Search
- Page Path
- HOME > Search
- Efficacy of hemostasis by gastroduodenal covered metal stent placement for hemorrhagic duodenal stenosis due to pancreatobiliary cancer invasion: a retrospective study
- Yasunari Sakamoto, Taku Sakamoto, Akihiro Ohba, Mitsuhito Sasaki, Shunsuke Kondo, Chigusa Morizane, Hideki Ueno, Yutaka Saito, Yasuaki Arai, Takuji Okusaka
- Received June 18, 2023 Accepted January 15, 2024 Published online June 14, 2024
- DOI: https://doi.org/10.5946/ce.2023.155 [Epub ahead of print]

-
 Graphical Abstract
Graphical Abstract
 Abstract
Abstract
 PDF
PDF PubReader
PubReader ePub
ePub 
- Background
/Aims: Advanced pancreatic and biliary tract cancers can invade the duodenum and cause duodenal hemorrhagic stenosis. This study aimed to evaluate the efficacy of covered self-expandable metal stents in the treatment of cancer-related duodenal hemorrhage with stenosis.
Methods
Between January 2014 and December 2016, metal stents were placed in 51 patients with duodenal stenosis. Among these patients, a self-expandable covered metal stent was endoscopically placed in 10 patients with hemorrhagic duodenal stenosis caused by pancreatobiliary cancer progression. We retrospectively analyzed the therapeutic efficacy of the stents by evaluating the technical and clinical success rates based on successful stent placement, degree of oral intake, hemostasis, stent patency, and overall survival.
Results
The technical and clinical success rates were 100%. All 10 patients achieved a Gastric Outlet Obstruction Scoring System score of three within two weeks after the procedure and had no recurrence of melena. The median stent patency duration and overall survival after stent placement were 52 days (range, 20–220 days) and 66.5 days (range, 31–220 days), respectively.
Conclusions
Endoscopic placement of a covered metal stent for hemorrhagic duodenal stenosis associated with pancreatic or biliary tract cancer resulted in duodenal hemostasis, recanalization, and improved quality of life.
- 416 View
- 12 Download

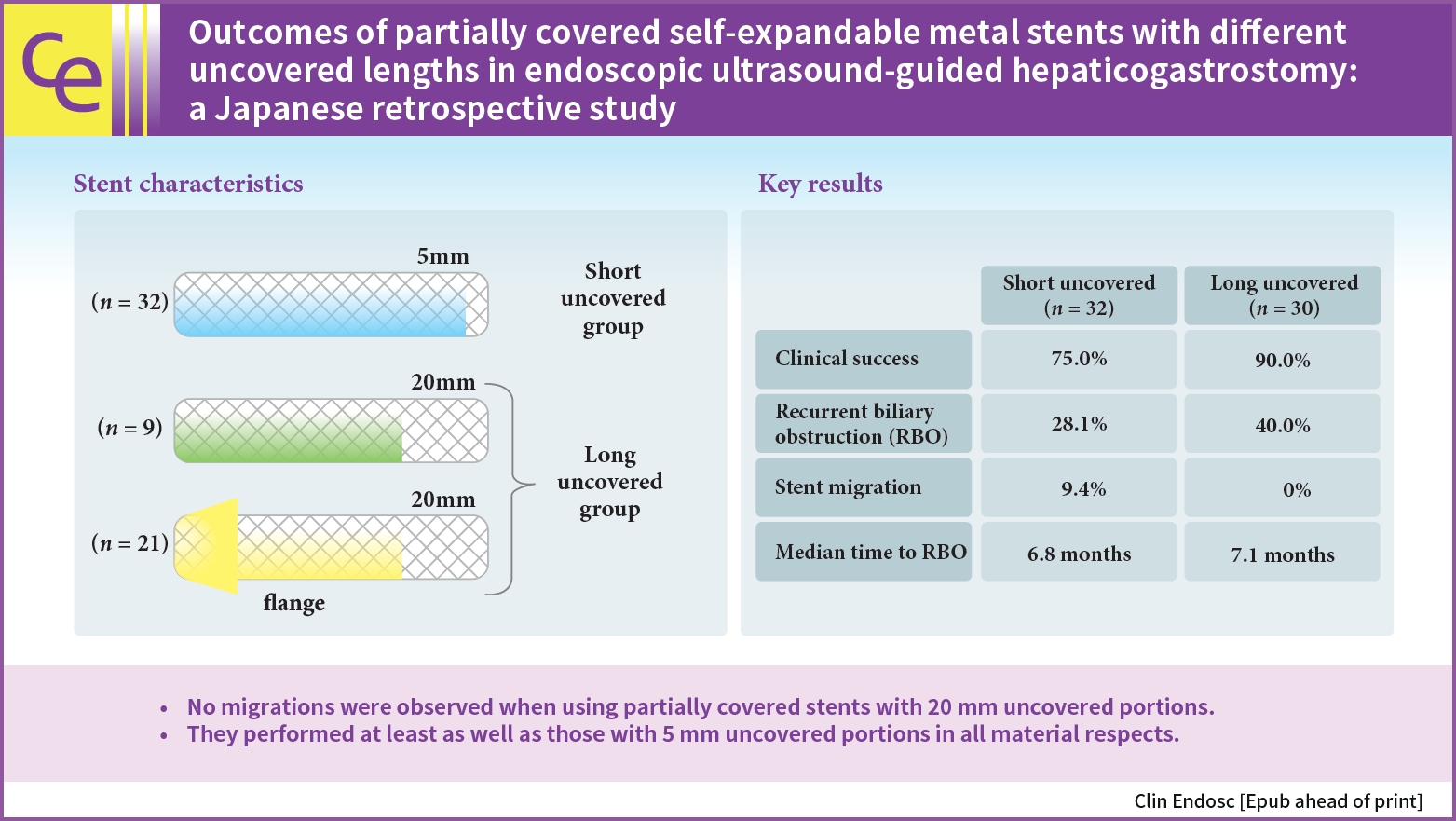
- Outcomes of partially covered self-expandable metal stents with different uncovered lengths in endoscopic ultrasound-guided hepaticogastrostomy: a Japanese retrospective study
- Takeshi Okamoto, Takashi Sasaki, Tsuyoshi Takeda, Tatsuki Hirai, Takahiro Ishitsuka, Manabu Yamada, Hiroki Nakagawa, Takafumi Mie, Takaaki Furukawa, Akiyoshi Kasuga, Masato Ozaka, Naoki Sasahira
- Received May 24, 2023 Accepted September 4, 2023 Published online May 10, 2024
- DOI: https://doi.org/10.5946/ce.2023.142 [Epub ahead of print]
-
 Graphical Abstract
Graphical Abstract
 Abstract
Abstract
 PDF
PDF Supplementary Material
Supplementary Material PubReader
PubReader ePub
ePub 
- Background
/Aims: The optimal length of the uncovered portion of partially covered self-expandable metal stents (PCSEMSs) used in endoscopic ultrasound-guided hepaticogastrostomy (EUS-HGS) remains unclear. This study investigated the safety and efficacy of PCSEMSs with different uncovered lengths, with a focus on stent migration and time to recurrent biliary obstruction (RBO).
Methods
Outcomes of patients undergoing EUS-HGS using PCSEMSs with 5-mm and 20-mm uncovered portions at our institution from January 2016 to December 2021 were compared.
Results
Sixty-two patients underwent EUS-HGS using PCSEMS (5/20-mm uncovered portions: 32/30). Stent migration occurred only in the 5-mm group. There were no differences in RBO rates (28.1% vs. 40.0%) or median time to RBO (6.8 vs. 7.1 months) between the two groups. Median overall survival (OS) was longer in the 20-mm group (3.1 vs. 4.9 months, p=0.037) due to the higher number of patients that resumed chemotherapy after EUS-HGS (56.7 vs. 28.1%, p=0.029). Good performance status, absence of hepatic metastases, and chemotherapy after EUS-HGS were independent predictors of longer OS.
Conclusions
No migration was observed in patients treated with PCSEMS with 20-mm uncovered portions. Patients treated with PCSEMS with 20-mm uncovered portions performed at least as well as those treated with 5-mm uncovered portions in all material respects.
- 1,432 View
- 35 Download

