(08): 850. CrossRef - Estimation and influence of blood loss under endoscope for percutaneous endoscopic lumbar discectomy (PELD): a clinical observational study combined with in vitro experiment
Dong Dong Sun, Dan Lv, Wei Zhou Wu, He Fei Ren, Bu He Bao, Qun Liu, Ming Lin Sun
Journal of Orthopaedic Surgery and Research.2020;[Epub] CrossRef
- Updates on the Prevention and Management of Post-Polypectomy Bleeding in the Colon
- 14,682 View
- 589 Download
- 18 Web of Science
- 22 Crossref

- Experience of the Endoscopists Matters in Endoscopic Retrograde Cholangiopancreatography in Billroth II Gastrectomy Patients
- Erkan Caglar, Deniz Atasoy, Mukaddes Tozlu, Engin Altınkaya, Serkan Dogan, Hakan Senturk
- Clin Endosc 2020;53(1):82-89. Published online September 3, 2019
- DOI: https://doi.org/10.5946/ce.2019.073

-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader ePub
ePub - Background
/Aims: Altered anatomy is a challenge in endoscopic retrograde cholangiopancreatography (ERCP) for patients with Billroth II anastomosis. In this study, we investigated the overall success and role of endoscopist experience.
Methods
Data of patients who underwent ERCP between 2014 and 2018 after a previous Billroth II operation were retrieved retrospectively from 2 tertiary ERCP centers. The procedures were performed by 2 endoscopists with different levels of experience. Clinical success was defined as extraction of the stone, placement of a stent through a malignant stricture, and clinical and laboratory improvements in patients.
Results
Seventy-five patients were included. The technical success rate was 83% for the experienced endoscopist and 75% for the inexperienced endoscopist (p=0.46). The mean (±standard deviation) procedure time was 23.8±5.7 min for the experienced endoscopist and 40.68±6.07 min for the inexperienced endoscopist (p<0.001). In total, 3 perforations (4%) were found. The rate of afferent loop perforation was 6.25% (1/16) for the inexperienced endoscopist and 0% (0/59) for the experienced endoscopist (p=0.053).
Conclusions
ERCP in patients who had undergone Billroth II gastrectomy was time consuming for the inexperienced endoscopist who should beware of the unique adverse events related to ERCP in patients with altered anatomy. -
Citations
Citations to this article as recorded by- Practical application of the modification in endoscopic retrograde cholangiopancreatography treated common bile duct stones in patients with Billroth II gastroenterostomy in Vietnam
Tran Thi Anh Tuyet, Nguyen Van Thai, Nguyen Tien Thinh, Mai Thanh Binh
Therapeutic Advances in Gastrointestinal Endoscopy.2024;[Epub] CrossRef - Endoscopic Retrograde Cholangiopancreatography Performed by Trainees Is Not Associated with Increased Immediate Adverse Events or Technical Failure Rates
Osayande Osagiede, Frank J. Lukens, Vivek Kumbhari, Juan E. Corral
Digestive Diseases and Sciences.2023; 68(5): 1747. CrossRef - Impact of center and endoscopist ERCP volume on ERCP outcomes: a systematic review and meta-analysis
Sara Teles de Campos, Apostolis Papaefthymiou, Theodosia Florou, Antonio Facciorusso, Marianna Arvanitakis, Jacques Devière, Paraskevas Gkolfakis
Gastrointestinal Endoscopy.2023; 98(3): 306. CrossRef - Unusual biliary gem: Cause of acute obstructive suppurative cholangitis and pancreatitis in a patient with Billroth II anastomosis
Koki Yamada, Susumu Shinoura
Annals of the Academy of Medicine, Singapore.2022; 51(3): 196. CrossRef - Increased ERCP volume improves cholangiogram interpretation: a new performance measure for ERCP training?
Shyam Vedantam, Sunil Amin, Ben Maher, Saqib Ahmad, Shanil Kadir, Saad Khalid Niaz, Mark Wright, Nadeem Tehami
Clinical Endoscopy.2022; 55(3): 426. CrossRef - Percutaneous transhepatic cholangiography vs endoscopic ultrasound-guided biliary drainage: A systematic review
Zeinab Hassan, Eyad Gadour
World Journal of Gastroenterology.2022; 28(27): 3514. CrossRef - Experience of endoscopic retrograde cholangiopancreatography with side-viewing duodenoscope in patients with previous gastric surgery
Mehmet Emin Gürbüz, Dursun Özgür Karakaş
Turkish Journal of Surgery.2022; 38(2): 149. CrossRef - A comparative study of side-viewing duodenoscope and forward-viewing gastroscope to perform endoscopic retrograde cholangiopancreatography in patients with Billroth II gastrectomy
Orhan Coşkun, Bülent Ödemiş
Surgical Endoscopy.2021; 35(8): 4222. CrossRef - Efficacy and safety of the rotatable sphincterotome during ERCP in patients with prior Billroth II gastrectomy (with videos)
Feng Zhu, Yaping Guan, Jing Wang
Surgical Endoscopy.2021; 35(8): 4849. CrossRef - Experience of Endoscopists in Endoscopic Retrograde Cholangiopancreatography in Surgically Altered Anatomy Patients
Chang-Hwan Park
Clinical Endoscopy.2020; 53(1): 7. CrossRef
- Practical application of the modification in endoscopic retrograde cholangiopancreatography treated common bile duct stones in patients with Billroth II gastroenterostomy in Vietnam
- 5,454 View
- 131 Download
- 12 Web of Science
- 10 Crossref

-
Removal of a Trigger Cord Stuck between Bands during Endoscopic Multiple-Band Ligation for Treating Esophageal Variceal Hemorrhage

- Nam Seok Ham, Danbi Lee, Sung Hyun Won, Jeongseok Kim, Seokjung Jo, Sangyoung Yi, Seol So
- Clin Endosc 2020;53(2):230-231. Published online July 24, 2019
- DOI: https://doi.org/10.5946/ce.2019.076
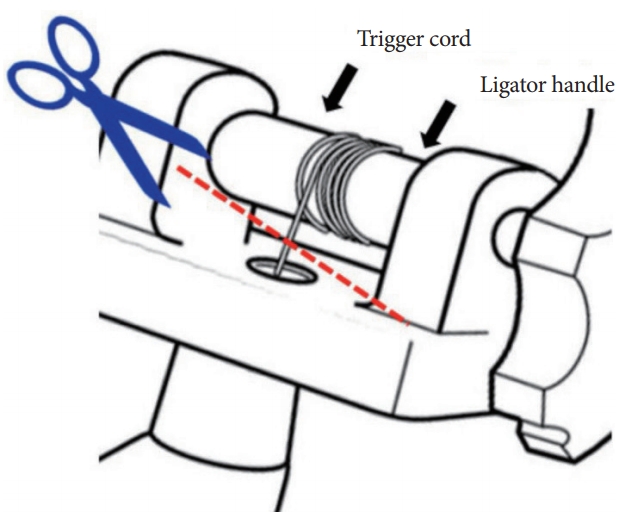
-
 Abstract
Abstract
 PDF
PDF Supplementary Material
Supplementary Material PubReader
PubReader ePub
ePub - Endoscopic variceal ligation is the preferred endoscopic treatment method for esophageal variceal bleeding. The incidence of complications such as chest pain, bleeding, stricture formation, and aspiration pneumonia is low. We report a case wherein a malfunctioning multiple-band ligator could have potentially caused damage to the esophageal varices and massive bleeding. The equipment was safely removed using scissors and forceps. To the best of our knowledge, this is the first published report detailing the management of a case of esophageal variceal bleeding.
- 4,558 View
- 118 Download

- Anesthetic Consideration for Peroral Endoscopic Myotomy
- Yun-Sic Bang, Chunghyun Park
- Clin Endosc 2019;52(6):549-555. Published online July 10, 2019
- DOI: https://doi.org/10.5946/ce.2019.033
-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader ePub
ePub - A recent achalasia guideline suggests that peroral endoscopic myotomy (POEM) is a safe option for achalasia that is as effective as Heller myotomy. It is recommended that POEM should be performed under general anesthesia. The incidence of adverse events such as bleeding, perforation, and carbon dioxide insufflation-related complications was lower in POEM under endotracheal general anesthesia than in POEM under sedation. Subcutaneous emphysema, pneumothorax, pneumomediastinum, pneumoperitoneum, and accompanying hemodynamic instability can be caused by carbon dioxide insufflation. Treatment of possible physiological changes and adverse events during the POEM procedure from the point of view of anesthesiologists may give endoscopists a new perspective on improving patient safety. The territory of therapeutic endoscopy can be expanded through cooperation with other departments, including anesthesia services. Efforts to understand different perspectives will certainly help not only secure patient safety but also expand the area of treatment.
-
Citations
Citations to this article as recorded by- Effectiveness and safety of peroral endoscopic myotomy in patients with achalasia
Nermin Mutlu Bilgiç, Zuhal Çalışkan, Oğuzhan Öztürk, Mehmet Ali Saruhan, Kamil Ozdil
Turkish Journal of Clinics and Laboratory.2024; 15(1): 123. CrossRef - Invasive CO2 monitoring with arterial line compared to end tidal CO2 during peroral endoscopic myotomy
Rodrigo Duarte-Chavez, Amy Tyberg, Avik Sarkar, Haroon M. Shahid, Bhargav Vemulapalli, Sardar Shah-Khan, Monica Gaidhane, Michel Kahaleh
Endoscopy International Open.2023; 11(05): E468. CrossRef - Demonstration of feasibility and technique of transesophageal endoscopic epicardial access in a porcine model
Zachary N. Weitzner, Steven Cha, Ronald Challita, Olujimi Ajijola, Shumpei Mori, Kalyanam Shivkumar, Erik Dutson, Alireza Sedarat
iGIE.2023; 2(4): 418. CrossRef - Our experience with peroral endoscopic myotomy complications: A case series
Shruti S Patil, Shantanu B Kulkarni
MGM Journal of Medical Sciences.2023; 10(4): 794. CrossRef - Anesthesia for Advanced Endoscopic Procedures
Basavana Goudra, Monica Saumoy
Clinical Endoscopy.2022; 55(1): 1. CrossRef - Effect of Drinking Warm Water on Esophageal Preparation Before Peroral Endoscopic Myotomy in Patients With Achalasia
Hong Jin Yoon, Young Hoon Youn, Sung Hwan Yoo, Seyeon Jeon, Hyojin Park
Journal of Neurogastroenterology and Motility.2022; 28(2): 231. CrossRef - Anesthesia for Per-oral endoscopic myotomy (POEM) – not so poetic!
Soumya Sarkar, Puneet Khanna, Deepak Gunjan
Journal of Anaesthesiology Clinical Pharmacology.2022; 38(1): 28. CrossRef - Third space endoscopy is a zone for teamwork
V.V. Subbotin, K.V. Shishin, I.Yu. Nedoluzhko, I.Yu. Larionov, A.A. Malakhova, I.S. Kanischev, I.I. Khvorova, S.S. Kazakova
Dokazatel'naya gastroenterologiya.2022; 11(3): 37. CrossRef - Drugs used for sedation in gastrointestinal endoscopy
Jun Kyu Lee
Journal of the Korean Medical Association.2022; 65(11): 735. CrossRef - Acquiring new complex endoscopic skills: Experience from the development of peroral endoscopic myotomy (POEM) in Malaysia
Shiaw Hooi Ho, Nik M A Nik Arsyad, Peng Choong Lau, Fadhil H Jamaludin, Sanjiv Mahadeva
JGH Open.2021; 5(7): 729. CrossRef - Challenges in Anesthesia Management for Peroral Endoscopic Myotomy: A Retrospective Analysis
Derya A. Yurtlu, Fatih Aslan
Surgical Laparoscopy, Endoscopy & Percutaneous Techniques.2021; 31(6): 729. CrossRef
- Effectiveness and safety of peroral endoscopic myotomy in patients with achalasia
- 8,080 View
- 294 Download
- 8 Web of Science
- 11 Crossref

- Management of Complications of Colorectal Submucosal Dissection
- Eun Ran Kim, Dong Kyung Chang
- Clin Endosc 2019;52(2):114-119. Published online March 29, 2019
- DOI: https://doi.org/10.5946/ce.2019.063
-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader ePub
ePub - Endoscopic submucosal dissection (ESD) is a useful procedure for the treatment of superficial gastrointestinal neoplasm. Compared with endoscopic mucosal resection (EMR), ESD has several benefits, which include resectability of various difficult lesion, accurate histologic assessment of specimen, and lower recurrence rate. However, the risk of procedure- related complications is higher with ESD than with EMR. Moreover, because the colon has a thin wall and limited endoscopic maneuverability, ESD is considered a more challenging and risky procedure when performed in the colon than in the stomach. ESD-related complications are more likely to occur. The significant complications associated with ESD are bleeding, perforation, coagulation syndrome and stenosis, most of which can be treated and prevented by endoscopic intervention and preparation. Therefore, it is important to know how to occur and manage the ESD related complication.
-
Citations
Citations to this article as recorded by- Feasibility and safety of 0.6% sodium alginate in endoscopic submucosal dissection for colorectal neoplastic lesion: A pilot study
Hajime Nakamura, Rie Morita, Ryo Ito, Akira Sakurada, Natsumi Tomita, Yuya Hirata, Yusuke Kanari, Yuya Komatsu, Kunihiro Takanashi, Tomonori Anbo, Shinichi Katsuki
DEN Open.2024;[Epub] CrossRef - Endoscopic Submucosal Dissection for Resections Larger than 10 cm: Outcomes from a Portuguese Center
Raquel R. Mendes, Pedro Barreiro, André Mascarenhas, Ana Rita Franco, Liliana Carvalho, Cristina Chagas
GE - Portuguese Journal of Gastroenterology.2024; 31(1): 33. CrossRef - A Phase II Clinical Trial to Study the Safety of Triamcinolone after Endoscopic Radial Incision and Cutting Dilatation for Benign Stenosis of the Lower Gastrointestinal Tract: A Study Protocol
RINTARO MOROI, HISASHI SHIGA, KOTARO NOCHIOKA, HIROFUMI CHIBA, YUSUKE SHIMOYAMA, MOTOYUKI ONODERA, TAKEO NAITO, MASAKI TOSA, YOICHI KAKUTA, YUICHIRO SATO, SHOICHI KAYABA, SEICHI TAKAHASHI, SATOSHI MIYATA, YOSHITAKA KINOUCHI, ATSUSHI MASAMUNE
The Kurume Medical Journal.2024;[Epub] CrossRef - Meta-Analysis of Endoscopic Full-Thickness Resection Versus Endoscopic Submucosal Dissection for Complex Colorectal Lesions
Sahib Singh, Babu P. Mohan, Rakesh Vinayek, Sudhir Dutta, Dushyant S. Dahiya, Manesh K. Gangwani, Vishnu C. Suresh Kumar, Ganesh Aswath, Ishfaq Bhat, Sumant Inamdar, Neil Sharma, Douglas G. Adler
Journal of Clinical Gastroenterology.2024;[Epub] CrossRef - Management of giant colorectal polyps (≥3 cm) by endoscopic submucosal dissection (ESD) versus surgery: a propensity score–based analysis
Lo Hau Ching Michelle, Poon Chi Ming Michael
Surgical Practice.2024;[Epub] CrossRef - The role of endoluminal surgery in a colorectal surgical practice. A global view
Ilker Ozgur, Fevzi Cengiz
Seminars in Colon and Rectal Surgery.2024; 35(2): 101023. CrossRef - Endoscopic submucosal dissection for colorectal polyps: outcome determining factors
Chi Woo Samuel Chow, Tak Lit Derek Fung, Pak Tat Chan, Kam Hung Kwok
Surgical Endoscopy.2023; 37(2): 1293. CrossRef - A novel strategy to perform endoscopic full-thickness resection at the ileocecal valve and securing the orifice with a double-pigtail catheter
Moritz Meiborg, Nicolae-Catalin Mechie, Tobias Blasberg, Marie Weber, Edris Wedi
Endoscopy.2023; 55(S 01): E375. CrossRef - A novel strategy to perform endoscopic full-thickness resection at the ileocecal valve and securing the orifice with a double-pigtail catheter
Moritz Meiborg, Nicolae-Catalin Mechie, Tobias Blasberg, Marie Weber, Edris Wedi
Endoscopy.2023; 55(06): 583. CrossRef - Experience of surgical treatment in a granular cell tumor in the qscending colon: a case report
In-Kyeong Kim, Young-Tae Ju, Han-Gil Kim, Jin-Kwon Lee, Dong-Chul Kim, Jae-Myung Kim, Jin Kyu Cho, Ji-Ho Park, Ju-Yeon Kim, Chi-Young Jeong, Soon-Chan Hong, Seung-Jin Kwag
Annals of Coloproctology.2023; 39(3): 275. CrossRef - Management of complications related to colorectal endoscopic submucosal dissection
Tae-Geun Gweon, Dong-Hoon Yang
Clinical Endoscopy.2023; 56(4): 423. CrossRef - Endoscopic Mucosal Resection and Endoscopic Submucosal Dissection
Sumeyye Yilmaz, Emre Gorgun
Clinics in Colon and Rectal Surgery.2023;[Epub] CrossRef - Colorectal Endoscopic Submucosal Dissection: Performance of a Novel Hybrid-Technology Knife in an Animal Trial
Jérémie Jacques, Horst Neuhaus, Markus D. Enderle, Ulrich Biber, Walter Linzenbold, Martin Schenk, Kareem Khalaf, Alessandro Repici
Diagnostics.2023; 13(21): 3347. CrossRef - Delayed Perforation of Colorectal Endoscopic Submucosal Dissection Treated by Endoscopic Ultrasound-Guided Drainage
Koichi Hamada, Yoshiki Shiwa, Akira Kurita, Yukitoshi Todate, Yoshinori Horikawa, Kae Techigawara, Masafumi Ishikawa, Takayuki Nagahashi, Yuki Takeda, Daizo Fukushima, Noriyuki Nishino, Hideo Sakuma, Michitaka Honda
Case Reports in Gastroenterology.2023; 17(1): 155. CrossRef - Colonoscopic‐assisted laparoscopic wedge resection versus segmental colon resection for benign colonic polyps: a comparative cost analysis
Julia Hanevelt, Laura W. Leicher, Leon M. G. Moons, Frank P. Vleggaar, Jelle F. Huisman, Henderik L. van Westreenen, Wouter H. de Vos tot Nederveen Cappel
Colorectal Disease.2023; 25(11): 2147. CrossRef - Temperature profile and residual heat of monopolar laparoscopic and endoscopic dissection instruments
Franz Brinkmann, Ronny Hüttner, Philipp J. Mehner, Konrad Henkel, Georgi Paschew, Moritz Herzog, Nora Martens, Andreas Richter, Sebastian Hinz, Justus Groß, Clemens Schafmayer, Jochen Hampe, Alexander Hendricks, Frank Schwandner
Surgical Endoscopy.2022; 36(6): 4507. CrossRef - A pilot study investigating the safety and feasibility of endoscopic dilation using a radial incision and cutting technique for benign strictures of the small intestine: a study protocol
Rintaro Moroi, Hisashi Shiga, Kotaro Nochioka, Yusuke Shimoyama, Masatake Kuroha, Yoichi Kakuta, Yoshitaka Kinouchi, Atsushi Masamune
Pilot and Feasibility Studies.2022;[Epub] CrossRef - Applicability of endoscopic submucosal dissection after unsuccessful endoscopic mucosal resection in colorectal laterally spreading tumors: a single center experience
Abdullah Murat BUYRUK, Ayten LİVAOĞLU, Aydın AKTAŞ
Ege Tıp Dergisi.2022; 61(2): 151. CrossRef - One thousand endoscopic submucosal dissections. Experience of the national center
S.I. Achkasov, Yu.A. Shelygin, A.A. Likutov, D.A. Mtvralashvili, V.V. Veselov, O.A. Mainovskaya, M.A. Nagudov, S.V. Chernyshov
Khirurgiya. Zhurnal im. N.I. Pirogova.2022; (8): 5. CrossRef - Post-polypectomy syndrome—a rare complication in colonoscopy procedures: a case report
Julián A Romo, Jorge David Peña, Laura A López, Carlos Figueroa, Horacio Garzon, Andrea Recamán
Journal of Surgical Case Reports.2022;[Epub] CrossRef - Clinical outcomes of endoscopic submucosal dissection for colorectal neoplasms: A single-center experience in Southern Taiwan
Chen-Yu Ko, Chih-Chien Yao, Yu-Chi Li, Lung-Sheng Lu, Yeh-Pin Chou, Ming-Luen Hu, Yi-Chun Chiu, Seng-Kee Chuah, Wei-Chen Tai, Hsu-Heng Yen
PLOS ONE.2022; 17(10): e0275723. CrossRef - Safety and feasibility of same-day discharge after esophageal endoscopic submucosal dissection
Yuri Hanada, Kenneth K. Wang
Gastrointestinal Endoscopy.2021; 93(4): 853. CrossRef - Evaluations on laser ablation of ex vivo porcine stomach tissue for development of Ho:YAG-assisted endoscopic submucosal dissection (ESD)
Hanjae Pyo, Hyeonsoo Kim, Hyun Wook Kang
Lasers in Medical Science.2021; 36(7): 1437. CrossRef - Evaluation of improved bi-manual endoscopic resection using a customizable 3D-printed manipulator system designed for use with standard endoscopes: a feasibility study using a porcine ex-vivo model
Benjamin Walter, Yannick S. Krieger, Dirk Wilhelm, Hubertus Feussner, Tim C. Lueth, Alexander Meining
Endoscopy International Open.2021; 09(06): E881. CrossRef - A patient-like swine model of gastrointestinal fibrotic strictures for advancing therapeutics
Ling Li, Mohamad I. Itani, Kevan J. Salimian, Yue Li, Olaya Brewer Gutierrez, Haijie Hu, George Fayad, Jean A. Donet, Min Kyung Joo, Laura M. Ensign, Vivek Kumbhari, Florin M. Selaru
Scientific Reports.2021;[Epub] CrossRef - Review on colorectal endoscopic submucosal dissection focusing on the technical aspect
Tak Lit Derek Fung, Chi Woo Samuel Chow, Pak Tat Chan, Kam Hung Kwok
Surgical Endoscopy.2020; 34(9): 3766. CrossRef - Endovascular hemostasis for endoscopic procedure-related gastrointestinal bleeding
Minho Park, Jong Woo Kim, Ji Hoon Shin
International Journal of Gastrointestinal Intervention.2019; 8(3): 134. CrossRef
- Feasibility and safety of 0.6% sodium alginate in endoscopic submucosal dissection for colorectal neoplastic lesion: A pilot study
- 8,806 View
- 339 Download
- 27 Web of Science
- 27 Crossref

- Air Embolism during Upper Endoscopy: A Case Report
- Yin Fang, Junbei Wu, Feng Wang, Lihong Cheng, Yunhong Lu, Xiaofei Cao
- Clin Endosc 2019;52(4):365-368. Published online March 13, 2019
- DOI: https://doi.org/10.5946/ce.2018.201

-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader ePub
ePub - Air embolism is a rare complication of upper endoscopy and potentially causes life-threatening events. A 67-year-old man with a history of surgery of cardiac carcinoma and pancreatic neuroendocrine tumor underwent painless upper endoscopy because of tarry stools. During the procedure, air embolism developed, which caused decreased pulse oxygen saturation and delayed sedation recovery. He recovered with some weakness of the left upper limb in the intensive care unit without hyperbaric oxygen therapy. The etiology, clinical manifestations, and treatments of air embolism are discussed based on the literature reports. Although air embolism is uncommon in endoscopic examinations, the patients’ outcomes could be improved if clinicians are alert to this potential complication, and promptly start proper diagnostic and therapeutic measures.
-
Citations
Citations to this article as recorded by- Anesthesia for Advanced Endoscopic Procedures
Basavana Goudra, Monica Saumoy
Clinical Endoscopy.2022; 55(1): 1. CrossRef - Cerebral Air Embolism After Endoscopic Variceal Band Ligation
Maria Azhar, Sunita Upreti, Bruce F. Sabath
ACG Case Reports Journal.2020; 7(8): e00443. CrossRef - Cerebral Air Embolism after Esophagogastroduodenoscopy: Insight on Pathophysiology, Epidemiology, Prevention and Treatment
Malik Ghannam, Azizullah Beran, Dana Ghazaleh, Tanner Ferderer, Brent Berry, Mona Al Banna, Leighton Mohl, Christopher Streib, Tapan Thacker, Ivan Matos
Journal of Stroke and Cerebrovascular Diseases.2019; 28(12): 104403. CrossRef
- Anesthesia for Advanced Endoscopic Procedures
- 4,305 View
- 117 Download
- 3 Web of Science
- 3 Crossref

- Gastrocolocutaneous Fistula: An Unusual Case of Gastrostomy Tube Malfunction with Diarrhea
- Junghwan Lee, Jinyoung Kim, Ha il Kim, Chung Ryul Oh, Sungim Choi, Soomin Noh, Hee Kyong Na, Hwoon-Yong Jung
- Clin Endosc 2018;51(2):196-200. Published online August 31, 2017
- DOI: https://doi.org/10.5946/ce.2017.062
-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader ePub
ePub - A gastrocolocutaneous fistula is a rare complication of percutaneous endoscopic gastrostomy (PEG). We report a case of a gastrocolocutaneous fistula presenting with intractable diarrhea and gastrostomy tube malfunction. A 62-year-old woman with a history of multiple system atrophy was referred to us because of PEG tube malfunction. Twenty days prior to presentation, the patient started developing sudden diarrhea within minutes after starting PEG feeding. Fluoroscopy revealed that the balloon of the PEG tube was located in the lumen of the transverse colon with the contrast material filling the colon. Subsequently, the PEG tube was removed and the opening of the gastric site was endoscopically closed using hemoclips. Clinicians should be aware of gastrocolocutaneous fistula as one of the complications of PEG insertion. Sudden onset of diarrhea, immediately after PEG feedings, might suggest this complication, which can be effectively treated with endoscopic closure.
-
Citations
Citations to this article as recorded by- A Rare Case of Severe Diarrhea: Gastrocolic Fistula Caused by Migration of Percutaneous Endoscopic Gastrostomy Tube
Maria Elena Pugliese, Riccardo Battaglia, Antonio Cerasa, Lucia Francesca Lucca
Healthcare.2023; 11(9): 1263. CrossRef - An unusual complication of percutaneous endoscopic gastrostomy and its endoscopic treatment
Noble Thomas, Cherukara Philip Thomas, C. Ganesh Pai
Indian Journal of Gastroenterology.2023; 42(4): 580. CrossRef - How far is the endoscopist to blame for a percutaneous endoscopic gastrostomy complication?
George Stavrou, Persefoni Gionga, George Chatziantoniou, Georgios Tzikos, Alexandra Menni, Stavros Panidis, Anne Shrewsbury, Katerina Kotzampassi
World Journal of Gastrointestinal Surgery.2023; 15(5): 940. CrossRef - Misplacement of the PEG tube through the transverse colon, an uncommon but possible complication
David Viso Vidal, Francisco Jorquera Plaza
Revista Española de Enfermedades Digestivas.2022;[Epub] CrossRef - Endoscopic management of enteral tubes in adult patients – Part 2: Peri- and post-procedural management. European Society of Gastrointestinal Endoscopy (ESGE) Guideline
Paraskevas Gkolfakis, Marianna Arvanitakis, Edward J. Despott, Asuncion Ballarin, Torsten Beyna, Kurt Boeykens, Peter Elbe, Ingrid Gisbertz, Alice Hoyois, Ofelia Mosteanu, David S. Sanders, Peter T. Schmidt, Stéphane M. Schneider, Jeanin E. van Hooft
Endoscopy.2021; 53(02): 178. CrossRef - Een laattijdige complicatie na het plaatsen van een PEG-sonde
H. DEDECKER, T. STEINHAUSER, S. BOUHADAN, O. PETERS, A. BEUNIS
Tijdschrift voor Geneeskunde.2021;[Epub] CrossRef - Complex gastro-colo-cutaneous fistula secondary to a gunshot injury, management and literature review
Maha Al Shaibi, Mohamed Al Abri, Ghaitha Al Mahruqi, Alok Mittal
Trauma Case Reports.2020; 28: 100313. CrossRef - Colocutaneous Fistula after Percutaneous Endoscopic Gastrostomy (PEG) Tube Insertion
Matthew Warner, Muhammad Durrani
Clinical Practice and Cases in Emergency Medicine.2020; 4(4): 632. CrossRef - Rectal Bleeding after Insertion of a Percutaneous Endoscopic Gastrostomy Tube
Ghadeer Alhazmi, Mroj Alsabri, Shahad Alsuwat, Adnan Al-Zangabi, Abdulaziz Al-Zahrani, Mohammed Kareemulla Shariff
Case Reports in Gastroenterology.2020; 14(3): 637. CrossRef - Long-Term Gastrocolocutaneous Fistula after Endoscopic Gastrostomy: How Concerned Should We Be?
Gonçalo Nunes, Gabriel Paiva de Oliveira, João Cruz, Carla Adriana Santos, Jorge Fonseca
GE - Portuguese Journal of Gastroenterology.2019; 26(6): 441. CrossRef - Update on endoscopic enteral access
Kamthorn Yolsuriyanwong, Bipan Chand
Techniques in Gastrointestinal Endoscopy.2018; 20(4): 172. CrossRef
- A Rare Case of Severe Diarrhea: Gastrocolic Fistula Caused by Migration of Percutaneous Endoscopic Gastrostomy Tube
- 9,388 View
- 151 Download
- 10 Web of Science
- 11 Crossref

- Endoscopic Approach for Major Complications of Bariatric Surgery
- Moon Kyung Joo
- Clin Endosc 2017;50(1):31-41. Published online December 23, 2016
- DOI: https://doi.org/10.5946/ce.2016.140
-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader ePub
ePub - As lifestyle and diet patterns have become westernized in East Asia, the prevalence of obesity has rapidly increased. Bariatric surgeries, such as Roux-en-Y gastric bypass (RYGB), sleeve gastrectomy (SG), and laparoscopic adjustable gastric banding (LAGB), are considered the first-line treatment option in patients with severe obesity. However, postoperative complications have increased and the proper management of these complications, including the use of endoscopic procedures, has become important. The most serious complications, such as leaks and fistulas, can be treated with endoscopic stent placement and injection of fibrin glue, and a novel full-thickness closure over-the-scope clip (OTSC) has been used for treatment of postoperative leaks. Stricture at the gastrojejunal (GJ) anastomosis site after RYGB or incisura angularis in SG can be managed using stents or endoscopic balloon dilation. Dilation of the GJ anastomosis or gastric pouch may lead to failure of weight loss, and the use of endoscopic sclerotherapy, novel endoscopic suturing devices, and OTSCs have been attempted. Intragastric migration of the gastric band can be successfully treated using various endoscopic tools. Endoscopy plays a pivotal role in the management of post-bariatric complications, and close cooperation between endoscopists and bariatric surgeons may further increase the success rate of endoscopic procedures.
-
Citations
Citations to this article as recorded by- Advanced endolumenal management of acute and chronic leaks after bariatric surgery
Andrew R. Harner, Francisco Jr Guerra, Shinil K. Shah, Kulvinder S. Bajwa, Peter A. Walker, Erik B. Wilson, Melissa M. Felinski
Mini-invasive Surgery.2024;[Epub] CrossRef - Current Management and Treatment Paradigms of Gastroesophageal Reflux Disease following Sleeve Gastrectomy
Muaaz Masood, Donald E. Low, Shanley B. Deal, Richard A. Kozarek
Journal of Clinical Medicine.2024; 13(5): 1246. CrossRef - Endoscopic Management of Post-Sleeve Gastrectomy Complications
Muaaz Masood, Donald E. Low, Shanley B. Deal, Richard A. Kozarek
Journal of Clinical Medicine.2024; 13(7): 2011. CrossRef - A Systemic Review on Photodynamic Therapy: Emerging Technology with
Healing Process
Prachi Varshney, Yogesh Kumar, Devdhar Yadav, Amit Singh, Naga Rani Kagithala, Pramod Kumar Sharma, Omji Porwal, Neeraj Kumar Fuloria, Pradeep Kumar Sharma, Ashok Kumar Gupta, G.S.N. Koteswara Rao
Current Cancer Therapy Reviews.2024; 20(3): 283. CrossRef - Endoscopic closure techniques of bariatric surgery complications: a meta-analysis
William N. Doyle, Alexander Netzley, Rahul Mhaskar, Abdul-Rahman F. Diab, Samer Ganam, Joseph Sujka, Christopher DuCoin, Salvatore Docimo
Surgical Endoscopy.2024; 38(5): 2894. CrossRef - Digestive neo-epithelialization after endoscopic stenting for upper digestive tract complete disunion
Sohaib Ouazzani, Arnaud Lemmers, Jean-Michel Gonzalez, Jean Closset, Imad El Moussaoui, Jacques Devière, Marc Barthet
Endoscopy.2024;[Epub] CrossRef - The Efficacy and Safety of Endoscopic Balloon Dilatation in the Treatment of Functional Post-Sleeve-Gastrectomy Stenosis
Mohamed A. Elsebaey, Mohamed Elsayed Enaba, Heba Elashry, Waleed Elrefaey, Rasha Youssef Hagag, Neveen A. Shalaby, Mohamed Sabry Aboelnasr, Mohamed Elsayed Sarhan, Omneya Mohamed Darrag, Assem Mohamed Elsokkary, Mohamed Abd Allah Alabd, Ahmed Mohamed El N
Medicina.2024; 60(5): 833. CrossRef - Bariatric Surgery Emergencies in Acute Care Surgery
Kalyana C. Nandipati, Kristin C. Bremer
Surgical Clinics of North America.2023; 103(6): 1113. CrossRef - Massive enlargement of gastric pouch as a complication of gastrojejunal anastomotic stenosis following one anastomosis laparoscopic gastric bypass: A case report
A. Martel-Vilchis, V. Gallardo-Chavez, P. León-Cabral, A. Paz-Fernández, E. Luna-Martinez, M. Sierra-Salazar
International Journal of Surgery Case Reports.2023; 110: 108557. CrossRef - Unexplained recurrent left lower lobe pneumonia, haematemesis and splenomegaly in a 32‐year‐old gentleman
Cynthuja Thilakanathan, Matthew Hall, Wassim Rahman, Mark Magdy, John Jorgensen
ANZ Journal of Surgery.2022; 92(1-2): 258. CrossRef - An innovative endoscopic management strategy for postoperative fistula after laparoscopic sleeve gastrectomy
Haiming Fang, Tingting Yao, Yating Chen, Yan Lu, Kangwei Xiong, Yuan Su, Yujue Zhang, Yong Wang, Lijiu Zhang
Surgical Endoscopy.2022; 36(9): 6439. CrossRef - Stent Management of Leaks After Bariatric Surgery: a Systematic Review and Meta-analysis
Andreu Martínez Hernández, Homero Beltrán Herrera, Vicente Martínez García, Miguel Ibáñez Belenguer, Raquel Queralt Martín, Ana Karina Maiocchi Segredo, Elena Aliaga Hilario, José Manuel Laguna Sastre
Obesity Surgery.2022; 32(4): 1034. CrossRef - Status of bariatric endoscopy–what does the surgeon need to know? A review
Diogo Turiani Hourneaux de Moura, Anna Carolina Batista Dantas, Igor Braga Ribeiro, Thomas R McCarty, Flávio Roberto Takeda, Marco Aurelio Santo, Sergio Carlos Nahas, Eduardo Guimarães Hourneaux de Moura
World Journal of Gastrointestinal Surgery.2022; 14(2): 185. CrossRef - Personalized Health Care Technology in Managing Postoperative Gastrointestinal Surgery Complications: Proof of Concept Study
Yaqeen Qudah, Mohammed Abdallah, Juan S. Barajas-Gamboa, Gabriel Diaz Del Gobbo, Juan Pablo Pantoja, Ricard Corcelles, John Rodriguez, Numan Balci, Matthew Kroh
Journal of Laparoendoscopic & Advanced Surgical Techniques.2022; 32(11): 1170. CrossRef - Role of Primary Use of Mega Stents Alone and Combined with Other Endoscopic Procedures for Early Leak and Stenosis After Bariatric Surgery, Single-Institution Experience
Mohamed Hany, Mohamed Ibrahim, Ahmed Zidan, Mohamed Samir, Amr Elsherif, Mohamed Selema, Mohamed Sharaan, Mohamed Elhashash
Obesity Surgery.2021; 31(5): 2050. CrossRef - A Comprehensive Review of Endoscopic Management of Sleeve Gastrectomy Leaks
Mihajlo Gjeorgjievski, Zaid Imam, Mitchell S. Cappell, Laith H. Jamil, Michel Kahaleh
Journal of Clinical Gastroenterology.2021; 55(7): 551. CrossRef - Foregut Issues After Bariatric Surgery
Fareed Cheema, Aurora D. Pryor
Foregut: The Journal of the American Foregut Society.2021; 1(4): 386. CrossRef - Endoscopic Stents in the Management of Bariatric Complications: Our Algorithm and Outcomes
Shyam Vedantam, Jay Roberts
Obesity Surgery.2020; 30(3): 1150. CrossRef - Mini gastric bypass for the management of gastrobronchial fistula: A case report
Abdulhamid Alharbi, Mohammed Alnaami, Abdulrahman Alsayyari, Mana Almuhaideb
International Journal of Surgery Case Reports.2020; 66: 192. CrossRef - Incidence and Efficacy of Stent Placement in Leak Management After Bariatric Surgery
Arielle E. Kanters, Sarah P. Shubeck, Oliver A. Varban, Justin B. Dimick, Dana A. Telem
Annals of Surgery.2020; 271(1): 134. CrossRef - Evolving procedural options for the treatment of obesity
Talar Tatarian, Kais A. Rona, Daniel H. Shin, Daniel G. Chen, Christopher G. Ducoin, Rachel L. Moore, Vitor O. Brunaldi, Manoel Galvão-Neto, Jessica Ardila-Gatas, Salvatore Docimo, Diogo T. Hourneax de Moura, Pichamol Jirapinyo, Christopher C. Thompson, H
Current Problems in Surgery.2020; 57(4): 100742. CrossRef - Endoscopic treatment of early leaks and strictures after laparoscopic one anastomosis gastric bypass
Fadi Younis, Mati Shnell, Nathan Gluck, Subhi Abu-Abeid, Shai Eldar, Sigal Fishman
BMC Surgery.2020;[Epub] CrossRef - Endoscopic balloon dilation for treatment of sleeve gastrectomy stenosis: a systematic review and meta-analysis
Steven H. Chang, Violeta B. Popov, Christopher C. Thompson
Gastrointestinal Endoscopy.2020; 91(5): 989. CrossRef - Long-term outcomes following endoscopic stenting in the management of leaks after foregut and bariatric surgery
Varun Krishnan, Kevin Hutchings, Andrew Godwin, Jonathan T. Wong, Julio Teixeira
Surgical Endoscopy.2019; 33(8): 2691. CrossRef - A Spanish Society joint SECO and SEEDO approach to the Post-operative management of the patients undergoing surgery for obesity
R Vilallonga, JL Pereira-Cunill, S Morales-Conde, I Alarcón, I Breton, E Domínguez-Adame, JV Ferrer, A Garcia Ruiz-de-Gordejuela, A Goday, A Lecube, E Martín García-Almenta, MÁ Rubio, FJ Tinahones, PP García-Luna
Obesity Surgery.2019; 29(12): 3842. CrossRef - Endoscopic Abscess Septotomy: A Less Invasive Approach for the Treatment of Sleeve Gastrectomy Leaks
Camila B. Ortega, Alfredo D. Guerron, Dana Portenier
Journal of Laparoendoscopic & Advanced Surgical Techniques.2018; 28(7): 859. CrossRef - Management of gastric fistula complicating laparoscopic sleeve gastrectomy with biological glue in a combined percutaneous and endoscopic approach
Ahmad Assalia, Anat Ilivitzki, Amos Ofer, Alain Suissa, Elias Manassa, Iyad Khamaysi, Ahmad Mahajna
Surgery for Obesity and Related Diseases.2018; 14(8): 1093. CrossRef - A Retrospective 2-Year Follow-up of Late Complications Treated Surgically and Endoscopically After Laparoscopic Roux-en-Y Gastric Bypass (LRYGB) and Laparoscopic Sleeve Gastrectomy (LSG) for Morbid Obesity
Mervi Javanainen, Anne Penttilä, Harri Mustonen, Anne Juuti, Tom Scheinin, Marja Leivonen
Obesity Surgery.2018; 28(4): 1055. CrossRef - Acute bleeding obstruction pancreatitis after Roux-en-Y anastomosis in total gastrectomy: a single center experience
J. Weindelmayer, S. Laiti, R. La Mendola, M. Bencivenga, L. Scorsone, V. Mengardo, S. Giacopuzzi
Updates in Surgery.2018; 70(2): 301. CrossRef - Management of Bariatric Complications Using Endoscopic Stents: a Multi-Center Study
Rena C. Moon, Andre F. Teixeira, Lyz Bezerra, Helga Cristina Almeida Wahnon Alhinho, Josemberg Campos, Luiz Gustavo de Quadros, Artagnan Menezes Barbosa de Amorim, Manoel Galvao Neto, Muhammad A. Jawad
Obesity Surgery.2018; 28(12): 4034. CrossRef - Endoscopic management of surgical complications
Robert J. Bowles-Cintron, Armando Perez-Ginnari, Jose M. Martinez
Techniques in Gastrointestinal Endoscopy.2018; 20(4): 182. CrossRef - A nutrition problem solved by a two-step endoscopic removal of a non-adjustable gastric band
Christer D Johansen, Jan Norum, Bernt E Engebretsen, Uwe Agledahl
Journal of Surgical Case Reports.2018;[Epub] CrossRef - Organization of future training in bariatric gastroenterology
Timothy R Koch, Timothy R Shope, Christopher J Gostout
World Journal of Gastroenterology.2017; 23(35): 6371. CrossRef
- Advanced endolumenal management of acute and chronic leaks after bariatric surgery
- 10,365 View
- 329 Download
- 31 Web of Science
- 33 Crossref