- Clinical utility of endoscopic ultrasound-guided tissue acquisition for comprehensive genomic profiling of pancreatic cancer
- Nozomi Okuno, Kazuo Hara, Nobumasa Mizuno, Shin Haba, Takamichi Kuwahara, Yasuhiro Kuraishi, Daiki Fumihara, Takafumi Yanaidani
- Clin Endosc 2023;56(2):221-228. Published online March 7, 2023
- DOI: https://doi.org/10.5946/ce.2022.086

-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader ePub
ePub - Background
/Aims: Endoscopic ultrasound-guided tissue acquisition (EUS-TA) is essential for the diagnosis of pancreatic cancer. The feasibility of comprehensive genomic profiling (CGP) using samples obtained by EUS-TA has been under recent discussion. This study aimed to evaluate the utility of EUS-TA for CGP in a clinical setting.
Methods
CGP was attempted in 178 samples obtained from 151 consecutive patients with pancreatic cancer at the Aichi Cancer Center between October 2019 and September 2021. We evaluated the adequacy of the samples for CGP and determined the factors associated with the adequacy of the samples obtained by EUS-TA retrospectively.
Results
The overall adequacy for CGP was 65.2% (116/178), which was significantly different among the four sampling methods (EUS-TA vs. surgical specimen vs. percutaneous biopsy vs. duodenal biopsy, 56.0% [61/109] vs. 80.4% [41/51] vs. 76.5% [13/17] vs. 100.0% [1/1], respectively; p=0.022). In a univariate analysis, needle gauge/type was associated with adequacy (22 G fine-needle aspiration vs. 22 G fine-needle biopsy [FNB] vs. 19 G-FNB, 33.3% (5/15) vs. 53.5% (23/43) vs. 72.5% (29/40); p=0.022). The sample adequacy of 19 G-FNB for CGP was 72.5% (29/40), and there was no significant difference between 19 G-FNB and surgical specimens (p=0.375).
Conclusions
To obtain adequate samples for CGP with EUS-TA, 19 G-FNB was shown to be the best in clinical practice. However, 19 G-FNB was not still sufficient, so further efforts are required to improve adequacy for CGP. -
Citations
Citations to this article as recorded by- Tissue acquisition for comprehensive genomic profiling of gallbladder cancer using a forward-viewing echoendoscope in a patient who underwent Roux-en-Y reconstruction
Michihiro Ono, Shutaro Oiwa, Atsushi Uesugi, Seiya Saito, Ryota Yokoyama, Makoto Usami, Tomoyuki Abe, Miri Fujita, Kohichi Takada, Masahiro Maeda
Clinical Journal of Gastroenterology.2024; 17(1): 164. CrossRef - Endoscopic ultrasound-guided tissue acquisition for comprehensive genomic profiling
Nozomi Okuno, Kazuo Hara
Journal of Medical Ultrasonics.2024; 51(2): 253. CrossRef - Oil blotting paper for formalin fixation increases endoscopic ultrasound‐guided tissue acquisition‐collected sample volumes on glass slides
Takuo Yamai, Kenji Ikezawa, Yusuke Seiki, Ko Watsuji, Yasuharu Kawamoto, Takeru Hirao, Kazuma Daiku, Shingo Maeda, Makiko Urabe, Yugo Kai, Ryoji Takada, Kaori Mukai, Tasuku Nakabori, Hiroyuki Uehara, Sayoko Tsuzaki, Ayumi Ryu, Satoshi Tanada, Shigenori Na
Cancer Medicine.2024;[Epub] CrossRef - Endoscopic ultrasound-guided tissue acquisition for personalized treatment in pancreatic adenocarcinoma
Sang Myung Woo
Clinical Endoscopy.2023; 56(2): 183. CrossRef - Comparison of the novel Franseen needle versus the fine‐needle aspiration needle in endoscopic ultrasound‐guided tissue acquisition for cancer gene panel testing: A propensity score‐matching analysis
Tomotaka Mori, Eisuke Ozawa, Akane Shimakura, Kosuke Takahashi, Satoshi Matsuo, Kazuaki Tajima, Yasuhiko Nakao, Masanori Fukushima, Ryu Sasaki, Satoshi Miuma, Hisamitsu Miyaaki, Shinji Okano, Kazuhiko Nakao
JGH Open.2023; 7(9): 652. CrossRef - Editorial: Endoscopic ultrasound‐guided tissue acquisition in the era of precision medicine
Tiing Leong Ang, James Weiquan Li, Lai Mun Wang
Journal of Gastroenterology and Hepatology.2023; 38(10): 1677. CrossRef
- Tissue acquisition for comprehensive genomic profiling of gallbladder cancer using a forward-viewing echoendoscope in a patient who underwent Roux-en-Y reconstruction
- 1,940 View
- 157 Download
- 4 Web of Science
- 6 Crossref

- A multicenter comparative study of endoscopic ultrasound-guided fine-needle biopsy using a Franseen needle versus conventional endoscopic ultrasound-guided fine-needle aspiration to evaluate microsatellite instability in patients with unresectable pancreatic cancer
- Tadayuki Takagi, Mitsuru Sugimoto, Hidemichi Imamura, Yosuke Takahata, Yuki Nakajima, Rei Suzuki, Naoki Konno, Hiroyuki Asama, Yuki Sato, Hiroki Irie, Jun Nakamura, Mika Takasumi, Minami Hashimoto, Tsunetaka Kato, Ryoichiro Kobashi, Yuko Hashimoto, Goro Shibukawa, Shigeru Marubashi, Takuto Hikichi, Hiromasa Ohira
- Clin Endosc 2023;56(1):107-113. Published online January 16, 2023
- DOI: https://doi.org/10.5946/ce.2022.019

-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader ePub
ePub - Background
/Aims: Immune checkpoint blockade has recently been reported to be effective in treating microsatellite instability (MSI)-high tumors. Therefore, sufficient sampling of histological specimens is necessary in cases of unresectable pancreatic cancer (UR-PC). This multicenter study investigated the efficacy of endoscopic ultrasound-guided fine-needle biopsy (EUS-FNB) using a Franseen needle for MSI evaluation in patients with UR-PC.
Methods
A total of 89 patients with UR-PC who underwent endoscopic ultrasound-guided fine-needle aspiration (EUS-FNA) or EUS-FNB using 22-G needles at three hospitals in Japan (2018–2021) were enrolled. Fifty-six of these patients (FNB 23 and FNA 33) were followed up or evaluated for MSI. Patient characteristics, UR-PC data, and procedural outcomes were compared between patients who underwent EUS-FNB and those who underwent EUS-FNA.
Results
No significant difference in terms of sufficient tissue acquisition for histology was observed between patients who underwent EUS-FNB and those who underwent EUS-FNA. MSI evaluation was possible significantly more with tissue samples obtained using EUS-FNB than with tissue samples obtained using EUS-FNA (82.6% [19/23] vs. 45.5% [15/33], respectively; p<0.01). In the multivariate analysis, EUS-FNB was the only significant factor influencing the possibility of MSI evaluation.
Conclusions
EUS-FNB using a Franseen needle is desirable for ensuring sufficient tissue acquisition for MSI evaluation. -
Citations
Citations to this article as recorded by- Oil blotting paper for formalin fixation increases endoscopic ultrasound‐guided tissue acquisition‐collected sample volumes on glass slides
Takuo Yamai, Kenji Ikezawa, Yusuke Seiki, Ko Watsuji, Yasuharu Kawamoto, Takeru Hirao, Kazuma Daiku, Shingo Maeda, Makiko Urabe, Yugo Kai, Ryoji Takada, Kaori Mukai, Tasuku Nakabori, Hiroyuki Uehara, Sayoko Tsuzaki, Ayumi Ryu, Satoshi Tanada, Shigenori Na
Cancer Medicine.2024;[Epub] CrossRef
- Oil blotting paper for formalin fixation increases endoscopic ultrasound‐guided tissue acquisition‐collected sample volumes on glass slides
- 2,158 View
- 125 Download
- 1 Crossref

- Endoscopic Ultrasound-Guided Local Therapy for Pancreatic Neoplasms
- Jun Seong Hwang, Hyun Don Joo, Tae Jun Song
- Clin Endosc 2020;53(5):535-540. Published online September 29, 2020
- DOI: https://doi.org/10.5946/ce.2020.181
-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader ePub
ePub - Surgical resection is considered the only treatment option for pancreatic cancer and other pancreatic neoplasms with malignant potential, such as neuroendocrine tumors, mucinous cystic neoplasms, and intraductal papillary mucinous neoplasms. However, only 10%–20% of all patients with pancreatic cancer present with resectable forms of the disease as the symptoms are rarely manifested during the early stages, and the disease tends to progress rapidly. Furthermore, pancreatic surgery is associated with high rates of morbidity and mortality. The development of linear-array endoscopic ultrasound (EUS) techniques has increased the indications of EUS-guided local therapy for pancreatic neoplasms. We assessed the studies that investigated various treatment modalities, such as fine-needle injection, radiofrequency ablation, irreversible electroporation, and radiotherapy, under EUS guidance to better understand the usefulness of these techniques with respect to the efficacy and associated complications.
-
Citations
Citations to this article as recorded by- Anticancer effect of a pyrrole‐imidazole polyamide‐triphenylphosphonium conjugate selectively targeting a common mitochondrial DNA cancer risk variant in cervical cancer cells
Jihang Yao, Keizo Takenaga, Nobuko Koshikawa, Yuki Kida, Jason Lin, Takayoshi Watanabe, Yoshiaki Maru, Yoshitaka Hippo, Seigi Yamamoto, Yuyan Zhu, Hiroki Nagase
International Journal of Cancer.2023; 152(5): 962. CrossRef - Endoscopic Ultrasound-Guided Local Ablative Therapies for the Treatment of Pancreatic Neuroendocrine Tumors and Cystic Lesions: A Review of the Current Literature
Alexander M. Prete, Tamas A. Gonda
Journal of Clinical Medicine.2023; 12(9): 3325. CrossRef - Response of Locally Advanced Pancreatic Cancer to Intratumoral Injection of Large Surface Area Microparticle Paclitaxel
Neil R. Sharma, Simon K. Lo, Andrew Hendifar, Mohamed O. Othman, Kalpesh Patel, Antonio Mendoza-Ladd, Shelagh Verco, Holly A. Maulhardt, James Verco, Alison Wendt, Alyson Marin, Christian Max Schmidt, Gere diZerega
Pancreas.2023; 52(3): e179. CrossRef - Multisite Is Superior to Single-Site Intratumoral Chemotherapy to Retard the Outcomes of Pancreatic Ductal Adenocarcinoma in a Murine Model
Janette Lazarovits, Ron Epelbaum, Jesse Lachter, Yaron Amikam, Jacob Ben Arie
Cancers.2023; 15(24): 5801. CrossRef - Endoscopic ultrasound-guided injectable therapy for pancreatic cancer: A systematic review
Jyotroop Kaur, Veeravich Jaruvongvanich, Vinay Chandrasekhara
World Journal of Gastroenterology.2022; 28(21): 2383. CrossRef
- Anticancer effect of a pyrrole‐imidazole polyamide‐triphenylphosphonium conjugate selectively targeting a common mitochondrial DNA cancer risk variant in cervical cancer cells
- 4,271 View
- 148 Download
- 5 Web of Science
- 5 Crossref