- Challenges of Endoscopic Management of Pancreaticobiliary Complications in Surgically Altered Gastrointestinal Anatomy
- Tin Moe Wai, Eun Young Kim
- Clin Endosc 2016;49(6):502-505. Published online November 29, 2016
- DOI: https://doi.org/10.5946/ce.2016.146
-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader ePub
ePub - Pancreaticobiliary complications following various surgical procedures, including liver transplantation, are not uncommon and are important causes of morbidity and mortality. Therapeutic endoscopy plays a substantial role in these patients and can help to avoid the need for reoperation. However, the endoscopic approach in patients with surgically altered gastrointestinal (GI) anatomy is technically challenging because of the difficulty in entering the enteral limb to reach the target orifice to manage pancreaticobiliary complications. Additional procedural complexity is due to the need of special devices and accessories to obtain successful cannulation and absence of an elevator in forward-viewing endoscopes, which is frequently used in this situation. Once bilioenteric anastomosis is reached, the technical success rates achieved in expert hands approach those of patients with intact GI anatomy. The success of endoscopic therapy in patients with surgically altered GI anatomy depends on multiple factors, including the expertise of the endoscopist, understanding of postoperative anatomic changes, and the availability of suitable scopes and accessories for endoscopic management. In this issue of Clinical Endoscopy, the focused review series deals with pancreatobiliary endoscopy in altered GI anatomy such as bilioenteric anastomosis and post-gastrectomy.
- 6,219 View
- 104 Download

- Clinical Outcomes of Endoscopic Submucosal Dissection for Superficial Esophageal Squamous Neoplasms
- Jung Soo Park, Young Hoon Youn, Jae Jun Park, Jie-Hyun Kim, Hyojin Park
- Clin Endosc 2016;49(2):168-175. Published online February 12, 2016
- DOI: https://doi.org/10.5946/ce.2015.080
-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader ePub
ePub - Background
/Aims: Endoscopic treatment has been broadly applied to superficial esophageal neoplasms. Endoscopic submucosal dissection (ESD) allows for high rates of en bloc resection, precise histological assessment, and low rates of local recurrence. The aim of this study was to evaluate the outcomes of ESD for superficial esophageal neoplasms.
Methods
We retrospectively reviewed 36 esophageal ESDs for superficial squamous neoplasms in 32 patients between March 2009 and August 2014 at Gangnam Severance Hospital.
Results
The median patient age was 64 years, and 30 men were included. The indications were early squamous cell carcinoma in 26 lesions, adenoma with high-grade dysplasia in five lesions, and low-grade dysplasia in five lesions. The en bloc resection and R0 resection rates were 97.2% (35 of 36) and 91.7% (33 of 36), respectively. Microperforation and post-ESD bleeding occurred in 5.6% (2 of 36) and 5.6% (2 of 36), respectively. Post-ESD esophageal strictures developed in five patients (13.9%). Five patients (15.6%) had an additional treatment after ESD (concurrent chemoradiation therapy in three, radiation therapy in one, and surgery in one patient). There was no disease-specific mortality during the median follow-up of 31 months.
Conclusions
Favorable clinical outcomes were observed in ESD for superficial esophageal squamous neoplasms. Esophageal ESD could be a good treatment option in terms of efficacy and safety. -
Citations
Citations to this article as recorded by- Predictors of technical difficulty for trainees in esophageal endoscopic submucosal dissection
Tomoya Ueda, Ryu Ishihara, Shunsuke Yoshii, James Weiquan Li, Yuya Asada, Daiki Kitagawa, Atsuko Kizawa, Takehiro Ninomiya, Yuki Okubo, Yushi Kawakami, Yasuhiro Tani, Satoki Shichijo, Takashi Kanesaka, Sachiko Yamamoto, Yoji Takeuchi, Koji Higashino, Nori
Esophagus.2024; 21(1): 58. CrossRef - A superficial esophageal cancer with a Rokitansky diverticulum treated by endoscopic submucosal dissection with dental floss clip traction: Letter to the editor
Xueyi Lin, Li Fan, Min Lin
Clinics and Research in Hepatology and Gastroenterology.2024; 48(6): 102368. CrossRef - Development and validation of a model to determine the risk of esophageal strictures after endoscopic submucosal dissection for esophageal neoplasms
Si-yuan Xia, Qing Lu, Zi-jing Wang, Tao Gan, Jin-lin Yang, Zhu Wang
Surgical Endoscopy.2023; 37(3): 2163. CrossRef - High-power green-light laser endoscopic submucosal dissection for non-muscle-invasive bladder cancer: A technical improvement and its initial application
Jilu Zheng, Feifan Liu, Keqin Zhang, Yuzhu Xiang, Lianjun Li, Haiyang Zhang, Yinan Zhang, Ning Suo, Zilong Wang, Chenglin Han, Xunbo Jin, Muwen Wang, Chunxiao Wei, Ji Chen
Journal of Cancer Research and Therapeutics.2023; 19(4): 945. CrossRef - Management of esophageal neoplasms by endoscopic submucosal dissection: experience over 100 consecutive procedures
Josué Aliaga Ramos, Yoshinori Morita, Takashi Toyonaga, Danilo Carvalho, Moises Salgado Pedrosa, Vitor N. Arantes
Clinical Endoscopy.2023; 56(5): 613. CrossRef - Recent approach for preventing complications in upper gastrointestinal endoscopic submucosal dissection
Waku Hatta, Tomoyuki Koike, Hiroko Abe, Yohei Ogata, Masahiro Saito, Xiaoyi Jin, Takeshi Kanno, Kaname Uno, Naoki Asano, Akira Imatani, Atsushi Masamune
DEN Open.2022;[Epub] CrossRef - Additional endoscopic treatments for patients with positive lateral margins after endoscopic resection of early esophageal squamous cell carcinoma
Yong Feng, Wei Wei, Shuo Guo, Bao-Qing Li
Oncology Letters.2022;[Epub] CrossRef - Prevention of delayed bleeding with vonoprazan in upper gastrointestinal endoscopic treatment
Hiroko Abe, Waku Hatta, Yohei Ogata, Tomoyuki Koike, Masahiro Saito, Xiaoyi Jin, Kenichiro Nakagawa, Takeshi Kanno, Kaname Uno, Naoki Asano, Akira Imatani, Tomohiro Nakamura, Naoki Nakaya, Kunio Tarasawa, Kenji Fujimori, Kiyohide Fushimi, Atsushi Masamune
Journal of Gastroenterology.2021; 56(7): 640. CrossRef - Comparison of Magnifying Endoscopy with Blue Light Imaging and Narrow Band Imaging for Determining the Invasion Depth of Superficial Esophageal Squamous Cell Carcinoma by the Japanese Esophageal Society’s Intrapapillary Capillary Loop Classification
Waku Hatta, Tomoyuki Koike, Yohei Ogata, Yutaka Kondo, Nobuyuki Ara, Kaname Uno, Naoki Asano, Akira Imatani, Atsushi Masamune
Diagnostics.2021; 11(11): 1941. CrossRef - Risk factors of postoperative stricture after endoscopic submucosal dissection for superficial esophageal neoplasms
Nan Lin, Jie Lin, Jinrong Gong
Medicine.2021; 100(51): e28396. CrossRef - Close Observation versus Additional Surgery after Noncurative Endoscopic Resection of Esophageal Squamous Cell Carcinoma
Byeong Geun Song, Ga Hee Kim, Charles J. Cho, Hyeong Ryul Kim, Yang Won Min, Hyuk Lee, Byung-Hoon Min, Ho June Song, Yong-Hee Kim, Jun Haeng Lee, Hwoon-Yong Jung, Jae Ill Zo, Young Mog Shim
Digestive Surgery.2021; 38(3): 247. CrossRef - Management following endoscopic resection in elderly patients with early‐stage upper gastrointestinal neoplasia
Waku Hatta, Takuji Gotoda, Tomoyuki Koike, Atsushi Masamune
Digestive Endoscopy.2020; 32(6): 861. CrossRef - Endoscopic resection of early squamous neoplasia of the oesophagus: long-term follow-up in a UK population from a tertiary hospital
Jen Yee Kuan, Sameul Baskind, Yeson Kim, Stephen McGrath, Ramakrishna Chaparala, Arash Assadsangabi, Neeraj Prasad, George Regi, Yeng Ang
European Journal of Gastroenterology & Hepatology.2020; 32(7): 789. CrossRef - Long-term outcomes of endoscopic submucosal dissection and comparison to surgery for superficial esophageal squamous cancer: a systematic review and meta-analysis
Jen-Hao Yeh, Ru-Yi Huang, Ching-Tai Lee, Chih-Wen Lin, Ming-Hung Hsu, Tsung-Chin Wu, Po-Jen Hsiao, Wen-Lun Wang
Therapeutic Advances in Gastroenterology.2020; 13: 175628482096431. CrossRef Prediction of Lymph Node Metastasis in Superficial Esophageal Cancer Using a Pattern Recognition Neural Network
Han Chen, Xiaoying Zhou, Xinyu Tang, Shuo Li, Guoxin Zhang
Cancer Management and Research.2020; Volume 12: 12249. CrossRef- Predictors of technical difficulty during endoscopic submucosal dissection of superficial esophageal cancer
Hiromasa Hazama, Masaki Tanaka, Naomi Kakushima, Yohei Yabuuchi, Masao Yoshida, Noboru Kawata, Kohei Takizawa, Sayo Ito, Kenichiro Imai, Kinichi Hotta, Hirotoshi Ishiwatari, Hiroyuki Matsubayashi, Keita Mori, Hiroyuki Ono
Surgical Endoscopy.2019; 33(9): 2909. CrossRef - A Case of Esophageal Squamous Cell Carcinoma in situ Arising from Esophageal Squamous Papilloma
Jae Yeong Cho, Dae Young Cheung, Tae Jung Kim, Jae Kwang Kim
Clinical Endoscopy.2019; 52(1): 72. CrossRef - Endoscopic Submucosal Single- or Multi-tunnel Dissection for Near-Circumferential and Circumferential Superficial Esophageal Neoplastic Lesions
Lin-Lin Zhu, Jun-Chao Wu, Yi-Ping Wang, Du He, Wen-Yan Zhang, Tao Gan, Jin-Lin Yang
Gastroenterology Research and Practice.2019; 2019: 1. CrossRef - Outcomes of endoscopic submucosal dissection for early oesophageal squamous cell neoplasia at a Western centre
Diane Lorenzo, Maximilien Barret, Sarah Leblanc, Benoit Terris, Frédéric Beuvon, Romain Coriat, Stanislas Chaussade, Frédéric Prat
United European Gastroenterology Journal.2019; 7(8): 1084. CrossRef - Commentary on “Efficacy of Endoscopic Submucosal Dissection of Esophageal Neoplasms under General Anesthesia”
Soo In Choi, Jun Chul Park
Clinical Endoscopy.2019; 52(3): 205. CrossRef - Endoscopic Treatment for Esophageal Cancer
Eun Jeong Gong, Do Hoon Kim
The Korean Journal of Helicobacter and Upper Gastrointestinal Research.2019; 19(3): 156. CrossRef - UEG Week 2019 Oral Presentations
United European Gastroenterology Journal.2019; 7(S8): 10. CrossRef - Does delayed esophagectomy after endoscopic resection affect outcomes in patients with stage T1 esophageal cancer? A propensity score-based analysis
Shengfei Wang, Yangle Huang, Juntao Xie, Lingdun Zhuge, Longlong Shao, Jiaqing Xiang, Yawei Zhang, Yihua Sun, Hong Hu, Sufeng Chen, Toni Lerut, James D. Luketich, Jie Zhang, Haiquan Chen
Surgical Endoscopy.2018; 32(3): 1441. CrossRef - Risk factors of electrocoagulation syndrome after esophageal endoscopic submucosal dissection
Dae Won Ma, Young Hoon Youn, Da Hyun Jung, Jae Jun Park, Jie-Hyun Kim, Hyojin Park
World Journal of Gastroenterology.2018; 24(10): 1144. CrossRef - Endoscopic Treatment for Esophageal Cancer
Yang Won Min
The Korean Journal of Gastroenterology.2018; 71(3): 116. CrossRef - Endoscopic submucosal dissection under general anesthesia for superficial esophageal squamous cell carcinoma is associated with better clinical outcomes
Byeong Geun Song, Yang Won Min, Ra Ri Cha, Hyuk Lee, Byung-Hoon Min, Jun Haeng Lee, Poong-Lyul Rhee, Jae J. Kim
BMC Gastroenterology.2018;[Epub] CrossRef - Long-Term Survival and Tumor Recurrence in Patients with Superficial Esophageal Cancer after Complete Non-Curative Endoscopic Resection: A Single-Center Case Series
Ji Wan Lee, Charles J. Cho, Do Hoon Kim, Ji Yong Ahn, Jeong Hoon Lee, Kee Don Choi, Ho June Song, Sook Ryun Park, Hyun Joo Lee, Yong Hee Kim, Gin Hyug Lee, Hwoon-Yong Jung, Sung-Bae Kim, Jong Hoon Kim, Seung-Il Park
Clinical Endoscopy.2018; 51(5): 470. CrossRef - Endoscopic submucosal dissection followed by chemoradiotherapy for superficial esophageal cancer: choice of new approach
Gen Suzuki, Hideya Yamazaki, Norihiro Aibe, Koji Masui, Naomi Sasaki, Daisuke Shimizu, Takuya Kimoto, Atsushi Shiozaki, Osamu Dohi, Hitoshi Fujiwara, Takeshi Ishikawa, Hirotaka Konishi, Yuji Naito, Eigo Otsuji, Kei Yamada
Radiation Oncology.2018;[Epub] CrossRef - Efficacy and safety of endoscopic submucosal dissection in elderly patients with esophageal squamous cell carcinoma
Byeong Geun Song, Yang Won Min, Jun Haeng Lee, Hyuk Lee, Byung-Hoon Min, Poong-Lyul Rhee, Jae J. Kim
Surgical Endoscopy.2017; 31(10): 3905. CrossRef - Comparison of endoscopic ultrasonography and magnifying endoscopy for assessment of the invasion depth of shallow gastrointestinal neoplasms: a systematic review and meta-analysis
Zhang Tao, Chen Yan, He Zhao, Jiawei Tsauo, Xiaowu Zhang, Bing Qiu, Yanqing Zhao, Xiao Li
Surgical Endoscopy.2017; 31(12): 4923. CrossRef - Endoscopic Submucosal Dissection for Superficial Esophageal Neoplasm: A Growing Body of Evidence
Eun Jeong Gong, Hwoon-Yong Jung
Clinical Endoscopy.2016; 49(2): 101. CrossRef - Esophageal subepithelial tumor: why tunneling?
Jong-Jae Park
Gastroenterology & Hepatology: Open Access.2016;[Epub] CrossRef
- Predictors of technical difficulty for trainees in esophageal endoscopic submucosal dissection
- 9,639 View
- 114 Download
- 31 Web of Science
- 32 Crossref

- Endoluminal Closure of Colon Perforation with Endoscopic Band Ligation: Technical Feasibility and Safety in an In Vivo Canine Model
- Joung-Ho Han, Myounghwan Kim, Tae Hoon Lee, Hyun Kim, Yunho Jung, Seon Mee Park, Heebok Chae, Seijin Youn, Ji Yun Shin, In-Kwang Lee, Tae Soo Lee, Seok Hwa Choi
- Clin Endosc 2015;48(6):534-541. Published online November 30, 2015
- DOI: https://doi.org/10.5946/ce.2015.48.6.534
-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader ePub
ePub - Background
/Aims: Endoscopic band ligation (EBL) is an accepted method in the management of variceal bleeding; however, there is little evidence on the safety and feasibility of EBL for the closure of bowel perforation. In this study, we aimed to evaluate the technical feasibility and efficacy of EBL in iatrogenic colon perforation by using a canine model.
Methods
We established an iatrogenic colon perforation model by using seven beagle dogs. Longitudinal 1.5- to 1.7-cm colon perforations were created with a needle knife and an insulated-tip knife, and the perforation was subsequently closed with EBL. During a 2-week follow-up period, the animals were carefully monitored and then euthanized for pathologic examination.
Results
The EBL of iatrogenic colon perforations was successful in all dogs. The mean procedure time for EBL closure with one to three bands was 191.7 seconds, and there were no immediate complications. One animal was euthanized after 3 days because of peritonitis. There were no clinical and laboratory features of sepsis or peritonitis in the remaining six animals. On necropsy, we did not find any fecal peritonitis, pericolonic abscess formation, or transmural dehiscence at the perforation site. Histopathology demonstrated inflamed granulation tissue and scar lesions replaced by fibrosis.
Conclusions
EBL might be a feasible and safe method for the management of iatrogenic colon perforations in an in vivo model. -
Citations
Citations to this article as recorded by- Endoscopic Management of Iatrogenic Colon Perforation
Yunho Jung
Clinical Endoscopy.2020; 53(1): 29. CrossRef - Endoluminal closure of an unrecognized penetrating stab wound of the duodenum with endoscopic band ligation: A case report
Dae Hoon Kim, Hanlim Choi, Ki Bae Kim, Hyo Yung Yun, Joung-Ho Han
World Journal of Clinical Cases.2019; 7(20): 3271. CrossRef - Endoscopic management of iatrogenic gastrointestinal perforations
Kan Wang, Jihao Shi, Linna Ye
Laparoscopic, Endoscopic and Robotic Surgery.2019; 2(2): 41. CrossRef - Endoscopic Band Ligation Is Able to Close Perforations Caused by Colonoscopy: A Porcine Model Study
Yidong Yang, Xianyi Lin, Siwei Tan, Xiaoli Huang, Zijun Xie, Xuan Xu, Yiming Lei, Bin Wu
Gastroenterology Research and Practice.2018; 2018: 1. CrossRef
- Endoscopic Management of Iatrogenic Colon Perforation
- 10,371 View
- 79 Download
- 3 Web of Science
- 4 Crossref