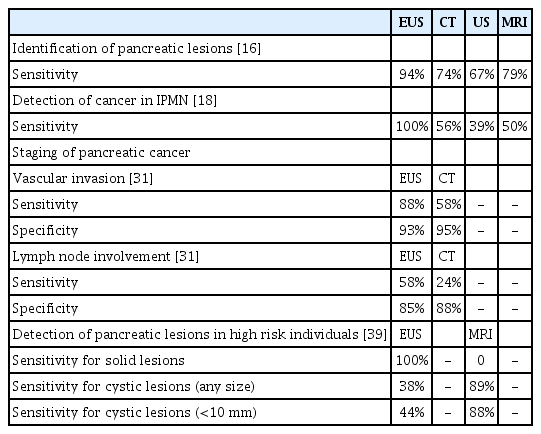
- Current Status of Endoscopic Ultrasound Techniques for Pancreatic Neoplasms
- Yousuke Nakai, Naminatsu Takahara, Suguru Mizuno, Hirofumi Kogure, Kazuhiko Koike
- Clin Endosc 2019;52(6):527-532. Published online July 24, 2019
- DOI: https://doi.org/10.5946/ce.2019.025
-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader ePub
ePub - Endoscopic ultrasound (EUS) now plays an important role in the management of pancreatic neoplasms. There are various types of pancreatic neoplasms, from benign to malignant lesions, and the role of EUS ranges from the imaging diagnosis to treatment. EUS is useful for the detection, characterization, and tissue acquisition of pancreatic lesions. Recent advancement of contrast-enhanced harmonic EUS and elastography enables better characterization of pancreatic lesions. In addition to these enhanced EUS imaging techniques, EUS-guided tissue acquisition is now the standard procedure to establish the pathological diagnosis of pancreatic neoplasms. While these diagnostic roles of EUS have been established, EUS-guided interventions such as ablation and drainage are also increasingly utilized in the management of pancreatic neoplasms. However, most of these EUS-guided interventions are not yet standardized in terms of techniques and devices and thus need further investigations.
-
Citations
Citations to this article as recorded by- Impact of endoscopic ultrasound-guided radiofrequency ablation in managing pancreatic malignancy
Cosmas Rinaldi Adithya Lesmana
World Journal of Gastrointestinal Surgery.2023; 15(2): 163. CrossRef - Artificial intelligence assisted endoscopic ultrasound for detection of pancreatic space-occupying lesion: a systematic review and meta-analysis
Arkadeep Dhali, Vincent Kipkorir, Bahadar S. Srichawla, Harendra Kumar, Roger B. Rathna, Ibsen Ongidi, Talha Chaudhry, Gisore Morara, Khulud Nurani, Doreen Cheruto, Jyotirmoy Biswas, Leonard R. Chieng, Gopal Krishna Dhali
International Journal of Surgery.2023; 109(12): 4298. CrossRef - Endotherapy in Pancreatic Diseases
Vaneet Jearth, Surinder S. Rana
Journal of Digestive Endoscopy.2022; 13(01): 019. CrossRef - Endoscopic Management of Pancreatobiliary Malignancies
Dong Wook Lee, Eun Young Kim
Digestive Diseases and Sciences.2022; 67(5): 1635. CrossRef - Bazo accesorio intrapancreático: reporte de caso resuelto con pancreatectomía distal robótica
Armando Pereyra-Talamantes, Juan Eduardo Flores-Martín, Marco Antonio Gallaga-Rojas, Jesús Emmanuel Rodríguez-Silverio, Erikc González-Azua, Mario Eduardo Alonso-Calamaco, Enrique Jiménez-Chavarría, Héctor F Noyola-Villalobos
Revista Mexicana de Cirugía Endoscópica.2022; 23(1-2): 41. CrossRef - Dynamic Doppler Ultrasound Assessment of Tissue Perfusion Is a Better Tool than a Single Vessel Doppler Examination in Differentiating Malignant and Inflammatory Pancreatic Lesions
Przemysław Dyrla, Arkadiusz Lubas, Jerzy Gil, Marek Saracyn, Maciej Gonciarz
Diagnostics.2021; 11(12): 2289. CrossRef - Differential diagnosis of solid pancreatic masses
Julio Iglesias-Garcia, Daniel de la Iglesia-Garcia, José M. Olmos-Martinez, José Lariño-Noia, J. Enrique Dominguez-Muñoz
Minerva Gastroenterologica e Dietologica.2020;[Epub] CrossRef
- Impact of endoscopic ultrasound-guided radiofrequency ablation in managing pancreatic malignancy
- 5,465 View
- 237 Download
- 6 Web of Science
- 7 Crossref

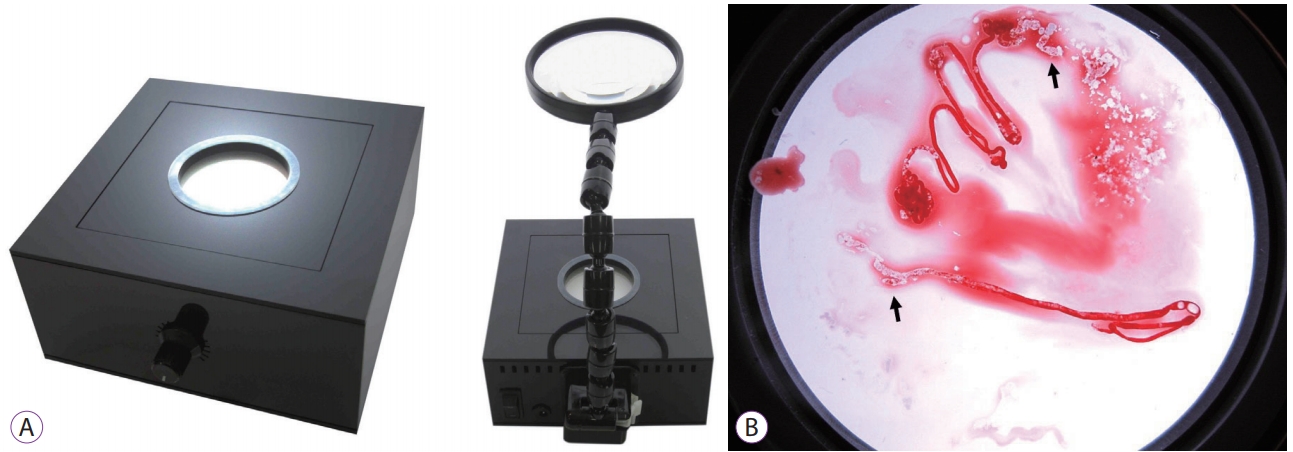
- A “Back Light System” for Identification of Sites for Endoscopic Ultrasound-Guided Fine-Needle Aspiration in Solid Pancreatic Masses: A Prospective, Randomized Study with a Crossover Design
- Ryo Harada, Hironari Kato, Soichiro Fushimi, Hirofumi Inoue, Daisuke Uchida, Yutaka Akimoto, Takeshi Tomoda, Kazuyuki Matsumoto, Yasuhiro Noma, Naoki Yamamoto, Shigeru Horiguchi, Koichiro Tsutsumi, Hiroyuki Okada
- Clin Endosc 2019;52(4):334-339. Published online May 16, 2019
- DOI: https://doi.org/10.5946/ce.2019.004
-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader ePub
ePub - Background
/Aims: We applied a back light system (BLS) with a magnifying glass to improve the ability to assess the adequacy of specimen sampling using endosonography. We conducted this study to evaluate the efficacy of the BLS in sampling of specimens by endoscopic ultrasound-guided fine needle aspiration of solid pancreatic masses.
Methods
This was a prospective, randomized, crossover, single-center clinical trial. An endosonographer evaluated adequacy on gross visual inspection and identified whitish specimen sampling sites with and without the BLS according to a randomization sequence in the first and second passes with a 25-G needle. On cytological evaluation, the presence of well-defined pancreatic ductal epithelium was evaluated by a cytopathologist who was blinded to any clinical information.
Results
A total of 80 consecutive patients were eligible during the study period. Adequacy was observed for 52 specimens (65%) with the BLS and 54 (68%) without the BLS (p=0.88). In assessment of specimen adequacy on gross examination, only fair agreement was observed both with and without BLS (kappa score 0.40 and 0.29, respectively).
Conclusions
The BLS did not influence the ability to identify specimen sampling sites or reliable assessment of specimen site adequacy using gross visual inspection. -
Citations
Citations to this article as recorded by- Tissue processing of endoscopic ultrasound-guided fine-needle aspiration specimens from solid pancreatic lesions
Kenji Notohara, Kaori Nakamura
Journal of Medical Ultrasonics.2024; 51(2): 261. CrossRef - Macroscopic qualitative evaluation of solid pancreatic lesion specimens from endoscopic ultrasound-guided fine needle aspiration/biopsies
Kaori Nakamura, Kenji Notohara, Ryoji Nishizaki, Etsuji Ishida, Midori Sato, Akemi Kodera, Junya Itakura, Motowo Mizuno
Pancreatology.2023; 23(8): 1028. CrossRef - Unfortunately, a “Back Light System” As a Global Positioning System Failed to Guide the Route in 25-G Fine-Needle Aspiration
Rungsun Rerknimitr, Phonthep Angsuwatcharakon
Clinical Endoscopy.2019; 52(4): 295. CrossRef
- Tissue processing of endoscopic ultrasound-guided fine-needle aspiration specimens from solid pancreatic lesions
- 4,297 View
- 81 Download
- 5 Web of Science
- 3 Crossref