- Factors for Endoscopic Submucosal Dissection in Early Colorectal Neoplasms: A Single Center Clinical Experience in China
- Yu-Qi He, Xin Wang, Ai-Qin Li, Lang Yang, Jian Zhang, Qian Kang, Shan Tang, Peng Jin, Jian-Qiu Sheng
- Clin Endosc 2015;48(5):405-410. Published online September 30, 2015
- DOI: https://doi.org/10.5946/ce.2015.48.5.405
-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader ePub
ePub Background/Aims Early colorectal (CR) neoplasm can be cured by endoscopic submucosal dissection (ESD), but clinical experience and factors associated with complications from ESD for CR neoplasms in China have not been reported.
Methods Seventy-eight cases of early CR neoplasm treated with endoscopic resection performed between December 2012 and December 2013 at Beijing Military General Hospital were included. Factors associated with ESD complications and procedure times were evaluated.
Results The
en bloc resection rate was 88.5% (69/78), tumor size was 32.1±10.7 mm, and procedure time was 71.8±49.5 minutes. The major complication was perforation, which occurred in 8.97% of the ESD procedures. Multivariate logistic regression analysis indicated that only tumor size (p =0.022) was associated with ESD perforation. Tumor size (p <0.001) and the non-lifting sign (p =0.017) were independent factors for procedure time, and procedure time (p =0.016) was a key factor foren bloc resection. After a median 10 months (range, 4 to 16) of follow-up, no patients had local recurrence.Conclusions This study indicated that ESD is an applicable method for large early CR neoplasm in the colon and rectum. Tumor size and the non-lifting sign might be considerable factors for increased complication rate and procedural time of ESD.
-
Citations
Citations to this article as recorded by- Risk factors for unsuccessful colorectal endoscopic submucosal dissection: A systematic review and meta-analysis
Feng Gu, Wei Jiang, Jingyi Zhu, Lei Ma, Boyuan He, Huihong Zhai
Digestive and Liver Disease.2023;[Epub] CrossRef - A large multicenter cohort on the use of full-thickness resection device for difficult colonic lesions
Y. Ichkhanian, K. Vosoughi, D. L. Diehl, I. S. Grimm, T. W. James, A. W. Templeton, K. Hajifathalian, J. L. Tokar, J. B. Samarasena, N. El Hage Chehade, J. Lee, K. Chang, M. Mizrahi, M. Barawi, S. Irani, S. Friedland, P. Korc, A. A. Aadam, M. A. Al-Haddad
Surgical Endoscopy.2021; 35(3): 1296. CrossRef - Evolving management of colorectal polyps
Yervant Ichkhanian, Tobias Zuchelli, Andrew Watson, Cyrus Piraka
Therapeutic Advances in Gastrointestinal Endoscopy.2021; 14: 263177452110470. CrossRef - Risk factors for adverse events of colorectal endoscopic submucosal dissection: a systematic review and meta-analysis
Juliana B. Santos, Moacyr R.C. Nobre, Cleyton Z. Oliveira, Adriana V. Safatle-Ribeiro, Fabio Kawaguti, Bruno Martins, Sergio C. Nahas, Ulysses Ribeiro, Lanjing Zhang, Fauze Maluf-Filho
European Journal of Gastroenterology & Hepatology.2021; 33(1S): e33. CrossRef - Colo-rectal endoscopic full-thickness resection (EFTR) with the over-the-scope device (FTRD®): A multicenter Italian experience
G. Andrisani, P. Soriani, M. Manno, M. Pizzicannella, F. Pugliese, M. Mutignani, R. Naspetti, L. Petruzziello, F. Iacopini, C. Grossi, P. Lagoussis, S. Vavassori, F. Coppola, A. La Terra, S. Ghersi, P. Cecinato, G. De Nucci, R. Salerno, M. Pandolfi, G. Co
Digestive and Liver Disease.2019; 51(3): 375. CrossRef - Comparing outcomes for endoscopic submucosal dissection between Eastern and Western countries: A systematic review and meta-analysis
Dane Christina Daoud, Nicolas Suter, Madeleine Durand, Mickael Bouin, Bernard Faulques, Daniel von Renteln
World Journal of Gastroenterology.2018; 24(23): 2518. CrossRef - Colonoscopic full-thickness resection using an over-the-scope device: a prospective multicentre study in various indications
Arthur Schmidt, Torsten Beyna, Brigitte Schumacher, Alexander Meining, Hans-Juergen Richter-Schrag, Helmut Messmann, Horst Neuhaus, David Albers, Michael Birk, Robert Thimme, Andreas Probst, Martin Faehndrich, Thomas Frieling, Martin Goetz, Bettina Riecke
Gut.2018; 67(7): 1280. CrossRef - Why attempt en bloc resection of non-pedunculated colorectal adenomas? A systematic review of the prevalence of superficial submucosal invasive cancer after endoscopic submucosal dissection
Lorenzo Fuccio, Alessandro Repici, Cesare Hassan, Thierry Ponchon, Pradeep Bhandari, Rodrigo Jover, Konstantinos Triantafyllou, Daniele Mandolesi, Leonardo Frazzoni, Cristina Bellisario, Franco Bazzoli, Prateek Sharma, Thomas Rösch, Douglas K Rex
Gut.2018; 67(8): 1464. CrossRef - Clinical outcomes after endoscopic submucosal dissection for colorectal neoplasia: a systematic review and meta-analysis
Lorenzo Fuccio, Cesare Hassan, Thierry Ponchon, Daniele Mandolesi, Andrea Farioli, Alessandro Cucchetti, Leonardo Frazzoni, Pradeep Bhandari, Cristina Bellisario, Franco Bazzoli, Alessandro Repici
Gastrointestinal Endoscopy.2017; 86(1): 74. CrossRef
- Risk factors for unsuccessful colorectal endoscopic submucosal dissection: A systematic review and meta-analysis
- 6,494 View
- 88 Download
- 8 Web of Science
- 9 Crossref

- Comparison of Clinical Outcomes Associated with Pull-Type and Introducer-Type Percutaneous Endoscopic Gastrostomies
- Sin Won Lee, Jeong Hoon Lee, Hyungjin Cho, Yeonjung Ha, Hyun Lim, Ji Yong Ahn, Kwi Sook Choi, Do Hoon Kim, Kee Don Choi, Ho June Song, Gin Hyug Lee, Hwoon-Yong Jung, Jin-Ho Kim
- Clin Endosc 2014;47(6):530-537. Published online November 30, 2014
- DOI: https://doi.org/10.5946/ce.2014.47.6.530
-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader ePub
ePub Background/Aims Percutaneous endoscopic gastrostomy (PEG) is a method of providing enteral nutrition using endoscopy. The PEG techniques differ according to the insertion method, and include the pull type, push type, and introducer type. The aim of this study was to compare the clinical outcomes associated with the pull-type and introducer-type PEG insertion techniques, which included the adverse events, at our tertiary care center in Korea.
Methods We retrospectively reviewed 141 cases that had undergone PEG insertion at our center from January 2009 to June 2012. The indications for PEG insertion and the acute and chronic complications caused by each type of PEG insertion were analyzed.
Results The indications for PEG insertion in our cohort included neurologic disease (58.7%), malignancy (21.7%), and other indications (19.6%). Successful PEG insertions were performed on 136 cases (96.5%), and there were no PEG-associated deaths. Bleeding was the most frequent acute complication (12.8%), and wound problems were the most frequent chronic complications (8.8%). There were no statistically significant differences between the pull-type and introducer-type PEG insertion techniques in relation to complication rates in our study population.
Conclusions PEG insertion is considered a safe procedure. The pull-type and introducer-type PEG insertion techniques produce comparable outcomes, and physicians may choose either of these approaches according to the circumstances.
-
Citations
Citations to this article as recorded by- Clinical Practice Guideline for Percutaneous Endoscopic Gastrostomy
Chung Hyun Tae, Ju Yup Lee, Moon Kyung Joo, Chan Hyuk Park, Eun Jeong Gong, Cheol Min Shin, Hyun Lim, Hyuk Soon Choi, Miyoung Choi, Sang Hoon Kim, Chul-Hyun Lim, Jeong-Sik Byeon, Ki-Nam Shim, Geun Am Song, Moon Sung Lee, Jong-Jae Park, Oh Young Lee
Gut and Liver.2024; 18(1): 10. CrossRef - Clinical practice guidelines for percutaneous endoscopic gastrostomy
Chung Hyun Tae, Ju Yup Lee, Moon Kyung Joo, Chan Hyuk Park, Eun Jeong Gong, Cheol Min Shin, Hyun Lim, Hyuk Soon Choi, Miyoung Choi, Sang Hoon Kim, Chul-Hyun Lim, Jeong-Sik Byeon, Ki-Nam Shim, Geun Am Song, Moon Sung Lee, Jong-Jae Park, Oh Young Lee
Clinical Endoscopy.2023; 56(4): 391. CrossRef - Clinical Practice Guideline for Percutaneous Endoscopic Gastrostomy
Chung Hyun Tae, Ju Yup Lee, Moon Kyung Joo, Chan Hyuk Park, Eun Jeong Gong, Cheol Min Shin, Hyun Lim, Hyuk Soon Choi, Miyoung Choi, Sang Hoon Kim, Chul-Hyun Lim, Jeong-Sik Byeon, Ki-Nam Shim, Geun Am Song, Moon Sung Lee, Jong-Jae Park, Oh Young Lee
The Korean Journal of Gastroenterology.2023; 82(3): 107. CrossRef - Buried T-Bar after Gastrostomy Placement in Children
Soon Chul Kim
Indian Journal of Pediatrics.2022; 89(8): 833. CrossRef - Nationwide Survey for Pediatric Gastrostomy Tube Placement in Korea
Sangwoo Lee, Byung-Ho Choe, Ben Kang, Soon Chul Kim
Journal of Korean Medical Science.2022;[Epub] CrossRef - Clinical Outcomes of Enteral Feeding Protocol Via Percutaneous Endoscopic Gastrostomy: A Single‐Center, Retrospective Study
Jin Hee Noh, Hee Kyong Na, Ji Yong Ahn, Suk‐Kyung Hong, Jiyoun Kim, Jina Yang, Hwoon‐Yong Jung
Nutrition in Clinical Practice.2021; 36(1): 225. CrossRef - Relationship of early acute complications and insertion site in push method percutaneous endoscopic gastrostomy
Hiroshi Suzuki, Satoru Joshita, Tadanobu Nagaya, Koichi Sato, Akihiro Ito, Tomoaki Suga, Takeji Umemura
Scientific Reports.2020;[Epub] CrossRef - The efficacy of a novel percutaneous endoscopic gastrostomy simulator using three‐dimensional printing technologies
Hee Kyong Na, Ji Yong Ahn, Gin Hyug Lee, Jeong Hoon Lee, Do Hoon Kim, Kee Wook Jung, Kee Don Choi, Ho June Song, Hwoon‐Yong Jung
Journal of Gastroenterology and Hepatology.2019; 34(3): 561. CrossRef - A large prospective audit of morbidity and mortality associated with feeding gastrostomies in the community
Emily Clarke, Narrie Pitts, Andrew Latchford, Stephen Lewis
Clinical Nutrition.2017; 36(2): 485. CrossRef - Antibacterial gauzes are effective in preventing infections after percutaneous endoscopic gastrostomy placement
Denise Strijbos, Erik J. Schoon, Wouter Curvers, Pieter Friederich, Hajo J. Flink, Arnold Stronkhorst, Lennard P.L. Gilissen
European Journal of Gastroenterology & Hepatology.2016; 28(3): 297. CrossRef - Advances in nutritional delivery interventions
Bryan Silon, John C. Fang
Techniques in Gastrointestinal Endoscopy.2015; 17(4): 152. CrossRef
- Clinical Practice Guideline for Percutaneous Endoscopic Gastrostomy
- 7,124 View
- 75 Download
- 13 Web of Science
- 11 Crossref

- Colonic Stent-Related Complications and Their Management
- Seung-Hee Han, Jong Hoon Lee
- Clin Endosc 2014;47(5):415-419. Published online September 30, 2014
- DOI: https://doi.org/10.5946/ce.2014.47.5.415
-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader ePub
ePub Since its introduction in the early 1990s, the self-expandable metal stent (SEMS) has been increasingly used for the management of malignant colorectal obstruction, not only as a palliative method but also as a preoperative treatment in surgical candidates. However, more recently, concerns have been raised over stent complication rates. Early complications include pain, perforation, and rectal bleeding, and late complications include stent migration and stent obstruction. With the increasing use of SEMS for treatment, physicians need to be more aware of complications occurring after the placement of these stents. This review covers the technical considerations and management of complications after colonic stenting.
-
Citations
Citations to this article as recorded by- Self-expandable metallic stents as a bridge to surgery in obstructive right- and left-sided colorectal cancer: a multicenter cohort study
Eui Myung Kim, Jun Ho Park, Byung Chun Kim, Il Tae Son, Jeong Yeon Kim, Jong Wan Kim
Scientific Reports.2023;[Epub] CrossRef - Arterial-colonic fistula secondary to colonic stent erosion into the left external iliac artery
Jacquelyn Dillon, Alexandra N Mills, Kate R Pawloski, Nicholas Scribetta, Alexander Greenstein
Journal of Surgical Case Reports.2023;[Epub] CrossRef - Interventional Radiology Management of Vascular Complications From Gastrointestinal Interventions
Harish A. Narayanan, Sailendra G. Naidu, Sadeer Alzubaidi, Indravadan Patel, Sasha Staack, Rahmi Oklu
Contemporary Diagnostic Radiology.2023; 46(4): 1. CrossRef - Acute malignant colorectal obstruction (K56.6; C18, C19, C20), adults
S. I. Achkasov, Z. A. Bagatelia, S. F. Bagnenko, A. M. Belyaev, Yu. A. Gevorkyan, V. L. Denisenko, I. I. Zatevakhin, A. D. Kaprin, A. M. Karachun, O. I. Kit, Z. Z. Mammedli, A. I. Moskalev, I. V. Nazarov, A. Sh. Revishvili, A. V. Sazhin, I. S. Stilidi, O.
Koloproktologia.2023; 22(2): 10. CrossRef - An Unusual Complication of Self-Expandable Metal Stent Placement in Malignant Sigmoid Obstruction
Qingjie Kang, Denghua Hu, Guangxu Wen, Zhengqiang Wei
Case Reports in Gastroenterology.2023; 17(1): 302. CrossRef - Abdominal compartment syndrome as a complication of endoscopic carbon dioxide insufflation in a patient with malignant bowel obstruction: a case report
Taro Tanabe, Genki Tsukuda, Takahiro Hobo, Noboru Yokoyama, Haruhiro Inoue
Surgical Case Reports.2023;[Epub] CrossRef - Case report: Stent-first strategy as a potential approach in the management of malignant right-sided colonic obstruction with cardiovascular risks
Tianyu Lin, Abdul Saad Bissessur, Pengfei Liao, Tunan Yu, Dingwei Chen
Frontiers in Surgery.2022;[Epub] CrossRef - Colovesical fistula with intravesical colonic stent migration
Chin Tang, Yu-Nung Chen, Yi-Wei Lee, Shu-Wei Tsai
Asian Journal of Surgery.2021; 44(12): 1581. CrossRef - A Study of 34 Cases of Palliative Colorectal Stenting for Colorectal Cancer Obstruction
Ippei YAMANA, Jun OHISHI, Kurumi SAHARA, Tatsuya HASHIMOTO, Hiroki TANI, Suguru HASEGAWA
Nihon Rinsho Geka Gakkai Zasshi (Journal of Japan Surgical Association).2021; 82(6): 1057. CrossRef - Colonic stenting as a bridge to surgery in malignant large bowel obstruction: oncological outcomes
N. E. Donlon, M. E. Kelly, F. Narouz, P. H. McCormick, J. O. Larkin, B. J. Mehigan
International Journal of Colorectal Disease.2019; 34(4): 613. CrossRef - Colorectal neuroendocrine carcinoma: A case report and review of the literature
Tomoaki Yoshida, Kenya Kamimura, Kazunori Hosaka, Koji Doumori, Hiromitsu Oka, Akito Sato, Yasuo Fukuhara, Shoji Watanabe, Tomomi Sato, Akira Yoshikawa, Takashi Tomidokoro, Shuji Terai
World Journal of Clinical Cases.2019; 7(14): 1865. CrossRef - Colonic stents for malignant bowel obstruction: current status and future prospects
Vittorio Maria Ormando, Rossella Palma, Alessandro Fugazza, Alessandro Repici
Expert Review of Medical Devices.2019; 16(12): 1053. CrossRef - Long-term outcomes after stenting as a bridge to surgery in patients with obstructing left-sided colorectal cancer
Jihye Park, Hyun Jung Lee, Soo Jung Park, Hyuk Hur, Byung Soh Min, Jae Hee Cheon, Tae Il Kim, Nam Kyu Kim, Won Ho Kim
International Journal of Colorectal Disease.2018; 33(6): 799. CrossRef - Three-dimensionally printed surface features to anchor endoluminal spring for distraction enterogenesis
Nhan Huynh, Genia Dubrovsky, Joshua D. Rouch, Andrew Scott, Elvin Chiang, Tommy Nguyen, Benjamin M. Wu, Shant Shekherdimian, Thomas M. Krummel, James C. Y. Dunn, Christina Chan
PLOS ONE.2018; 13(7): e0200529. CrossRef - Colorectal stenting in cancer patients
I. N. Yurichev, I. A. Karasev, V. V. Vereschak, M. S. Burdyukov, O. A. Malikhova, A. G. Malikhov
Colorectal Oncology.2018; 8(2): 55. CrossRef - A methodology for the customized design of colonic stents based on a parametric model
S. Puértolas, D. Navallas, A. Herrera, E. López, J. Millastre, E. Ibarz, S. Gabarre, J.A. Puértolas, L. Gracia
Journal of the Mechanical Behavior of Biomedical Materials.2017; 71: 250. CrossRef - Self-Expanding Metallic Stents (SEMS) in Left-Sided Colonic Cancer—a Cancer Center Experience
Kamran Muddasar Saeed, Waleed Zafar, Muhammad Adnan Masood, Shahid Khattak, Aamir Ali Syed, Muhammed Aasim Yusuf
Journal of Gastrointestinal Cancer.2016; 47(1): 69. CrossRef - Value in palliative cancer surgery: A critical assessment
Ian W. Folkert, Robert E. Roses
Journal of Surgical Oncology.2016; 114(3): 311. CrossRef - Comparison of through-the-scope stent insertion with standard stent insertion for the management of malignant colorectal obstruction: a prospective study
Y. Wan, Y.-Q. Zhu, N.-W. Chen, Z.-G. Wang, Y.-S. Cheng, J. Shi
Techniques in Coloproctology.2016; 20(10): 707. CrossRef - Obstructive Left Colon Cancer Should Be Managed by Using a Subtotal Colectomy Instead of Colonic Stenting
Chung Ki Min, Hyung Ook Kim, Donghyoun Lee, Kyung Uk Jung, Sung Ryol Lee, Hungdai Kim, Ho-Kyung Chun
Annals of Coloproctology.2016; 32(6): 215. CrossRef - Innate Immunity and Biomaterials at the Nexus: Friends or Foes
Susan N. Christo, Kerrilyn R. Diener, Akash Bachhuka, Krasimir Vasilev, John D. Hayball
BioMed Research International.2015; 2015: 1. CrossRef - Endoprótesis colónica para el manejo paliativo de la obstrucción intestinal por cáncer. Reporte de caso
Víctor Manuel Noriega Usi, Leopoldo Gutiérrez Rodríguez, Carlos González de Cosío Corredor
Endoscopia.2015; 27(3): 129. CrossRef
- Self-expandable metallic stents as a bridge to surgery in obstructive right- and left-sided colorectal cancer: a multicenter cohort study
- 8,802 View
- 105 Download
- 22 Web of Science
- 22 Crossref

- Complications Related to Gastric Endoscopic Submucosal Dissection and Their Managements
- Itaru Saito, Yosuke Tsuji, Yoshiki Sakaguchi, Keiko Niimi, Satoshi Ono, Shinya Kodashima, Nobutake Yamamichi, Mitsuhiro Fujishiro, Kazuhiko Koike
- Clin Endosc 2014;47(5):398-403. Published online September 30, 2014
- DOI: https://doi.org/10.5946/ce.2014.47.5.398
-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader ePub
ePub Endoscopic submucosal dissection (ESD) for early gastric cancer is a well-established procedure with the advantage of resection in an en bloc fashion, regardless of the size, shape, coexisting ulcer, and location of the lesion. However, gastric ESD is a more difficult and meticulous technique, and also requires a longer procedure time, than conventional endoscopic mucosal resection. These factors naturally increase the risk of various complications. The two most common complications accompanying gastric ESD are bleeding and perforation. These complications are known to occur both intraoperatively and postoperatively. However, there are other rare but serious complications related to gastric ESD, including aspiration pneumonia, stenosis, venous thromboembolism, and air embolism. Endoscopists should have sufficient knowledge about such complications and be prepared to deal with them appropriately, as successful management of complications is necessary for the successful completion of the entire ESD procedure.
-
Citations
Citations to this article as recorded by- Management of high risk T1 gastric adenocarcinoma following endoscopic resection
Jéssica Chaves, Diogo Libânio, Pedro Pimentel-Nunes
Best Practice & Research Clinical Gastroenterology.2024; 68: 101887. CrossRef - Incidence and risk factors for fever after endoscopic submucosal dissection and its derivative technology for gastric lesions
Yongkang Lai, Qian Zhang, Foqiang Liao, Xiaolin Pan, Zhenhua Zhu, Shunhua Long, Xiaojiang Zhou, Guohua Li, Yin Zhu, Youxiang Chen, Xu Shu
Heliyon.2024; 10(4): e25748. CrossRef - BEST-J Score: Validation of a Predicting Model for Delayed Bleeding After Gastric Endoscopic Submucosal Dissection on a European Sample
Vítor Macedo Silva, Ana Isabel Ferreira, Tiago Lima Capela, Sofia Xavier, Pedro Boal Carvalho, José Cotter
Digestive Diseases and Sciences.2024; 69(4): 1372. CrossRef - Endoscopic resection and laparoscopic lymph node dissection for early gastric cancer beyond conventional endoscopic treatment indications: a 10-year outcome study
Ah Young Lee, Yong Jin Kim, Sungwoo Cho, Tae Hee Lee, Jun-Young Seo, Seong Hwan Kim, Joo Young Cho
Surgical Endoscopy.2024; 38(5): 2533. CrossRef - Efficacy of using red dichromatic imaging throughout endoscopic submucosal dissection procedure
Aoi Kita, Shiko Kuribayashi, Yuki Itoi, Keigo Sato, Yu Hashimoto, Kengo Kasuga, Hirohito Tanaka, Hiroko Hosaka, Kazue Nagai, Hemchand Ramberan, Toshio Uraoka
Surgical Endoscopy.2023; 37(1): 503. CrossRef - Efficacy and safety of one-step knife compared to conventional insulated-tip knife for endoscopic submucosal dissection: a preliminary study with prospective data collection and retrospective review
Dae Gon Ryu, Su Jin Kim, Cheol Woong Choi, Dae Hwan Kang, Hyung Wook Kim, Su Bum Park, Hyeong Seok Nam
Surgical Endoscopy.2023; 37(1): 329. CrossRef - Management of Adverse Events of Submucosal Endoscopy
Manu Venkat, Kavel Visrodia
Gastrointestinal Endoscopy Clinics of North America.2023; 33(1): 183. CrossRef - Associate factors for endoscopic submucosal dissection operation time and postoperative delayed hemorrhage of early gastric cancer
Ren-Song Cai, Wei-Zhong Yang, Guang-Rui Cui
World Journal of Gastrointestinal Surgery.2023; 15(1): 94. CrossRef - Design and validation of performance-oriented injectable chitosan thermosensitive hydrogels for endoscopic submucosal dissection
Jia Liu, Panxianzhi Ni, Yi Wang, Zhengkui Zhou, Junlin Li, Tianxu Chen, Tun Yuan, Jie Liang, Yujiang Fan, Jing Shan, Xiaobin Sun, Xingdong Zhang
Biomaterials Advances.2023; 146: 213286. CrossRef - Short-Term and Long-Term Outcomes of Liver Cirrhosis in Gastric Neoplasm Patients Undergoing Endoscopic Submucosal Dissection
Xu-Rui Liu, Lian-Shuo Li, Fei Liu, Zi-Wei Li, Xiao-Yu Liu, Wei Zhang, Dong Peng
Journal of Laparoendoscopic & Advanced Surgical Techniques.2023; 33(7): 640. CrossRef - Clinical characteristics of early esophageal cancer and squamous intraepithelial neoplasia in patients of different ages
Ke-Yu Chen, Yan-Qi Huang, Ling-Li Zhang
World Chinese Journal of Digestology.2023; 31(6): 238. CrossRef - Risk factors for conversion from endoscopic resection to laparoscopic resection for gastric gastrointestinal stromal tumors
Luojie Liu, Xiaodan Xu, Ye Ye, Dongtao Shi, Rui Li, Weichang Chen
Journal of International Medical Research.2023; 51(4): 030006052311677. CrossRef - Multifunctional two-component in-situ hydrogel for esophageal submucosal dissection for mucosa uplift, postoperative wound closure and rapid healing
Xiong-Xin Lei, Juan-Juan Hu, Chen-Yu Zou, Yan-Lin Jiang, Long-Mei Zhao, Xiu-Zhen Zhang, Ya-Xing Li, An-Ni Peng, Yu-Ting Song, Li-Ping Huang, Jesse Li-Ling, Hui-Qi Xie
Bioactive Materials.2023; 27: 461. CrossRef - A 6-year nationwide population-based study on the current status of gastric endoscopic resection in Korea using administrative data
Jae Yong Park, Mi-Sook Kim, Beom Jin Kim, Jae Gyu Kim
Scientific Reports.2023;[Epub] CrossRef - Automated machine learning to predict the difficulty for endoscopic resection of gastric gastrointestinal stromal tumor
Luojie Liu, Rufa Zhang, Dongtao Shi, Rui Li, Qinghua Wang, Yunfu Feng, Fenying Lu, Yang Zong, Xiaodan Xu
Frontiers in Oncology.2023;[Epub] CrossRef - Development and validation of a preoperative difficulty scoring system for endoscopic resection of gastric gastrointestinal stromal tumor: a multi-center study
Luojie Liu, Mei Han, Dongtao Shi, Qinghua Wang, Yunfu Feng, Fenying Lu, Rui Li, Xiaodan Xu
Surgical Endoscopy.2023; 37(8): 6255. CrossRef - Postoperative Bleeding Risk after Gastric Endoscopic Submucosal Dissection in Patients Receiving a P2Y12 Receptor Antagonist
Ryosuke Hirai, Seiji Kawano, Shoko Inoo, Sakiko Kuraoka, Shotaro Okanoue, Takuya Satomi, Kenta Hamada, Yoshiyasu Kono, Hiromitsu Kanzaki, Masaya Iwamuro, Yoshiro Kawahara, Hiroyuki Okada
Gut and Liver.2023; 17(3): 404. CrossRef - Is proton-pump inhibitor effective in preventing postoperative bleeding after esophageal endoscopic submucosal dissection?
Ippei Tanaka, Kunio Tarasawa, Hiroaki Saito, Dai Hirasawa, Kenji Fujimori, Kiyohide Fushimi, Tomoki Matsuda
Diseases of the Esophagus.2023;[Epub] CrossRef - Effect of Sarcopenia on Pneumonia after Endoscopic Submucosal Resection in Patients Aged ≥65 Years: A Retrospective Study
Min-Yu Kim, So Yeon Kim, Hye Jung Shin, Ki Hong Kweon, Jooeun Park, Na Young Kim
Cancers.2023; 15(19): 4753. CrossRef - The efficacy of newly proposed gastric open peroral endoscopic myotomy (GO-POEM) in preventing post-endoscopic submucosal dissection stenosis: A comparison with non-GO-POEM group
Bong Ju Cho, Won Dong Lee, Jae Sun Song, Min A. Yang, Byung Sun Kim, Sung Yeol Yang, Gum Mo Jung, Ji Woong Kim, Yong Keun Cho, Jin Woong Cho
Medicine.2023; 102(52): e36755. CrossRef - Asymmetric Rolling Contact Joint for Enhanced Payload Capabilities
Jeongdo Ahn, Minho Hwang, Dukyoo Kong, Joonhwan Kim, Dong-Soo Kwon
IEEE/ASME Transactions on Mechatronics.2023; : 1. CrossRef - Recent approach for preventing complications in upper gastrointestinal endoscopic submucosal dissection
Waku Hatta, Tomoyuki Koike, Hiroko Abe, Yohei Ogata, Masahiro Saito, Xiaoyi Jin, Takeshi Kanno, Kaname Uno, Naoki Asano, Akira Imatani, Atsushi Masamune
DEN Open.2022;[Epub] CrossRef - Clinical usefulness of red dichromatic imaging in hemostatic treatment during endoscopic submucosal dissection: First report from a multicenter, open‐label, randomized controlled trial
Ai Fujimoto, Yutaka Saito, Seiichiro Abe, Syu Hoteya, Kosuke Nomura, Hiroshi Yasuda, Yasumasa Matsuo, Toshio Uraoka, Shiko Kuribayashi, Yosuke Tsuji, Daisuke Ohki, Tadateru Maehata, Motohiko Kato, Naohisa Yahagi
Digestive Endoscopy.2022; 34(2): 379. CrossRef - A Novel One-Step Knife Approach Can Reduce the Submucosal Injection Time of Endoscopic Submucosal Dissection: A Single-Blinded Randomized Multicenter Clinical Trials
Hyunil Kim, Jin Woo Kim, Hong Jun Park, Su Young Kim, Hyun-Soo Kim, Gwang Ho Baik, Sung Chul Park, Sang Jin Lee, Tae-Hwa Go
Gut and Liver.2022; 16(1): 44. CrossRef - Endoscopic Resection of Upper Gastrointestinal Subepithelial Tumours: Our Clinical Experience and Results
Mehmet Zeki Buldanlı, Oktay Yener
Prague Medical Report.2022; 123(1): 20. CrossRef - A Novel Knife for Endoscopic Submucosal Dissection in Early Gastric Cancer
Seokin Kang, Jeong Hoon Lee
The Korean Journal of Helicobacter and Upper Gastrointestinal Research.2022; 22(1): 3. CrossRef - Endoscopic Applications of Near-Infrared Photoimmunotherapy (NIR-PIT) in Cancers of the Digestive and Respiratory Tracts
Hideyuki Furumoto, Takuya Kato, Hiroaki Wakiyama, Aki Furusawa, Peter L. Choyke, Hisataka Kobayashi
Biomedicines.2022; 10(4): 846. CrossRef - Utility of a deep learning model and a clinical model for predicting bleeding after endoscopic submucosal dissection in patients with early gastric cancer
Ji Eun Na, Yeong Chan Lee, Tae Jun Kim, Hyuk Lee, Hong-Hee Won, Yang Won Min, Byung-Hoon Min, Jun Haeng Lee, Poong-Lyul Rhee, Jae J Kim
World Journal of Gastroenterology.2022; 28(24): 2721. CrossRef - Endoscopic submucosal dissection of an early-stage gastric tumor with minimal bleeding using full-time red dichromatic imaging
Yohei Ikenoyama, Ayaka Iri, Hajime Imai, Hayato Nakagawa
Endoscopy.2022; 54(S 02): E1001. CrossRef - Prediction model of bleeding after endoscopic submucosal dissection for early
gastric cancer: BEST-J score
Waku Hatta, Yosuke Tsuji, Toshiyuki Yoshio, Naomi Kakushima, Shu Hoteya, Hisashi Doyama, Yasuaki Nagami, Takuto Hikichi, Masakuni Kobayashi, Yoshinori Morita, Tetsuya Sumiyoshi, Mikitaka Iguchi, Hideomi Tomida, Takuya Inoue, Tomoyuki Koike, Tatsuya Mikami
Gut.2021; 70(3): 476. CrossRef - Clinical benefit of the multibending endoscope for gastric endoscopic submucosal dissection: a randomized controlled trial
Koichi Hamada, Yoshinori Horikawa, Yoshiki Shiwa, Kae Techigawara, Takayuki Nagahashi, Daizo Fukushima, Shinya Nishida, Ryota Koyanagi, Koichiro Kawano, Noriyuki Nishino, Michitaka Honda
Endoscopy.2021; 53(07): 683. CrossRef - Clinical feasibility and oncologic safety of primary endoscopic submucosal dissection for clinical submucosal invasive early gastric cancer
Ji Eun Na, Hyuk Lee, Yang Won Min, Byung-Hoon Min, Jun Haeng Lee, Poong-Lyul Rhee, Kyoung-Mee Kim, Jae J. Kim
Journal of Cancer Research and Clinical Oncology.2021; 147(10): 3051. CrossRef - Rebleeding in patients with delayed bleeding after endoscopic submucosal dissection for early gastric cancer
Minami Hashimoto, Waku Hatta, Yosuke Tsuji, Toshiyuki Yoshio, Yohei Yabuuchi, Shu Hoteya, Hisashi Doyama, Yasuaki Nagami, Takuto Hikichi, Masakuni Kobayashi, Yoshinori Morita, Tetsuya Sumiyoshi, Mikitaka Iguchi, Hideomi Tomida, Takuya Inoue, Tatsuya Mikam
Digestive Endoscopy.2021; 33(7): 1120. CrossRef - Endoscopic instruments and techniques in endoscopic submucosal dissection for early gastric cancer
Mitsuru Esaki, Eikichi Ihara, Takuji Gotoda
Expert Review of Gastroenterology & Hepatology.2021; 15(9): 1009. CrossRef - A patient-like swine model of gastrointestinal fibrotic strictures for advancing therapeutics
Ling Li, Mohamad I. Itani, Kevan J. Salimian, Yue Li, Olaya Brewer Gutierrez, Haijie Hu, George Fayad, Jean A. Donet, Min Kyung Joo, Laura M. Ensign, Vivek Kumbhari, Florin M. Selaru
Scientific Reports.2021;[Epub] CrossRef - Efficacy and safety of endoscopic resection in treatment of small gastric stromal tumors: A state-of-the-art review
Ze-Ming Chen, Min-Si Peng, Li-Sheng Wang, Zheng-Lei Xu
World Journal of Gastrointestinal Oncology.2021; 13(6): 462. CrossRef - Long- and short-term outcomes of early gastric cancer after endoscopic resection: a retrospective study from China
Qing-Wei Zhang, Jin-Nan Chen, Zhao-Rong Tang, Yun-Jie Gao, Zhi-Zheng Ge, Xiao-Bo Li
Endoscopy International Open.2021; 09(07): E1086. CrossRef - Endoscopic hemostatic spray for uncontrolled bleeding after complicated endoscopic mucosal resection or endoscopic submucosal dissection: a report of 2 cases
Kayla M. Hartz, Roland Y. Lee, Leonard T. Walsh, Matthew E.B. Dixon, Matthew T. Moyer
VideoGIE.2021; 6(10): 481. CrossRef - Vonoprazan versus lansoprazole in the treatment of artificial gastric ulcers after endoscopic submucossal dissection: a randomized, open-label trial
Daisuke Kawai, Ryuta Takenaka, Mikako Ishiguro, Shotaro Okanoue, Tatsuhiro Gotoda, Yoshiyasu Kono, Koji Takemoto, Hirofumi Tsugeno, Shigeatsu Fujiki
BMC Gastroenterology.2021;[Epub] CrossRef - Endoscopic Resection for Gastric Subepithelial Tumor with Backup Laparoscopic Surgery: Description of a Single-Center Experience
Wei-Jung Chang, Lien-Cheng Tsao, Hsu-Heng Yen, Chia-Wei Yang, Joseph Lin, Kuo-Hua Lin
Journal of Clinical Medicine.2021; 10(19): 4423. CrossRef - Efficacy of vonoprazan against bleeding from endoscopic submucosal dissection-induced gastric ulcers under antithrombotic medication: A cross-design synthesis of randomized and observational studies
Yu Hidaka, Toru Imai, Tomoki Inaba, Tomo Kagawa, Katsuhiro Omae, Shiro Tanaka, Sanjiv Mahadeva
PLOS ONE.2021; 16(12): e0261703. CrossRef - Skeletal Muscle Depletion: A Risk Factor for Pneumonia following Gastric Endoscopic Submucosal Dissection in Elderly Patients
Masamichi Arao, Taku Mizutani, Noritaka Ozawa, Tatsunori Hanai, Jun Takada, Masaya Kubota, Kenji Imai, Takashi Ibuka, Makoto Shiraki, Hiroshi Araki, Takuma Ishihara, Masahito Shimizu
Digestive Diseases.2021; 39(5): 435. CrossRef - Prophylactic antibiotics may be unnecessary in gastric endoscopic submucosal dissection due to the low incidence of bacteremia
Yang Liu, Youxiang Chen, Xu Shu, Yin Zhu, Guohua Li, Junbo Hong, Conghua Song, Yue Guan, Xiaojiang Zhou
Surgical Endoscopy.2020; 34(9): 3788. CrossRef - A case of gastric pseudoaneurysm following endoscopic submucosal dissection of early gastric cancer
Ryoichiro Kobashi, Takuto Hikichi, Hidemichi Imamura, Takeaki Hashimoto, Shinji Mukai, Hiromasa Ohira
Clinical Journal of Gastroenterology.2020; 13(3): 354. CrossRef - Efficacy and safety of endoscopic submucosal dissection for large gastric stromal tumors
Qiaofeng Chen, Mingju Yu, Yupeng Lei, Chang Zhong, Zhijian Liu, Xiaojiang Zhou, Guohua Li, Xiaodong Zhou, Youxiang Chen
Clinics and Research in Hepatology and Gastroenterology.2020; 44(1): 90. CrossRef - A Single-Center Early Experience of Endoscopic Submucosal Dissection for Gastric Lesions in Thailand
Prasit Mahawongkajit
Gastroenterology Research and Practice.2020; 2020: 1. CrossRef - Bleeding after endoscopic submucosal dissection of gastric lesions
Chao Hu Yang, Yu Qiu, Xiao Li, Rui Hua Shi
Journal of Digestive Diseases.2020; 21(3): 139. CrossRef - EMR/ESD: Techniques, Complications, and Evidence
Yahya Ahmed, Mohamed Othman
Current Gastroenterology Reports.2020;[Epub] CrossRef - Life on a knife edge: the optimal approach to the management of perforations during endoscopic submucosal dissection (ESD)
Shria Kumar, Young Hoon Youn, Jeffrey H. Lee
Expert Review of Gastroenterology & Hepatology.2020; 14(10): 965. CrossRef - Pneumothorax Following Gastric Endoscopic Mucosal Resection
Myeongseok Koh, Jin Seok Jang, Jae Hwang Cha
The Korean Journal of Gastroenterology.2020; 76(2): 83. CrossRef - Endoscopic submucosal dissection under D‐sorbitol solution in an animal model
Asahiro Morishita, Hideki Kobara, Tsutomu Masaki
Digestive Endoscopy.2019;[Epub] CrossRef - Safety and effectiveness of endoscopic mucosal resection or endoscopic submucosal dissection for gastric neoplasia within 2 days’ hospital stay
Joon Young Choi, Young Soo Park, Gyeongjae Na, Sung Jae Park, Hyuk Yoon, Cheol Min Shin, Nayoung Kim, Dong Ho Lee
Medicine.2019; 98(32): e16578. CrossRef - Endoscopic full-thickness resection using suture loop needle T-tag tissue anchors in the porcine stomach (with video)
Akira Dobashi, Elizabeth Rajan, Mary A. Knipschield, Christopher J. Gostout
Gastrointestinal Endoscopy.2018; 87(2): 590. CrossRef - Endoskopische Techniken bei Frühkarzinomen im oberen und unteren Gastrointestinaltrakt
A. Probst, A. Ebigbo, H. Messmann
Der Chirurg.2018; 89(5): 365. CrossRef - Endoscopic Submucosal Dissection of Early Gastric Cancer in Patients with Liver Cirrhosis
Won Hyeok Choe, Jeong Hwan Kim, Jung Ho Park, Heung Up Kim, Dae Hyeon Cho, Sang Pyo Lee, Tae Yoon Lee, Sun-Young Lee, In Kyung Sung, Hyung Seok Park, Chan Sup Shim
Digestive Diseases and Sciences.2018; 63(2): 466. CrossRef - USEFULNESS OF GASTRIC SUBMUCOSAL DISSECTION DEPTH TO EVALUATE SKILL ACQUIREMENT IN SHORT TERM TRAINING COURSES IN ESD: AN EXPERIMENTAL STUDY
Kendi YAMAZAKI, Eduardo Guimarães Hourneaux de MOURA, Mariana Matera VERAS, Luiz Henrique MESTIERI, Paulo SAKAI
Arquivos de Gastroenterologia.2018; 55(3): 221. CrossRef - Multicenter Prospective Study on the Safety of Upper Gastrointestinal Endoscopic Procedures in Antithrombotic Drug Users
Yoshiyasu Kono, Minoru Matsubara, Tatsuya Toyokawa, Ryuta Takenaka, Seiyu Suzuki, Junichirou Nasu, Masao Yoshioka, Masahiro Nakagawa, Motowo Mizuno, Hiroyuki Sakae, Makoto Abe, Tatsuhiro Gotoda, Ko Miura, Hiromitsu Kanzaki, Masaya Iwamuro, Keisuke Hori, T
Digestive Diseases and Sciences.2017; 62(3): 730. CrossRef - Is the Reinitiation of Antiplatelet Agents Safe at 1 Week after Gastric Endoscopic Submucosal Dissection? Assessment of Bleeding Risk Using the Forrest Classification
Jong Yeul Lee, Chan Gyoo Kim, Soo-Jeong Cho, Young-Il Kim, Il Ju Choi
Gut and Liver.2017; 11(4): 489. CrossRef - Preoperative Pulmonary Function Tests Predict Aspiration Pneumonia After Gastric Endoscopic Submucosal Dissection
Akihiro Matsumi, Ryuta Takenaka, Chihiro Ando, Yuki Sato, Kensuke Takei, Eriko Yasutomi, Shotaro Okanoue, Shohei Oka, Daisuke Kawai, Junro Kataoka, Koji Takemoto, Hirofumi Tsugeno, Shigeatsu Fujiki, Yoshiro Kawahara
Digestive Diseases and Sciences.2017; 62(11): 3084. CrossRef - Acute Phlegmonous Gastritis Developing after Endoscopic Submucosal Dissection That Was Successfully Treated by Antibiotics Alone
Yoo-Min Park, Jae-Young Jang, Hyo-Jung Ha, Da Rae Kim, Sun-Hee Park, A-Ri Shin, Ja-Won Koo
The Korean Journal of Medicine.2016; 90(2): 127. CrossRef - Feasibility of optical coherence tomography for the evaluation of Barrett's mucosa buried underneath esophageal squamous epithelium
Waku Hatta, Kaname Uno, Tomoyuki Koike, Nobuyuki Ara, Naoki Asano, Katsunori Iijima, Akira Imatani, Fumiyoshi Fujishima, Tooru Shimosegawa
Digestive Endoscopy.2016; 28(4): 427. CrossRef - Current Practices in the Management of Antithrombotic Therapy During the Periendoscopic Period for Patients With Cardiovascular Disease
Satoshi Ono, Itaru Saito, Yuichi Ikeda, Mitsuhiro Fujishiro, Issei Komuro, Kazuhiko Koike
International Heart Journal.2016; 57(5): 530. CrossRef - Endoscopic resection of gastric and esophageal cancer
Bryan Balmadrid, Joo Ha Hwang
Gastroenterology Report.2015; : gov050. CrossRef - Prevention of gastrointestinal events in patients on antithrombotic therapy in the peri‐endoscopy period: Review of new evidence and recommendations from recent guidelines
Raymond S. Y. Tang, Francis K. L. Chan
Digestive Endoscopy.2015; 27(5): 562. CrossRef - A Case of Pneumothorax Following Gastric Endoscopic Submucosal Dissection
Yu Rim Lee, Jun Heo, Min Kyu Jung, Sung Kook Kim, Eun Jeong Kang, Seong Jae Yeo, Hye Yoon Park
Korean Journal of Medicine.2015; 88(1): 54. CrossRef - Giant Solitary Fibrous Tumor of Esophagus Resected by Endoscopic Submucosal Dissection
Xiao-San Zhu, Yi-Chen Dai, Zhang-Xing Chen
The Annals of Thoracic Surgery.2015; 100(6): 2340. CrossRef - Endoscopic Resection Compared with Gastrectomy to Treat Early Gastric Cancer: A Systematic Review and Meta-Analysis
Shuanhu Wang, Zongbing Zhang, Mulin Liu, Shiqing Li, Congqiao Jiang, John Green
PLOS ONE.2015; 10(12): e0144774. CrossRef - International Digestive Endoscopy Network 2014: Turnpike to the Future
Eun Young Kim, Kwang An Kwon, Il Ju Choi, Ji Kon Ryu, Ki Baik Hahm
Clinical Endoscopy.2014; 47(5): 371. CrossRef
- Management of high risk T1 gastric adenocarcinoma following endoscopic resection
- 8,198 View
- 189 Download
- 67 Web of Science
- 68 Crossref