- Endoscopic Palliation for Biliary and Pancreatic Malignancies: Recent Advances
- Zaheer Nabi, D. Nageshwar Reddy
- Clin Endosc 2019;52(3):226-234. Published online January 22, 2019
- DOI: https://doi.org/10.5946/ce.2019.003
-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader ePub
ePub - Malignancies of the pancreatobiliary system are usually unresectable at the time of diagnosis. As a consequence, a majority of these cases are candidates for palliative care. With advances in chemotherapeutic agents and multidisciplinary care, the survival rate in pancreatobiliary malignancies has improved. Therefore, there is a need to provide an effective and long-lasting palliative care for these patients. Endoscopic palliation is preferred to surgery as the former is associated with equal efficacy and reduced morbidity. The main role of endoscopic palliation in the vast majority of pancreatobiliary malignancies includes biliary and enteral stenting for malignant obstructive jaundice and gastric outlet obstruction, respectively. Recent advances in endoscopic palliation appear promising in imparting long-lasting relief of symptoms. Use of radiofrequency ablation and photodynamic therapy in malignant biliary obstruction has been shown to improve the survival rates as well as the patency of biliary stents. The emergence of endoscopic ultrasound (EUS) as a therapeutic tool has enhanced the capability of minimally invasive palliation in pancreatobiliary cancers. EUS is a valuable alternative to endoscopic retrograde cholangiopancreatography for the palliation of obstructive jaundice. More recently, EUS is emerging as an effective primary modality for biliary and gastric bypass.
-
Citations
Citations to this article as recorded by- International Digestive Endoscopy Network consensus on the management of antithrombotic agents in patients undergoing gastrointestinal endoscopy
Seung Joo Kang, Chung Hyun Tae, Chang Seok Bang, Cheol Min Shin, Young-Hoon Jeong, Miyoung Choi, Joo Ha Hwang, Yutaka Saito, Philip Wai Yan Chiu, Rungsun Rerknimitr, Christopher Khor, Vu Van Khien, Kee Don Choi, Ki-Nam Shim, Geun Am Song, Oh Young Lee
Clinical Endoscopy.2024; 57(2): 141. CrossRef - Palliative Interventions and Best Supportive Care in Biliary Malignancy
Christine Chung, Lauren Wancata
Surgical Clinics of North America.2024;[Epub] CrossRef - Gamma-Glutamyl Transferase: A Friend against Cholestatic Itch? A Retrospective Observational Data Analysis in Patients with Extrahepatic Cholestasis
Floris W. Haijer, Cornelis B. Van Vliet, Marjolein G. J. Brusse-Keizer, Job A. M. Van der Palen, Marjo J. Kerbert-Dreteler, Jeroen J. Kolkman, Dirk Uhlmann
International Journal of Hepatology.2023; 2023: 1. CrossRef - Endoscopic and Endosonographic Palliation for Triple Obstruction Caused by Recurrent Gallbladder Cancer: A Case Report
Young Rong Kim, Chi Hyuk Oh, Min Jae Yang
The Korean Journal of Pancreas and Biliary Tract.2023; 28(1): 19. CrossRef - Endoscopic Management of Pancreatobiliary Malignancies
Dong Wook Lee, Eun Young Kim
Digestive Diseases and Sciences.2022; 67(5): 1635. CrossRef - Angle of covered self-expandable metallic stents after placement is a risk factor for recurrent biliary obstruction
Kojiro Tanoue, Hirotsugu Maruyama, Yuki Ishikawa-Kakiya, Yosuke Kinoshita, Kappei Hayashi, Masafumi Yamamura, Masaki Ominami, Yuji Nadatani, Shusei Fukunaga, Koji Otani, Shuhei Hosomi, Fumio Tanaka, Noriko Kamata, Yasuaki Nagami, Koichi Taira, Toshio Wata
World Journal of Hepatology.2022; 14(5): 993. CrossRef - Angle of covered self-expandable metallic stents after placement is a risk factor for recurrent biliary obstruction
Kojiro Tanoue, Hirotsugu Maruyama, Yuki Ishikawa-Kakiya, Yosuke Kinoshita, Kappei Hayashi, Masafumi Yamamura, Masaki Ominami, Yuji Nadatani, Shusei Fukunaga, Koji Otani, Shuhei Hosomi, Fumio Tanaka, Noriko Kamata, Yasuaki Nagami, Koichi Taira, Toshio Wata
World Journal of Hepatology.2022; 14(5): 992. CrossRef - Pancreatic Cancer: Challenges and Opportunities in Locoregional Therapies
Alaa Y. Bazeed, Candace M. Day, Sanjay Garg
Cancers.2022; 14(17): 4257. CrossRef - Endoscopic retrograde cholangiopancreatography: Current practice and future research
David J Sanders, Shivanand Bomman, Rajesh Krishnamoorthi, Richard A Kozarek
World Journal of Gastrointestinal Endoscopy.2021; 13(8): 260. CrossRef - Early malfunction of a biliary self-expandable metal stent with an antireflux valve
Sang Hoon Kim, Chi Hyuk Oh, Jae Min Lee, Seong Ji Choi, Hyuk Soon Choi, Eun Sun Kim, Bora Keum, Yoon Tae Jeen, Hoon Jai Chun, Hong Sik Lee, Chang Duck Kim
Medicine.2020; 99(16): e19750. CrossRef - Endosonography-guided Radiofrequency Ablation in Pancreatic Diseases
Giuseppe Vanella, Gabriele Capurso, Paolo G. Arcidiacono
Journal of Clinical Gastroenterology.2020; 54(7): 591. CrossRef - Efficacy and safety of radiofrequency ablation in patients with unresectable malignant biliary strictures
Martha Claudia Galindo Orozco, Angélica Hernández Guerrero, Juan Octavio Alonso Larraga, Eduardo Ramírez Solis, José Guillermo de la Mora Levy, María del Carmen Manzano Robleda
Revista Española de Enfermedades Digestivas.2020;[Epub] CrossRef
- International Digestive Endoscopy Network consensus on the management of antithrombotic agents in patients undergoing gastrointestinal endoscopy
- 6,347 View
- 312 Download
- 9 Web of Science
- 12 Crossref