- Preparation of High-Risk Patients and the Choice of Guidewire for a Successful Endoscopic Retrograde Cholangiopancreatography Procedure
- Tae Hoon Lee, Young Kyu Jung, Sang-Heum Park
- Clin Endosc 2014;47(4):334-340. Published online July 28, 2014
- DOI: https://doi.org/10.5946/ce.2014.47.4.334
-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader ePub
ePub Endoscopic retrograde cholangiopancreatography (ERCP) is an essential technique for the diagnosis and treatment of pancreatobiliary diseases. However, ERCP-related complications such as pancreatitis, cholangitis, hemorrhage, and perforation may be problematic. For a successful and safe ERCP, preprocedural evaluations of the patients and intervention-related risk factors are needed. Furthermore, in light of the recent population aging and increase in chronic cardiopulmonary diseases in Korea, precautions including endoscopic sedation and prevention of cardiopulmonary complications should be considered. In this literature review, we describe these risk factors and the use of endoscopic sedation. In addition, we reviewed the commonly available guidewires, including their materials and options, used as a basic accessory for ERCP procedures.
-
Citations
Citations to this article as recorded by- Structural factors influencing the clinical performance of 0.025-inch guidewires for pancreatobiliary endoscopy: An experimental study
Takehiko Koga, Naoaki Tsuchiya, Yusuke Ishida, Takanori Kitaguchi, Keisuke Matsumoto, Makoto Fukuyama, Satoki Kojima, Norihiro Kojima, Fumihito Hirai
Endoscopy International Open.2024; 12(05): E666. CrossRef - Racial disparities in endoscopic retrograde cholangiopancreatography (ERCP) utilization in the United States: are we getting better?
Dushyant Singh Dahiya, Abhilash Perisetti, Neil Sharma, Sumant Inamdar, Hemant Goyal, Amandeep Singh, Laura Rotundo, Rajat Garg, Chin-I Cheng, Sailaja Pisipati, Mohammad Al-Haddad, Madhusudhan Sanaka
Surgical Endoscopy.2023; 37(1): 421. CrossRef - Comparison of guidewires for successful cannulation of biliary stenosis and targeting of biliary branches in ERCP
Masanori Kobayashi, Hiromune Katsuda, Kazuo Ohtsuka, Ryuichi Okamoto
Endoscopy International Open.2023; 11(09): E805. CrossRef - Prevention of Post-ERCP Pancreatitis: Pro-gress in Different Procedural Techniques
永烜 张
Advances in Clinical Medicine.2022; 12(11): 10124. CrossRef - Bedside Percutaneous Approach in a Critically Ill ICU Patient with Complex Pancreatobiliary Disorder Followed by Endoscopic Approach: Lessons Learnt from a Tertiary Referral Center
Cosmas Rinaldi Adithya Lesmana, Caecilia Herjuningtyas, Sri Inggriani, Yulia Estu Pratiwi, Laurentius A. Lesmana
Case Reports in Gastroenterology.2021; 15(1): 210. CrossRef - Technical Reports of Endoscopic Retrograde Cholangiopancreatography Guidewires on the Basis of Physical Properties
Chang-Il Kwon, Dong Hee Koh, Tae Jun Song, Won Suk Park, Dong Hang Lee, Seok Jeong
Clinical Endoscopy.2020; 53(1): 65. CrossRef - PREDICTIVE FACTORS FOR POST-ERCP BLEEDING. INFLUENCE OF DIRECT ORAL ANTICOAGULANTS
Ernesto Parras Castañera, Pelayo Rodríguez López, Alberto Álvarez Delgado, Fernando Muñoz Núñez, Fernando Geijo Martínez, Antonio Velasco Guardado
Revista Española de Enfermedades Digestivas.2020;[Epub] CrossRef - Current approaches to the treatment of complications of endoscopic transpapillary interventions
S. G. Shapovaliyants, S. A. Budzinskiy, E. D. Fedorov, M. V. Bordikov, M. A. Zakharova
Annaly khirurgicheskoy gepatologii = Annals of HPB Surgery.2019; 24(2): 74. CrossRef - Efficacy of midazolam‐ versus propofol‐based sedations by non‐anesthesiologists during therapeutic endoscopic retrograde cholangiopancreatography in patients aged over 80 years
Su Jung Han, Tae Hoon Lee, Sang‐Heum Park, Young Sin Cho, Yun Nah Lee, Yunho Jung, Hyun Jong Choi, Il‐Kwun Chung, Sang‐Woo Cha, Jong Ho Moon, Young Deok Cho, Sun‐Joo Kim
Digestive Endoscopy.2017; 29(3): 369. CrossRef - Pancreatic Stenting Reduces Post-ERCP Pancreatitis and Biliary Sepsis in High-Risk Patients: A Randomized, Controlled Study
He-Kun Yin, Hai-En Wu, Qi-Xiang Li, Wei Wang, Wei-Lin Ou, Harry Hua-Xiang Xia
Gastroenterology Research and Practice.2016; 2016: 1. CrossRef - Optimal Use of Wire-Assisted Techniques and Precut Sphincterotomy
Tae Hoon Lee, Sang-Heum Park
Clinical Endoscopy.2016; 49(5): 467. CrossRef - Highlights from the 50th Seminar of the Korean Society of Gastrointestinal Endoscopy
Eun Young Kim, Il Ju Choi, Kwang An Kwon, Ji Kon Ryu, Seok Ho Dong, Ki Baik Hahm
Clinical Endoscopy.2014; 47(4): 285. CrossRef
- Structural factors influencing the clinical performance of 0.025-inch guidewires for pancreatobiliary endoscopy: An experimental study
- 7,785 View
- 84 Download
- 10 Web of Science
- 12 Crossref

- Fatal Cerebral Air Embolism Due to a Patent Foramen Ovale during Endoscopic Retrograde Cholangiopancreatography
- Adam Bastovansky, Claudia Stöllberger, Josef Finsterer
- Clin Endosc 2014;47(3):275-280. Published online May 31, 2014
- DOI: https://doi.org/10.5946/ce.2014.47.3.275
-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader ePub
ePub Fatal air embolism to the cerebrum during an endoscopic retrograde cholangiopancreatography (ERCP) has not been reported in a patient with a biliodigestive anastomosis and multiresistant extended-spectrum β-lactamase
Escherichia coli (ESBL) bacteremia. A 59-year-old woman with a history of laparoscopic cholecystectomy and iatrogenic injury of the right choledochal duct, choledochojejunostomy (biliodigestive anastomosis), recurrent cholangitis, revision of the biliodigestive anastomosis, recurrent liver abscesses, and recurrent stenting of stenotic bile ducts, was admitted because of fever and tenderness of the right upper quadrant. On ERCP, a previously deployed covered Wallstent was replaced. Blood cultures grew ESBL. After stent removal 8 days later, the patient did not wake up and developed arterial hypotension and respiratory insufficiency, requiring mechanical ventilation. Computed tomography scans showed extensive air embolism to the liver, heart, and cerebrum. She died 1 day later. Although the exact pathogenesis of the fatal cerebral air embolism remains speculative, the nonphysiological anatomy and chronic infection with ESBL may have been contributory factors.-
Citations
Citations to this article as recorded by- Carbon Dioxide Embolism Resulting From Liver Laceration During Peritoneal Optical Trocar Entry
Andrea C Lin, Elizabeth J Olecki, Meghan L Good, Christopher Cowart, Jeffery S Scow
Cureus.2022;[Epub] CrossRef - Fatal air embolism during gastrointestinal endoscopy in a 5 -year- old girl
Kalagi Dana, Alalawi Shahd, Al Shawa M. Anas, Habib Zakaria
Journal of Pediatric Surgery Case Reports.2021; 74: 102017. CrossRef - A rare but lethal complication: Post-endoscopic retrograde cholangiopancreatography cerebral arterial gas embolism
Hin San Chow, Clarence Mak, Wai Yin Chu, Kwok Fai Cheung
International Journal of Gastrointestinal Intervention.2021; 10(4): 192. CrossRef - When in Trouble Think of the Bubble: Paradoxical Cerebral Arterial Gas Embolism after Endoscopic Retrograde Cholangiopancreatography
Konstantinos Ekmektzoglou, Georgios Alexandrakis, Konstantinos Dimopoulos, Panagiotis Tsibouris, Chrysostomos Kalantzis, Erasmia Vlachou, Periklis Apostolopoulos
Case Reports in Gastroenterology.2021; 15(1): 456. CrossRef - Massive cerebral air embolism following percutaneous transhepatic biliary drainage
Jae Ho Lee, Ha Young Lee, Myung Kwan Lim, Young Hye Kang
Medicine.2021; 100(52): e28389. CrossRef - Systemic air embolism after endoscopy without vessel injury – A summary of reported cases
Peter Voigt, Stefan Schob, Sebastian Gottschling, Thomas Kahn, Alexey Surov
Journal of the Neurological Sciences.2017; 376: 93. CrossRef - Multiple small hemorrhagic infarcts in cerebral air embolism: a case report
Masaya Togo, Taku Hoshi, Ryosuke Matsuoka, Yukihiro Imai, Nobuo Kohara
BMC Research Notes.2017;[Epub] CrossRef - Acute Air Embolism during Retrograde Cholangiography for Postoperative Recurrent Cholangitis Due to Intraductal Residual Meals after Choledochoduodenostomy
Osamu Miyoshi, Shu Nakano
The Japanese Journal of Gastroenterological Surger.2015; 48(5): 463. CrossRef
- Carbon Dioxide Embolism Resulting From Liver Laceration During Peritoneal Optical Trocar Entry
- 6,851 View
- 68 Download
- 7 Web of Science
- 8 Crossref

- A Needle Penetrating the Stomach Cavity after Acupuncture
- Sin Won Lee, Ji Yong Ahn, Won Jung Choi, Eun Jin Kim, Seung-Hyeon Bae, Yun Sik Choi, Hwoon-Yong Jung, Jin-Ho Kim
- Clin Endosc 2014;47(3):258-261. Published online May 31, 2014
- DOI: https://doi.org/10.5946/ce.2014.47.3.258
-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader ePub
ePub Although acupuncture is known as a safe procedure that is widely used in many countries, complications including infection, hemorrhage, hematoma, pneumothorax, nerve damage, and cardiac tamponade have been reported. A needle penetrating the stomach after acupuncture, however, is very rare. Here, we report the case of 47-year-old woman who experienced abdominal pain 2 days after receiving acupuncture. Upper gastrointestinal endoscopy identified an approximately 2.5-cm long needle in the posterior wall of the antrum. The needle was removed endoscopically using rat tooth forceps with no complications.
-
Citations
Citations to this article as recorded by- Review on Intraperitoneal Acupuncture and Needling Depth
Soyeon Kim
Korean Journal of Acupuncture.2023; 40(3): 55. CrossRef - An Observational Study Using Ultrasound to Assess Allowable Needle Insertion Range of Acupoint CV12
Hongmin Chu, Jaehyo Kim, Seongjun Park, Jaehyun Kim, Jung-Han Lee, Won-Bae Ha, Hyun-Jong Jung, Seung-bum Yang, Cheol-hyun Kim, Jun Yong Park, Kyung-ho Kang, Sangkwan Lee, Sanghun Lee
Healthcare.2022; 10(9): 1707. CrossRef - Literature Review on Adverse Events (2012-2015) associated with Acupuncture and Moxibustion
Nobutatsu FURUSE, Akihito UEHARA, Masaaki SUGAWARA, Toshiya YAMAZAKI, Hisashi SHINBARA, Hitoshi YAMASHITA
Zen Nihon Shinkyu Gakkai zasshi (Journal of the Japan Society of Acupuncture and Moxibustion).2017; 67(1): 29. CrossRef - Retrospective study using MRI to measure depths of acupuncture points in neck and shoulder region
Pei-Chi Chou, Yu-Chuen Huang, Chun-Jen Hsueh, Jaung-Geng Lin, Heng-Yi Chu
BMJ Open.2015; 5(7): e007819. CrossRef - An Alternative to Current Therapies of Functional Dyspepsia: Self-Administrated Transcutaneous Electroacupuncture Improves Dyspeptic Symptoms
Ting Ji, Xueliang Li, Lin Lin, Liuqin Jiang, Meifeng Wang, Xiaopin Zhou, Ranran Zhang, Jiande DZ Chen
Evidence-Based Complementary and Alternative Medicine.2014; 2014: 1. CrossRef
- Review on Intraperitoneal Acupuncture and Needling Depth
- 7,320 View
- 65 Download
- 5 Web of Science
- 5 Crossref