- Future Perspectives on Endoscopic Ultrasonography-Guided Therapy for Pancreatic Neoplasm
- Woo Hyun Paik, Sang Hyub Lee, Sunguk Jang
- Clin Endosc 2018;51(3):229-234. Published online May 18, 2018
- DOI: https://doi.org/10.5946/ce.2018.063
-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader ePub
ePub - Endoscopic ultrasonography (EUS)-guided therapy with ethanol injection or catheter-based radiofrequency ablation for pancreatic neoplasm has been conducted as a potential alternate treatment modality for patients who are not eligible for surgery. On the basis of the limited number of studies available, EUS-guided ablation therapy with the aforementioned methods for small pancreatic neoplasms has demonstrated promising technical feasibility and safety profiles. To be considered as a legitimate alternative option to surgery, however, EUS-guided ablation therapy must provide a long-term efficacy profile along with the consensus among experts regarding its treatment parameter. This review focuses on the clinical issues and future perspectives of EUS-guided therapy for pancreatic neoplasm.
-
Citations
Citations to this article as recorded by- An updated review on ablative treatment of pancreatic cystic lesions
Andrew Canakis, Ryan Law, Todd Baron
Gastrointestinal Endoscopy.2020; 91(3): 520. CrossRef - Endosonography-guided Radiofrequency Ablation in Pancreatic Diseases
Giuseppe Vanella, Gabriele Capurso, Paolo G. Arcidiacono
Journal of Clinical Gastroenterology.2020; 54(7): 591. CrossRef
- An updated review on ablative treatment of pancreatic cystic lesions
- 5,558 View
- 134 Download
- 3 Web of Science
- 2 Crossref

- Usefulness of Endoscopic Transpapillary Tissue Sampling for Malignant Biliary Strictures and Predictive Factors of Diagnostic Accuracy
- Hiroki Tanaka, Shimpei Matsusaki, Youichirou Baba, Yoshiaki Isono, Tomohiro Sase, Hiroshi Okano, Tomonori Saito, Katsumi Mukai, Tetsuya Murata, Hiroki Taoka
- Clin Endosc 2018;51(2):174-180. Published online August 31, 2017
- DOI: https://doi.org/10.5946/ce.2017.082
-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader ePub
ePub - Background
/Aims: It is sometimes difficult to distinguish between malignant and benign biliary strictures using imaging studies alone, and pathological diagnosis is necessary. The aim of this study was to determine the usefulness of endoscopic transpapillary tissue sampling and factors predictive of diagnostic accuracy.
Methods
From April 2008 to December 2014, 136 patients underwent endoscopic transpapillary tissue sampling for malignant biliary strictures. The cytological and histological findings were reported as negative, suspicious, or positive. Suspicious and positive findings were defined as pathologically positive.
Results
The sensitivity was 65.0% for forceps biopsy, 49.5% for brush cytology, 46.2% for bile aspiration cytology, and 21.9% for endoscopic nasobiliary drainage cytology. The combination of these procedures improved the sensitivity (72.8%). Endoscopic transpapillary tissue sampling was more sensitive for lesions of biliary origin (91.4%) than for extrabiliary lesions (66.3%). In surgical cases, the sensitivity for tumors with an infiltrative growth pattern (53.3%) was significantly lower than for a tumor with an expanding or intermediate growth pattern (87.5%).
Conclusions
Combining procedures can improve diagnostic accuracy. It may be possible to predict the sensitivity of endoscopic transpapillary tissue sampling by evaluating the etiology and tumor growth pattern using preoperative imaging studies. -
Citations
Citations to this article as recorded by- A new tool for bile duct tissue sampling: ex vivo clinical evaluation of intraductal cryobiopsy for cholangioscopy
Lukas Wirsing, Walter Linzenbold, Simon U. Jaeger, Phillip Stahl, German Ott, Tobias Leibold, Markus Enderle, Jörg Albert, Jan Peveling-Oberhag
Endoscopy International Open.2022; 10(06): E809. CrossRef - Increased accuracy of FNA-based cytological diagnosis of pancreatic lesions by use of an ethanol-based fixative system: A STROBE compliant study
Martin Bürger, Antje Heidrich, Iver Petersen, Andreas Stallmach, Carsten Schmidt
Medicine.2022; 101(36): e30449. CrossRef - Comparison of the Diagnostic Performance of Novel Slim Biopsy Forceps with Conventional Biopsy Forceps for Biliary Stricture: A Multicenter Retrospective Study
Eun Suk Jung, Se Woo Park, Jung Hee Kim, Jang Han Jung, Min Jae Yang, Da Hae Park
Journal of Personalized Medicine.2021; 11(1): 55. CrossRef - Factors affecting the diagnostic yield of endoscopic transpapillary forceps biopsy in patients with malignant biliary strictures
Min Jae Yang, Jae Chul Hwang, Dakeun Lee, Young Bae Kim, Byung Moo Yoo, Jin Hong Kim
Journal of Gastroenterology and Hepatology.2021; 36(8): 2324. CrossRef - Tissue sampling for biliary strictures using novel elbow biopsy forceps
Huahui Zhang, Chunyan Huo, Yongxin Guo, Keyuan Zhu, Fengdong Li, Jin Huang
Scientific Reports.2021;[Epub] CrossRef - Tips and tricks for the diagnosis and management of biliary stenosis-state of the art review
Giovanna Del Vecchio Blanco, Michelangela Mossa, Edoardo Troncone, Renato Argirò, Andrea Anderloni, Alessandro Repici, Omero Alessandro Paoluzi, Giovanni Monteleone
World Journal of Gastrointestinal Endoscopy.2021; 13(10): 473. CrossRef - Ethanol-based fixation is superior to conventional brush cytology in the evaluation of indeterminate biliary strictures by endoscopic retrograde cholangiography
Martin Bürger, Antje Besser, Iver Petersen, Andreas Stallmach, Carsten Schmidt
Medicine.2020; 99(5): e18920. CrossRef - Endoscopic retrograde cholangiopancreatography guided interventions in the management of pancreatic cancer
Muhammad Nadeem Yousaf, Hamid Ehsan, Ahsan Wahab, Ahmad Muneeb, Fizah S Chaudhary, Richard Williams, Christopher J Haas
World Journal of Gastrointestinal Endoscopy.2020; 12(10): 323. CrossRef - Percutaneous transhepatic cholangiobiopsy
Thiago Franchi Nunes, Tiago Kojun Tibana, Rômulo Florêncio Tristão Santos, Bernardo Bacelar de Faria, Edson Marchiori
Radiologia Brasileira.2019; 52(1): 41. CrossRef - Factors Associated with Malignant Biliary Strictures in Patients with Atypical or Suspicious Cells on Brush Cytology
Ji Young Park, Tae Joo Jeon
Clinical Endoscopy.2019; 52(2): 168. CrossRef - Diagnosis of Malignant Biliary Stricture: More is Better
Hyun Jik Lee, Kwang Bum Cho
Clinical Endoscopy.2018; 51(2): 115. CrossRef
- A new tool for bile duct tissue sampling: ex vivo clinical evaluation of intraductal cryobiopsy for cholangioscopy
- 6,895 View
- 126 Download
- 12 Web of Science
- 11 Crossref