- Predictors of Esophageal Stricture Formation Post Endoscopic Mucosal Resection
- Bashar Qumseya, Abraham M. Panossian, Cynthia Rizk, David Cangemi, Christianne Wolfsen, Massimo Raimondo, Timothy Woodward, Michael B. Wallace, Herbert Wolfsen
- Clin Endosc 2014;47(2):155-161. Published online March 31, 2014
- DOI: https://doi.org/10.5946/ce.2014.47.2.155
-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader ePub
ePub Background/Aims Stricture formation is a common complication after endoscopic mucosal resection. Predictors of stricture formation have not been well studied.
Methods We conducted a retrospective, observational, descriptive study by using a prospective endoscopic mucosal resection database in a tertiary referral center. For each patient, we extracted the age, sex, lesion size, use of ablative therapy, and detection of esophageal strictures. The primary outcome was the presence of esophageal stricture at follow-up. Multivariate logistic regression was used to analyze the association between the primary outcome and predictors.
Results Of 136 patients, 27% (
n =37) had esophageal strictures. Thirty-two percent (n =44) needed endoscopic dilation to relieve dysphagia (median, 2; range, 1 to 8). Multivariate logistic regression analysis showed that the size of the lesion excised is associated with increased odds of having a stricture (odds ratio, 1.6; 95% confidence interval, 1.1 to 2.3;p =0.01), when controlling for age, sex, and ablative modalities. Similarly, the number of lesions removed in the index procedure was associated with increased odds of developing a stricture (odds ratio, 2.3; 95% confidence interval, 1.3 to 4.2;p =0.007).Conclusions Stricture formation after esophageal endoscopic mucosal resection is common. Risk factors for stricture formation include large mucosal resections and the resection of multiple lesions on the initial procedure.
-
Citations
Citations to this article as recorded by- Simplified Versus Standard Radiofrequency Ablation Protocols for Barrett's Esophagus: A Systematic Review and Meta-Analysis
Sagar Shah, Mary Kathryn Roccato, Samuel Ji, Neil Jariwalla, Spencer Kozik, Ronald Dungca Ortizo, Anastasia Chahine, Jennifer M. Kolb, Jason B. Samarasena
Techniques and Innovations in Gastrointestinal Endoscopy.2022; 24(1): 45. CrossRef - A reliable nomogram model for predicting esophageal stricture after endoscopic submucosal dissection
Guodong Yang, Zhao Mu, Ke Pu, Yulin Chen, Luoyao Zhang, Haiyue Zhou, Peng Luo, Xiaoying Zhang
Medicine.2022; 101(5): e28741. CrossRef - Management of esophageal strictures after endoscopic resection for early neoplasia
Einas Abou Ali, Arthur Belle, Rachel Hallit, Benoit Terris, Frédéric Beuvon, Mahaut Leconte, Anthony Dohan, Sarah Leblanc, Solène Dermine, Lola-Jade Palmieri, Romain Coriat, Stanislas Chaussade, Maximilien Barret
Therapeutic Advances in Gastroenterology.2021; 14: 175628482098529. CrossRef - Lesion size and circumferential range identified as independent risk factors for esophageal stricture after endoscopic submucosal dissection
Meihong Chen, Yini Dang, Chao Ding, Jiajia Yang, Xinmin Si, Guoxin Zhang
Surgical Endoscopy.2020; 34(9): 4065. CrossRef - Risk factors for serious adverse events associated with multiband mucosectomy in Barrett’s esophagus: an international multicenter analysis of 3827 endoscopic resection procedures
Kamar Belghazi, Norman Marcon, Christopher Teshima, Kenneth K. Wang, Reza V. Milano, Nahid Mostafavi, Michael B. Wallace, Pujan Kandel, Lady Katherine Mejía Pérez, Michael J. Bourke, Farzan Bahin, Martin A. Everson, Rehan Haidry, Gregory G. Ginsberg, Gene
Gastrointestinal Endoscopy.2020; 92(2): 259. CrossRef - Advances in Endoscopic Resection in the Management of Esophageal Neoplasia
Don C. Codipilly, Prasad G. Iyer
Current Treatment Options in Gastroenterology.2020; 18(2): 308. CrossRef - Issues and controversies in esophageal inlet patch
Adriana Ciocalteu, Petrica Popa, Mircea Ionescu, Dan Ionut Gheonea
World Journal of Gastroenterology.2019; 25(30): 4061. CrossRef - Radiofrequency ablation in patients with large cervical heterotopic gastric mucosa and globus sensation: Closing the treatment gap
Ivan Kristo, Erwin Rieder, Matthias Paireder, Katrin Schwameis, Gerd Jomrich, Werner Dolak, Thomas Parzefall, Martin Riegler, Reza Asari, Sebastian F. Schoppmann
Digestive Endoscopy.2018; 30(2): 212. CrossRef - Endoscopic submucosal dissection for early Barrett’s neoplasia: a meta-analysis
Dennis Yang, Fei Zou, Sican Xiong, Justin J. Forde, Yu Wang, Peter V. Draganov
Gastrointestinal Endoscopy.2018; 87(6): 1383. CrossRef - Endoscopic eradication therapy for Barrett’s esophagus: Adverse outcomes, patient values, and cost-effectiveness
Swarup Kumar, Prasad G. Iyer
Techniques in Gastrointestinal Endoscopy.2018; 20(2): 75. CrossRef - Recent advances in Barrett's esophagus
John Inadomi, Hani Alastal, Luigi Bonavina, Seth Gross, Richard H. Hunt, Hiroshi Mashimo, Massimiliano di Pietro, Horace Rhee, Marmy Shah, Salvatore Tolone, David H. Wang, Shao‐Hua Xie
Annals of the New York Academy of Sciences.2018; 1434(1): 227. CrossRef - Endoscopic therapy for Barrett’s esophagus and early esophageal cancer: Where do we go from here?
Tavankit Singh, Madhusudhan R Sanaka, Prashanthi N Thota
World Journal of Gastrointestinal Endoscopy.2018; 10(9): 165. CrossRef - Ablation Therapy for Barrett’s Esophagus: New Rules for Changing Times
Nour Hamade, Prateek Sharma
Current Gastroenterology Reports.2017;[Epub] CrossRef - Endoscopic Resection and Ablation for Early-Stage Esophageal Cancer
Stephanie Worrell, Steven R. DeMeester
Thoracic Surgery Clinics.2016; 26(2): 173. CrossRef - Advances in the Endoscopic Diagnosis of Barrett Esophagus
Ashley H. Davis-Yadley, Kevin G. Neill, Mokenge P. Malafa, Luis R. Peña
Cancer Control.2016; 23(1): 67. CrossRef - Endoscopic mucosal resection
Joo Ha Hwang, Vani Konda, Barham K. Abu Dayyeh, Shailendra S. Chauhan, Brintha K. Enestvedt, Larissa L. Fujii-Lau, Sri Komanduri, John T. Maple, Faris M. Murad, Rahul Pannala, Nirav C. Thosani, Subhas Banerjee
Gastrointestinal Endoscopy.2015; 82(2): 215. CrossRef - When Is Pre-Emptive Treatment Necessary after Endoscopic Mucosal Resection of Early Esophageal Neoplasm?
Hyung Gil Kim
Clinical Endoscopy.2014; 47(2): 124. CrossRef
- Simplified Versus Standard Radiofrequency Ablation Protocols for Barrett's Esophagus: A Systematic Review and Meta-Analysis
- 8,257 View
- 61 Download
- 23 Web of Science
- 17 Crossref

- Late Complications and Stone Recurrence Rates after Bile Duct Stone Removal by Endoscopic Sphincterotomy and Large Balloon Dilation are Similar to Those after Endoscopic Sphincterotomy Alone
- Ka Young Kim, Jimin Han, Ho Gak Kim, Byeong Suk Kim, Jin Tae Jung, Joong Goo Kwon, Eun Young Kim, Chang Hyeong Lee
- Clin Endosc 2013;46(6):637-642. Published online November 19, 2013
- DOI: https://doi.org/10.5946/ce.2013.46.6.637
-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader ePub
ePub Background/Aims Between endoscopic sphincterotomy (ES) alone and combined endoscopic sphincterotomy and large balloon dilation (ES-LBD) groups, efficacy and long-term complications, difference in biliary stone recurrence rate, and risk factors of stone recurrence were compared.
Methods Medical records of 222 patients who underwent ERCP for biliary stone removal were retrospectively reviewed. Patients with dilated CBD ≥11 mm and follow-up longer than 6 months were included.
Results There were 101 patients in ES-LBD group and 121 patients in ES group. Mean follow-up duration was 25.0 (6-48) months and 13.0 (6-43) months, respectively (
p =0.001). There was no difference in number of ERCP sessions, brown pigment stones, angle between mid and distal common bile duct (CBD angle) <135°, and lithotripsy rate. Complete retrieval success rate was excellent in both groups (100% vs. 99%). Early complication rate of ES-LBD and ES alone group was 4 and 4.1%, respectively (p =1.000). One patient in ES-LBD group died from delayed bleeding. Late complication rate was 5.9 and 3.3%, respectively (p =1.000). Stone recurrence rate was 6.9% and 5.8%, respectively (p =0.984). The only Independent risk factor of stone recurrence was presence of periampullary diverticulum.Conclusions Late complication and stone recurrence rates were similar between ES-LBD and ES alone groups.
-
Citations
Citations to this article as recorded by- Recurrence of common bile duct stones after endoscopic clearance and its predictors: A systematic review
Marko Kozyk, Suprabhat Giri, Sidharth Harindranath, Manan Trivedi, Kateryna Strubchevska, Rakesh Kumar Barik, Sridhar Sundaram
DEN Open.2024;[Epub] CrossRef - Characterization of biliary and duodenal microbiota in patients with primary and recurrent choledocholithiasis
Fang Liu, Zi-Kai Wang, Ming-Yang Li, Xiu-li Zhang, Feng-Chun Cai, Xiang-Dong Wang, Xue-Feng Gao, Wen Li
Health Information Science and Systems.2024;[Epub] CrossRef - Evidence-based clinical practice guidelines for cholelithiasis 2021
Naotaka Fujita, Ichiro Yasuda, Itaru Endo, Hiroyuki Isayama, Takuji Iwashita, Toshiharu Ueki, Kenichiro Uemura, Akiko Umezawa, Akio Katanuma, Yu Katayose, Yutaka Suzuki, Junichi Shoda, Toshio Tsuyuguchi, Toshifumi Wakai, Kazuo Inui, Michiaki Unno, Yoshifu
Journal of Gastroenterology.2023; 58(9): 801. CrossRef - Endoclip papillaplasty (ECPP) versus limited EST plus EPLBD for a decrease in recurrent choledocholithiasis: a prospective cohort study
Xiaofang Lu, Yingchun Wang, Wenzheng Liu, Yaopeng Zhang, Wei Zheng, Xiue Yan, Hong Chang, Yonghui Huang
Surgical Endoscopy.2023; 37(10): 7790. CrossRef - The Clinical Presentations of Liver Abscess Development After Endoscopic Retrograde Cholangiopancreatography with Choledocholithiasis: A 17-Year Follow-Up
An-Che Liu, Wei-Chen Tai, Shao-Ming Chiu, Fai-Meng Sou, Shih-Cheng Yang, Lung-Sheng Lu, Chung-Mou Kuo, Yi-Chun Chiu, Seng-Kee Chuah, Chih-Ming Liang, Cheng-Kun Wu
Infection and Drug Resistance.2023; Volume 16: 6167. CrossRef - Low insertion of cystic duct increases risk for common bile duct stone recurrence
Seong Ji Choi, Jai Hoon Yoon, Dong Hee Koh, Hang Lak Lee, Dae Won Jun, Ho Soon Choi
Surgical Endoscopy.2022; 36(5): 2786. CrossRef - New common bile duct morphological subtypes: Risk predictors of common bile duct stone recurrence
Xu Ji, Zhuo Yang, Shu-Ren Ma, Wen Jia, Qian Zhao, Lu Xu, Ying Kan, Yang Cao, Yao Wang, Bao-Jun Fan
World Journal of Gastrointestinal Surgery.2022; 14(3): 236. CrossRef - Endoscopic Papillary Large Balloon Dilation Reduces Further Recurrence in Patients With Recurrent Common Bile Duct Stones: A Randomized Controlled Trial
Xu Wang, Xiangping Wang, Hao Sun, Gui Ren, Biaoluo Wang, Shuhui Liang, Linhui Zhang, Xiaoyu Kang, Qin Tao, Xuegang Guo, Hui Luo, Yanglin Pan
American Journal of Gastroenterology.2022; 117(5): 740. CrossRef - Angle of covered self-expandable metallic stents after placement is a risk factor for recurrent biliary obstruction
Kojiro Tanoue, Hirotsugu Maruyama, Yuki Ishikawa-Kakiya, Yosuke Kinoshita, Kappei Hayashi, Masafumi Yamamura, Masaki Ominami, Yuji Nadatani, Shusei Fukunaga, Koji Otani, Shuhei Hosomi, Fumio Tanaka, Noriko Kamata, Yasuaki Nagami, Koichi Taira, Toshio Wata
World Journal of Hepatology.2022; 14(5): 992. CrossRef - Angle of covered self-expandable metallic stents after placement is a risk factor for recurrent biliary obstruction
Kojiro Tanoue, Hirotsugu Maruyama, Yuki Ishikawa-Kakiya, Yosuke Kinoshita, Kappei Hayashi, Masafumi Yamamura, Masaki Ominami, Yuji Nadatani, Shusei Fukunaga, Koji Otani, Shuhei Hosomi, Fumio Tanaka, Noriko Kamata, Yasuaki Nagami, Koichi Taira, Toshio Wata
World Journal of Hepatology.2022; 14(5): 993. CrossRef - Recent developments in antibacterial or antibiofilm compound coating for biliary stents
Tao Wu, Yan Yang, He Su, Yuanhui Gu, Quanming Ma, Yan Zhang
Colloids and Surfaces B: Biointerfaces.2022; 219: 112837. CrossRef - Effect of stent placement on stone recurrence and post-procedural cholangitis after endoscopic removal of common bile duct stones
Jung-Hye Choi, Tae-Yoon Lee, Young-Koog Cheon
The Korean Journal of Internal Medicine.2021; 36(Suppl 1): S27. CrossRef - Need to identify the risk factor for stone recurrence after common bile duct exploration
Kee-Hwan Kim
The Journal of Minimally Invasive Surgery.2021; 24(1): 8. CrossRef - Should Common Bile Duct Exploration for Choledocholithiasis Be a Specialist-Only Procedure?
Russell Hodgson, Daniel Heathcock, Chien-Tse Kao, Rosemary Seagar, Mark Tacey, Jiun Miin Lai, Tuck Leong Yong, Nezor Houli, David Bird
Journal of Laparoendoscopic & Advanced Surgical Techniques.2021; 31(7): 743. CrossRef - Increased Risk of Pyogenic Liver Abscess after Endoscopic Sphincterotomy for Treatment of Choledocholithiasis
Cheng-Kun Wu, Chien-Ning Hsu, Wei-Ru Cho, Shih-Cheng Yang, An-Che Liu, Wei-Chen Tai, Chen-Hsiang Lee, Yao-Hsu Yang, Seng-Kee Chuah, Chih-Ming Liang
Infection and Drug Resistance.2021; Volume 14: 2121. CrossRef - Long-term Outcomes of Endoscopic Papillary Large-balloon Dilation for Common Bile Duct Stones
Toji Murabayashi, Yoshihide Kanno, Shinsuke Koshita, Takahisa Ogawa, Hiroaki Kusunose, Toshitaka Sakai, Kaori Masu, Keisuke Yonamine, Kazuaki Miyamoto, Fumisato Kozakai, Kazuki Endo, Yutaka Noda, Kei Ito
Internal Medicine.2020; 59(7): 891. CrossRef - Clinical significance of different periampullary diverticulum classifications for endoscopic retrograde cholangiopancreatography cannulation
Ping Yue, Ke-Xiang Zhu, Hai-Ping Wang, Wen-Bo Meng, Jian-Kang Liu, Lei Zhang, Xiao-Liang Zhu, Hui Zhang, Long Miao, Zheng-Feng Wang, Wen-Ce Zhou, Azumi Suzuki, Kiyohito Tanaka, Xun Li
World Journal of Gastroenterology.2020; 26(19): 2402. CrossRef - Clinical significance of different periampullary diverticulum classifications for endoscopic retrograde cholangiopancreatography cannulation
Ping Yue, Ke-Xiang Zhu, Hai-Ping Wang, Wen-Bo Meng, Jian-Kang Liu, Lei Zhang, Xiao-Liang Zhu, Hui Zhang, Long Miao, Zheng-Feng Wang, Wen-Ce Zhou, Azumi Suzuki, Kiyohito Tanaka, Xun Li
World Journal of Gastroenterology.2020; 26(19): 2403. CrossRef - Laparoscopic and endoscopic cooperative surgery for cholecystogastric fistula: A case report
Goshi Fujimoto
International Journal of Surgery Case Reports.2020; 71: 116. CrossRef - Risk factors of stone recurrence after endoscopic retrograde cholangiopancreatography for common bile duct stones
Peng Lujian, Cheng Xianneng, Zhang Lei
Medicine.2020; 99(27): e20412. CrossRef - Novel risk factors for recurrent biliary obstruction and pancreatitis after metallic stent placement in pancreatic cancer
Tsuyoshi Takeda, Takashi Sasaki, Takafumi Mie, Takaaki Furukawa, Ryo Kanata, Akiyoshi Kasuga, Masato Matsuyama, Masato Ozaka, Naoki Sasahira
Endoscopy International Open.2020; 08(11): E1603. CrossRef - Clinical Impact of Common Bile Duct Angulation for Recurrence of Bile Duct Stones
Se Woo Park
The Korean Journal of Gastroenterology.2020; 76(4): 177. CrossRef - Clinical Impact of Common Bile Duct Angulation on the Recurrence of Common Bile Duct Stone: A Meta-analysis and Review
Seongyul Ryu, Ik Hyun Jo, Seonhoo Kim, Yeon-Ji Kim, Woo Chul Chung
The Korean Journal of Gastroenterology.2020; 76(4): 199. CrossRef - Best Procedure for the Management of Common Bile Duct Stones via the Papilla: Literature Review and Analysis of Procedural Efficacy and Safety
Shigeto Ishii, Hiroyuki Isayama, Mako Ushio, Sho Takahashi, Wataru Yamagata, Yusuke Takasaki, Akinori Suzuki, Kazushige Ochiai, Ko Tomishima, Ryo Kanazawa, Hiroaki Saito, Toshio Fujisawa, Shuichiro Shiina
Journal of Clinical Medicine.2020; 9(12): 3808. CrossRef - Presence of Periampullary Diverticulum is Not a Hurdle to Successful Endoscopic Retrograde Cholangiopancreatography
Jimin Han
Clinical Endoscopy.2019; 52(1): 7. CrossRef - Causes associated with recurrent choledocholithiasis following therapeutic endoscopic retrograde cholangiopancreatography: A large sample sized retrospective study
Feng Deng, Mi Zhou, Ping-Ping Liu, Jun-Bo Hong, Guo-Hua Li, Xiao-Jiang Zhou, You-Xiang Chen
World Journal of Clinical Cases.2019; 7(9): 1028. CrossRef - A nationwide population-based study of common bile duct stone recurrence after endoscopic stone removal in Korea
Byung Kyu Park, Jeong Hun Seo, Han Ho Jeon, Jong Won Choi, Sun Young Won, Yong Suk Cho, Chun Kyon Lee, Haeyong Park, Dong Wook Kim
Journal of Gastroenterology.2018; 53(5): 670. CrossRef - Japan Gastroenterological Endoscopy Society guidelines for endoscopic papillary large balloon dilation
Takao Itoi, Shomei Ryozawa, Akio Katanuma, Yoshinobu Okabe, Hironori Kato, Jun Horaguchi, Takayoshi Tsuchiya, Takuji Gotoda, Naotaka Fujita, Kenjiro Yasuda, Yoshinori Igarashi, Kazuma Fujimoto
Digestive Endoscopy.2018; 30(3): 293. CrossRef - Recurrent common bile duct stones as a late complication of endoscopic sphincterotomy
Tatenda C. Nzenza, Yahya Al-Habbal, Glen R. Guerra, S. Manolas, Tuck Yong, Trevor McQuillan
BMC Gastroenterology.2018;[Epub] CrossRef - Comparison of late adverse events after endoscopic sphincterotomy versus endoscopic papillary large balloon dilation for common bile duct stones: A propensity score‐based cohort analysis
Akinori Maruta, Takuji Iwashita, Shinya Uemura, Kensaku Yoshida, Keisuke Iwata, Tsuyoshi Mukai, Shinpei Doi, Ichiro Yasuda, Kenji Imai, Masahito Shimizu
Digestive Endoscopy.2018; 30(4): 493. CrossRef - Comparison of the Long-Term Outcomes of Endoscopic Papillary Large Balloon Dilation Alone versus Endoscopic Sphincterotomy for Removal of Bile Duct Stones
Tao Li, Jun Wen, Like Bie, Biao Gong
Gastroenterology Research and Practice.2018; 2018: 1. CrossRef - Long-term recurrence of bile duct stones after endoscopic papillary large balloon dilation with sphincterotomy: 4-year extended follow-up of a randomized trial
Gregorios A. Paspatis, Konstantina Paraskeva, Emmanouil Vardas, Vasilios Papastergiou, Aikaterini Tavernaraki, Maria Fragaki, Angeliki Theodoropoulou, Gregorios Chlouverakis
Surgical Endoscopy.2017; 31(2): 650. CrossRef - Advances of recurrent risk factors and management of choledocholithiasis
Jian-Shan Cai, Sun Qiang, Yin Bao-Bing
Scandinavian Journal of Gastroenterology.2017; 52(1): 34. CrossRef - Endoscopic Papillary Large Balloon Dilatation Without Sphincterotomy for the Treatment of Large Common Bile Duct Stone: Long-Term Outcomes at a Single Center
Jin-Seok Park, Seok Jeong, Byung Wook Bang, Ae Ra Kang, Don Haeng Lee
Digestive Diseases and Sciences.2016; 61(10): 3045. CrossRef - The Wire-Grasping Method as a New Technique for Forceps Biopsy of Biliary Strictures: A Prospective Randomized Controlled Study of Effectiveness
Yasunobu Yamashita, Kazuki Ueda, Yuki Kawaji, Takashi Tamura, Masahiro Itonaga, Takeichi Yoshida, Hiroki Maeda, Hirohito Magari, Takao Maekita, Mikitaka Iguchi, Hideyuki Tamai, Masao Ichinose, Jun Kato
Gut and Liver.2016; 10(4): 642. CrossRef - Abdominal manifestations of histiocytic disorders in adults: imaging perspective
Abhijit Sunnapwar, Christine O Menias, Vijaynadh Ojili, Maria Policarpio Nicolas, Rashmi Katre, Kiran Gangadhar, Arpit Nagar
The British Journal of Radiology.2016; 89(1065): 20160221. CrossRef - Short-term and long-term outcomes after endoscopic sphincterotomy versus endoscopic papillary balloon dilation for bile duct stones
Yi Lu, Jia-Chuan Wu, Lei Liu, Li-Ke Bie, Biao Gong
European Journal of Gastroenterology & Hepatology.2014; 26(12): 1367. CrossRef - Long-Term Outcome of Endoscopic Papillary Large Balloon Dilatation
Chang-Il Kwon
Clinical Endoscopy.2013; 46(6): 601. CrossRef
- Recurrence of common bile duct stones after endoscopic clearance and its predictors: A systematic review
- 7,400 View
- 85 Download
- 38 Crossref