- Endoscopic Ultrasound-Guided Direct Intervention for Solid Pancreatic Tumors
- Jimin Han, Kenneth J. Chang
- Clin Endosc 2017;50(2):126-137. Published online March 30, 2017
- DOI: https://doi.org/10.5946/ce.2017.034
-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader ePub
ePub - Development and use of linear-array echoendoscope and endoscopic ultrasound-guided fine needle aspiration (EUS-FNA) have made endoscopic ultrasound (EUS) more of an interventional procedure than a purely diagnostic procedure. This is a literature review of previously published clinical studies on EUS-guided direct intervention for solid pancreatic tumors, including EUS-guided fine needle injection (EUS-FNI) of antitumor agents, EUS-guided fiducial marker placement, EUS-guided brachytherapy and EUS-guided tumor ablation.
-
Citations
Citations to this article as recorded by- Endoscopic Ultrasound-Guided Antitumor Therapy
Yousuke Nakai
Gastrointestinal Endoscopy Clinics of North America.2024; 34(1): 79. CrossRef - Direct image-guided retroperitoneal approach and treatment of the pancreas by using natural orifice transluminal endoscopic surgery after EUS sugar-assisted radiofrequency ablation (with video)
Leonardo Sosa-Valencia, Giulia Pecorella, Gerlinde Averous, Julieta Montanelli, Fanélie Wanert, Lee Swanström
Gastrointestinal Endoscopy.2022; 95(3): 573. CrossRef - Endoscopic Management of Pancreatobiliary Malignancies
Dong Wook Lee, Eun Young Kim
Digestive Diseases and Sciences.2022; 67(5): 1635. CrossRef - Estimation of porcine pancreas optical properties in the 600–1100 nm wavelength range for light-based therapies
Pranav Lanka, Leonardo Bianchi, Andrea Farina, Martina De Landro, Antonio Pifferi, Paola Saccomandi
Scientific Reports.2022;[Epub] CrossRef - Gold Nanoparticles-Mediated Photothermal Therapy of Pancreas Using GATE: A New Simulation Platform
Somayeh Asadi, Leonardo Bianchi, Martina De Landro, Paola Saccomandi
Cancers.2022; 14(22): 5686. CrossRef - Endoscopic Ultrasound–Guided Fiducial Placement for Stereotactic Body Radiation Therapy in Pancreatic Malignancy
Seong-Hun Kim, Eun Ji Shin
Clinical Endoscopy.2021; 54(3): 314. CrossRef - Diagnostic and Interventional Role of Endoscopic Ultrasonography for the Management of Pancreatic Neuroendocrine Neoplasms
Giuseppinella Melita, Socrate Pallio, Andrea Tortora, Stefano Francesco Crinò, Antonio Macrì, Gianlorenzo Dionigi
Journal of Clinical Medicine.2021; 10(12): 2638. CrossRef - Advanced Endoscopic Techniques for the Diagnosis of Pancreatic Cancer and Management of Biliary and GastricOutlet Obstruction
Yousuke Nakai, Zachary Smith, Kenneth J. Chang, Kulwinder S. Dua
Surgical Oncology Clinics of North America.2021; 30(4): 639. CrossRef - New Isotopes for the Treatment of Pancreatic Cancer in Collaboration With CERN: A Mini Review
Claudia Burkhardt, Léo Bühler, David Viertl, Thierry Stora
Frontiers in Medicine.2021;[Epub] CrossRef - PID Controlling Approach Based on FBG Array Measurements for Laser Ablation of Pancreatic Tissues
Sanzhar Korganbayev, Annalisa Orrico, Leonardo Bianchi, Davide Paloschi, Alexey Wolf, Alexander Dostovalov, Paola Saccomandi
IEEE Transactions on Instrumentation and Measurement.2021; 70: 1. CrossRef - Endoscopic retrograde cholangiopancreatography guided interventions in the management of pancreatic cancer
Muhammad Nadeem Yousaf, Hamid Ehsan, Ahsan Wahab, Ahmad Muneeb, Fizah S Chaudhary, Richard Williams, Christopher J Haas
World Journal of Gastrointestinal Endoscopy.2020; 12(10): 323. CrossRef - Necrosis volume and Choi criteria predict the response to endoscopic ultrasonography-guided HybridTherm ablation of locally advanced pancreatic cancer
Sabrina Gloria Giulia Testoni, Gabriele Capurso, Maria Chiara Petrone, Maurizio Barbera, Walter Linzenbold, Markus Enderle, Simone Gusmini, Roberto Nicoletti, Emanuel Della Torre, Alberto Mariani, Gemma Rossi, Livia Archibugi, Francesco De Cobelli, Michel
Endoscopy International Open.2020; 08(10): E1511. CrossRef - EUS-guided fine-needle injection for pancreatic cancer: back to the future
Yousuke Nakai, Kenneth J. Chang
Gastrointestinal Endoscopy.2020; 92(5): 1053. CrossRef - EUS-guided irreversible electroporation using endoscopic needle-electrode in porcine pancreas
Jae Min Lee, Hyuk Soon Choi, Hoon Jai Chun, Eun Sun Kim, Bora Keum, Yeon Seok Seo, Yoon Tae Jeen, Hong Sik Lee, Soon Ho Um, Chang Duck Kim, Hong Bae Kim
Surgical Endoscopy.2019; 33(2): 658. CrossRef - Management of Gastrointestinal Neuroendocrine Tumors
Rongzhi Wang, Rui Zheng-Pywell, H Alexander Chen, James A Bibb, Herbert Chen, J Bart Rose
Clinical Medicine Insights: Endocrinology and Diabetes.2019; 12: 117955141988405. CrossRef - Current Status of Endoscopic Ultrasound Techniques for Pancreatic Neoplasms
Yousuke Nakai, Naminatsu Takahara, Suguru Mizuno, Hirofumi Kogure, Kazuhiko Koike
Clinical Endoscopy.2019; 52(6): 527. CrossRef - Future Perspectives on Endoscopic Ultrasonography-Guided Therapy for Pancreatic Neoplasm
Woo Hyun Paik, Sang Hyub Lee, Sunguk Jang
Clinical Endoscopy.2018; 51(3): 229. CrossRef - Diagnosis of Pancreatic Neuroendocrine Tumors
Dong Wook Lee, Michelle Kang Kim, Ho Gak Kim
Clinical Endoscopy.2017; 50(6): 537. CrossRef
- Endoscopic Ultrasound-Guided Antitumor Therapy
- 9,215 View
- 219 Download
- 20 Web of Science
- 18 Crossref