- Unusual Complications Related to Endoscopic Retrograde Cholangiopancreatography and Its Endoscopic Treatment
- Chang-Il Kwon, Sang Hee Song, Ki Baik Hahm, Kwang Hyun Ko
- Clin Endosc 2013;46(3):251-259. Published online May 31, 2013
- DOI: https://doi.org/10.5946/ce.2013.46.3.251
-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader ePub
ePub Endoscopic retrograde cholangiopancreatography (ERCP)-induced complications, once occurred, can lead to significant morbidity. Commonly 5% to 10% of patients experience procedure related complications such as post-ERCP pancreatitis, biliary hemorrhage, and cholangitis, in descending order. However, complications such as perforation, pneumothorax, air embolism, splenic injury, and basket impaction are rare but are associated with high mortality if occurred. Such unexpected unusual complications might extend the length of hospitalization, require urgent surgical intervention, and put the patient in miserable condition leading to permanent disability or mortality. Although these ERCP-induced complications can be minimized by a skilled operator using advanced techniques and devices, the occurrence of unusual complications are hard to expect and induce very difficult management condition. In this review, we will focus on the uncommon complications related to ERCP. This review is also aimed at suggesting optimal endoscopic treatment strategies for several complications based on our institutional experiences.
-
Citations
Citations to this article as recorded by- Cystic duct disimpaction for acute cholecystitis in the high-risk cholecystectomy patient: Case report
Humzah Iqbal, Aalam Sohal, Kanana Aburayyan, Bandhul Hans, Juliana Yang
SAGE Open Medical Case Reports.2024;[Epub] CrossRef - Emergency digital cholangioscopy-assisted electrohydraulic lithotripsy for basket impaction with an entrapped bile duct stone
Akane Hara, Kosuke Minaga, Yasuo Otsuka, Hidekazu Tanaka, Mamoru Takenaka, Masatoshi Kudo
Endoscopy International Open.2024; 12(02): E271. CrossRef - A Study on the Spectrum of Imaging Findings of Post-ERCP-Specific Complications: A Retrospective Descriptive Study
Ruchira Mukherji, Manoj Gopinath
Indian Journal of Radiology and Imaging.2024;[Epub] CrossRef - Management of ERCP complications
Partha Pal, Mohan Ramchandani
Best Practice & Research Clinical Gastroenterology.2024; 69: 101897. CrossRef - Outcomes of ERCP in Patients With Cystic Fibrosis
Salman Haider, Daryl Ramai, Saira Shah, Nayna D. Riyat, Marco Spadaccini, Saurabh Chandan, Marcello Maida, Asad Ur Rehman, Monique T. Barakat
Journal of Clinical Gastroenterology.2024;[Epub] CrossRef - Prophylactic cholecystectomy offers best outcomes following ERCP clearance of common bile duct stones: a meta-analysis
Gearóid Mc Geehan, Conor Melly, Niall O’ Connor, Gary Bass, Shahin Mohseni, Magda Bucholc, Alison Johnston, Michael Sugrue
European Journal of Trauma and Emergency Surgery.2023; 49(5): 2257. CrossRef - Subcapsular Biloma following Endoscopic Retrograde Cholangiopancreatography and Endoscopic Biliary Sphincterotomy: A Case Report with a Mini Review of Literature
Natalia Valeria Pentara, Aristidis Ioannidis, Georgios Tzikos, Leonidas Kougias, Eleni Karlafti, Angeliki Chorti, Despoina Tsalkatidou, Antonios Michalopoulos, Daniel Paramythiotis
Diagnostics.2023; 13(5): 831. CrossRef - HEMATOMA POST-ENDOSCOPIC RETROGRADE CHOLANGIOPANCREATOGRAPHY. AN ANALYSIS OF THREE CASES
Sandra Díez Ruiz, Irene Latras Cortés, Sandra Borrego Rivas, Víctor Blázquez Ávila, Marcos Jiménez Palacios, Rubén Díez Rodríguez, Jesús Espinel Díez, Francisco Jorquera Plaza
Revista Española de Enfermedades Digestivas.2023;[Epub] CrossRef - Gas spread following endoscopic sphincterotomy: Challenging diagnosis and management: A case reports
Asma Sghaier, Khalil Fradi, Amine El Ghali, Khaireddine Dhouioui, Fehmi Hamila, Sabri Youssef
International Journal of Surgery Case Reports.2023; 108: 108487. CrossRef - Gas Embolism After a Patient's Ninth ERCP Procedure
Helal Said Ahmad, Sari Anne Cohen, Tawfik Khoury, Riad Tome, Haitam Zeibak, Wisam Abboud, Amir Mari
ACG Case Reports Journal.2023; 10(8): e01124. CrossRef - ERCP in patients over 90 years old: Safety and efficacy comparison with a younger cohort
Ana E Colmenero Gargari, Fernando E Melgar Somoza, Jorge Vera, Carlos G Micames
Endoscopy International Open.2023; 11(09): E893. CrossRef - Endoscopic management of upper gastrointestinal perforations, leakage and fistula
Hee Seok Moon
Journal of Innovative Medical Technology.2023; 1(1): 15. CrossRef - Rare post-endoscopic retrograde cholangiopancreatography complications: Can we avoid them?
Marta Aleksandra Przybysz, Rafał Stankiewicz
World Journal of Meta-Analysis.2022; 10(3): 122. CrossRef - Fatal Venous Gas Embolism During Endoscopic Retrograde Cholangiopancreatography After Simultaneous Deployment of 2 Self-Expandable Metallic Stents
Justin Chuang, Rebecca Kuang, Ajit Ramadugu, Dipen Patel, Sachit Sharma, Kishan Shrestha, Jordan Burlen, Ali Nawras
ACG Case Reports Journal.2022; 9(10): e00873. CrossRef - Best practices for prevention of post-endoscopic retrograde cholangiopancreatography pancreatitis
Simcha Weissman, Mohamed Ahmed, Matthew R Baniqued, Dean Ehrlich, James H Tabibian
World Journal of Gastrointestinal Endoscopy.2021; 13(6): 161. CrossRef - Nonsurgical rescue after basket-stone impaction by endoscopic cutting of a metallic lithotripter and laser lithotripsy
Toshiaki Terauchi, Satoshi Shinozaki, Masazumi Inoue, Alan Kawarai Lefor, Hiroharu Shinozaki
VideoGIE.2021; 6(9): 416. CrossRef - Splenic laceration following endoscopic retrograde cholangiopancreatography: A literature review and our experience
Kan Wang, Yanfei Fang, Aihua Huang, Min Gao
Laparoscopic, Endoscopic and Robotic Surgery.2020; 3(3): 80. CrossRef - Gallstone Dislodgement in the Airway during ERCP: A Case Report and Review of the Literature
Hisham Hurreiz, Amira Babikir, Edward Black
Case Reports in Gastrointestinal Medicine.2020; 2020: 1. CrossRef - Experiencia de cinco años en el manejo de pacientes con alto riesgo de coledocolitiasis
Josué Israel Olivares del Moral, José Augusto Rodríguez Osuna, Danyel Chávez Fernández, José Cruz De la Torre Gonzáles, Ramiro Gómez-Arámbulo, Efrén Flores-Álvarez, José de Jesús Marín-López
Revista Mexicana de Cirugía Endoscópica.2020; 21(1): 26. CrossRef - Retracted: Endoscopic Treatment of Endoscopic Retrograde Cholangiopancreatography-Related Duodenal Perforations
Ding Shi, Jian feng Yang, Yong pan Liu
Journal of Laparoendoscopic & Advanced Surgical Techniques.2019; 29(3): 385. CrossRef - Complications of endoscopic retrograde cholangiopancreatography: an imaging review
Dinesh Manoharan, Deep Narayan Srivastava, Arun Kumar Gupta, Kumble Seetharama Madhusudhan
Abdominal Radiology.2019; 44(6): 2205. CrossRef -
COMPARATIVE EVALUATION OF RADIcitation_listOGICAL IMAGING RESULTS OF CHcitation_listEDOCHcitation_listITHIASIS IN PATIENTS WITH ACUTE CALCULOUS CHcitation_listECYSTITIS
V. I. Kolomiytsev, O. M. Terletskiy, M. M. Bufan
Bulletin of Problems Biology and Medicine.2019; 2.1(150): 236. CrossRef - Intrahepatic subcapsular biloma after endoscopic retrograde cholangiopancreatography treated by endoscopic biliary drainage
Hiroaki Igarashi, Hiroko Yamashita, Kiyoshi Tsuchiya, Dai Sugimoto, Itsuro Ogata
Clinical Journal of Gastroenterology.2018; 11(2): 167. CrossRef - Embolía pulmonar aérea secundaria a colangiopancreatografía retrógrada endoscópica en una paciente con trasplante hepático. Reporte de caso.
Gustavo Adolfo Reyes, Renzo Pinto Carta, Fernando Sierra Arango, Luis Felipe Cabrera Vargas
Revista Colombiana de Gastroenterología.2018; 33(4): 464. CrossRef - Cerebral air embolism after ERCP
Sonia Trabanco, Sara Pardo, Mónica Williams, Javier Diaz, Cristina Ruiz
Journal of Clinical Anesthesia.2017; 36: 133. CrossRef - Bilateral Pneumothoraces as a Complication of Endoscopic Retrograde Cholangiopancreatography
Douglas E. Rappaport, Joshua J. Solano, Jonathan A. Edlow
The Journal of Emergency Medicine.2017; 52(4): 573. CrossRef - Cholangitis after endoscopic retrograde cholangiopancreatography: a rare complication?
Armando Peixoto, Marco Silva, Guilherme Macedo
Revista Española de Enfermedades Digestivas.2017;[Epub] CrossRef - Training in Endoscopy: Endoscopic Retrograde Cholangiopancreatography
Jaihwan Kim
Clinical Endoscopy.2017; 50(4): 334. CrossRef - The role of endoscopic ultrasound in children with Pancreatobiliary and gastrointestinal disorders: a single center series and review of the literature
Alessandro Fugazza, Barbara Bizzarri, Federica Gaiani, Marco Manfredi, Alessia Ghiselli, Pellegrino Crafa, Maria Clotilde Carra, Nicola de’Angelis, Gian Luigi de’Angelis
BMC Pediatrics.2017;[Epub] CrossRef - ERCP–Related Duodenal Perforation; The Prevention and Management
Hong Ja Kim, Seon Mee Park
Korean Journal of Pancreas and Biliary Tract.2016; 21(2): 61. CrossRef - Upper gastrointestinal endoscopy: expected post-procedural findings and adverse events
Tarek N. Hanna, Saurabh Rohatgi, Haris N. Shekhani, Fatima Shahid, Vijayanadh Ojili, Faisal Khosa
Emergency Radiology.2016; 23(5): 503. CrossRef - Recent Advanced Endoscopic Management of Endoscopic Retrograde Cholangiopancreatography Related Duodenal Perforations
Seon Mee Park
Clinical Endoscopy.2016; 49(4): 376. CrossRef - Complications of ERCP
Rupjyoti Talukdar
Best Practice & Research Clinical Gastroenterology.2016; 30(5): 793. CrossRef - Parenchymal Guidewire Perforation during ERCP: An Unappreciated Injury
M. Ezzedien Rabie, Saad Al Faris, Ali Nasser, Abdul Aziz Shahir, Yasser Al Mahdi, Mansour Youssef Al Asmari
Case Reports in Surgery.2015; 2015: 1. CrossRef - Retroperitoneales Emphysem nach endoskopischer retrograder Cholangiopankreatikographie
T. Vowinkel, N. Senninger
Der Chirurg.2015; 86(5): 462. CrossRef - Duodenal Perforations After Endoscopic Retrograde Cholangiopancreatography
María Desirée Armas Ojeda, Vanesa Ojeda Marrero, Cristina Roque Castellano, José Carlos Cabrera Marrero, María del Pino Mathías Gutierrez, Daniel Ceballos Santos, Joaquín Marchena Gómez
Cirugía Española (English Edition).2015; 93(6): 403. CrossRef - Perforaciones duodenales tras colangiopancreatografía retrógrada endoscópica
María Desirée Armas Ojeda, Vanesa Ojeda Marrero, Cristina Roque Castellano, José Carlos Cabrera Marrero, María del Pino Mathías Gutierrez, Daniel Ceballos Santos, Joaquín Marchena Gómez
Cirugía Española.2015; 93(6): 403. CrossRef - Huge biloma after endoscopic retrograde cholangiopancreatography and endoscopic biliary sphincterotomy
Harith M. Alkhateeb, Thaer J. Aljanabi, Khairallh H. Al-azzawi, Taha A. Alkarboly
International Journal of Surgery Case Reports.2015; 16: 7. CrossRef - Cross-sectional imaging of common and unusual complications after endoscopic retrograde cholangiopancreatography
Massimo Tonolini, Alessandra Pagani, Roberto Bianco
Insights into Imaging.2015; 6(3): 323. CrossRef - Acute Air Embolism during Retrograde Cholangiography for Postoperative Recurrent Cholangitis Due to Intraductal Residual Meals after Choledochoduodenostomy
Osamu Miyoshi, Shu Nakano
The Japanese Journal of Gastroenterological Surger.2015; 48(5): 463. CrossRef - Early endoscopic ultrasonography in acute biliary pancreatitis: A prospective pilot study
Andrea Anderloni
World Journal of Gastroenterology.2015; 21(36): 10427. CrossRef - ERCP-induced duodenal perforation successfully treated with endoscopic purse-string suture: a case report
Quanpeng Li, Jie Ji, Fei Wang, Xianxiu Ge, Junjie Nie, Boming Xu, Xiuhua Zhang, Guobing Jiang, Lin Miao
Oncotarget.2015; 6(19): 17847. CrossRef - Development of a swine bile duct dilation model using endoclips or a detachable snare under cap-assisted endoscopy
Jin-Seok Park, Chang-Il Kwon, Seok Jeong, Kwangil Kim, Jong Ho Moon, Don Haeng Lee
Gastrointestinal Endoscopy.2014; 80(2): 325. CrossRef - Rare and underappreciated complications of endoscopic retrograde cholangiopancreatography
Ji Young Bang, Gregory A. Coté
Techniques in Gastrointestinal Endoscopy.2014; 16(4): 195. CrossRef - Air Embolism after Endoscopic Retrograde Cholangiopancreatography in a Patient with Budd Chiari Syndrome
Beatriz Wills-Sanin, Yenny R. Cárdenas, Lucas Polanco, Oscar Rivero, Sebastian Suarez, Andrés F. Buitrago
Case Reports in Critical Care.2014; 2014: 1. CrossRef - Endoscopic retrograde cholangiopancreatography-related perforation: Management and prevention
Varayu Prachayakul
World Journal of Clinical Cases.2014; 2(10): 522. CrossRef - Emergencies after endoscopic procedures
Carla Rolanda, Ana C. Caetano, Mário Dinis-Ribeiro
Best Practice & Research Clinical Gastroenterology.2013; 27(5): 783. CrossRef - Endoscopic Treatments of Endoscopic Retrograde Cholangiopancreatography-Related Duodenal Perforations
Tae Hoon Lee, Joung-Ho Han, Sang-Heum Park
Clinical Endoscopy.2013; 46(5): 522. CrossRef
- Cystic duct disimpaction for acute cholecystitis in the high-risk cholecystectomy patient: Case report
- 9,649 View
- 167 Download
- 48 Crossref


 KSGE
KSGE


 First
First Prev
Prev