- Extragastroesophageal Malignancy-Associated Secondary Achalasia: A Rare Association of Pancreatic Cancer Rendering Alarm Manifestation
- Hong Min Kim, Ji Min Chu, Won Hee Kim, Sung Pyo Hong, Ki Baik Hahm, Kwang Hyun Ko
- Clin Endosc 2015;48(4):328-331. Published online July 24, 2015
- DOI: https://doi.org/10.5946/ce.2015.48.4.328
-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader ePub
ePub Secondary achalasia or pseudoachalasia is a rare esophageal motor abnormality, which mimics primary achalasia; it is not easily distinguishable from idiopathic achalasia by manometry, radiological examination, or endoscopy. Although the majority of reported pseudoachalasia cases are associated with neoplasms at or near the esophagogastric (EG) junction, other neoplastic processes or even chronic illnesses such as rheumatoid arthritis can lead to the development of pseudoachalasia, for example, mediastinal masses, gastrointestinal (GI) tumors of the liver and biliary tract, and non-GI malignancies. Therefore, even if a patient presents with the typical findings of achalasia, we should be alert to the possibility of other GI malignancies besides EG tumors. For instance, pancreatic cancer was found in the case reported here; only four such cases have been reported in the literature. A 47-year-old man was admitted to our center with a 3-month history of dysphagia. His endoscopic and esophageal manometric findings were compatible with primary achalasia. However, unresponsiveness to diverse conventional achalasia treatments led us to suspect secondary achalasia. An active search led to a diagnosis of pancreatic mucinous cystadenocarcinoma invading the gastric fundus and EG junction. This rare case of pseudoachalasia caused by pancreatic carcinoma emphasizes the need for suspecting GI malignancies other than EG tumors in patients refractory to conventional achalasia treatment.
-
Citations
Citations to this article as recorded by- Delayed Presentation of Malignancy-Associated Pseudoachalasia of the Gastric Cardia
Clive J Miranda, Farhan Azad, Ross R Moyer, Sasikanth N Ravi, Gina M Sparacino
Cureus.2024;[Epub] CrossRef - Is it necessary to perform a morphological assessment for an esophageal motility disorder? A retrospective descriptive study
Sofya Latrache, Chloe Melchior, Charlotte Desprez, Sabrina Sidali, Julien Recton, Olivier Touchais, Elise van der Eecken, Fabien Wuestenberghs, Cloe Charpentier, Anne Marie Leroi, Guillaume Gourcerol
Clinics and Research in Hepatology and Gastroenterology.2021; 45(6): 101633. CrossRef - When a Late Metastasis Is Hard to Swallow
Catarina Negrão, Rita Sismeiro, Margarida Monteiro, Filipa G Pereira, Marta Jonet
Cureus.2021;[Epub] CrossRef - Development of pseudoachalasia following magnetic sphincter augmentation (MSA) with restoration of peristalsis after endoscopic dilation
Katrin Schwameis, Shahin Ayazi, Ali H. Zaidi, Toshitaka Hoppo, Blair A. Jobe
Clinical Journal of Gastroenterology.2020; 13(5): 697. CrossRef - Burkitt’s Lymphoma of the Gastrohepatic Omentum: A Malignant Presentation of Pseudoachalasia
Eric Omar Then, Andrew Ofosu, Prashanth Rawla, Tagore Sunkara, Sriharsha Dadana, Andrea Culliford, Vinaya Gaduputi
Case Reports in Gastrointestinal Medicine.2019; 2019: 1. CrossRef
- Delayed Presentation of Malignancy-Associated Pseudoachalasia of the Gastric Cardia
- 7,128 View
- 57 Download
- 6 Web of Science
- 5 Crossref

- Endoscopic Ultrasound-Guided Treatment of Pancreatic Cystic and Solid Masses
- Jaihwan Kim
- Clin Endosc 2015;48(4):308-311. Published online July 24, 2015
- DOI: https://doi.org/10.5946/ce.2015.48.4.308
-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader ePub
ePub Pancreatic tumor is one of the most difficult diseases to diagnose and treat because of its anatomical location and characteristics. Recently, there have been several innovative trials on the treatment of pancreatic tumors using endoscopic ultrasound (EUS) because it allows selective access to the difficult to reach target organ along the gastrointestinal tract and can differentiate vessels by color Doppler. Among these trials, several have investigated EUS-guided ethanol lavage with or without paclitaxel for pancreatic cystic tumors. These studies show a 33% to 79% complete resolution rate with a favorable safety profile. Compared to EUS-guided ethanol lavage for pancreatic cystic tumors, EUS-guided radiofrequency ablation is considered a less invasive treatment method for pancreatic cancer. Although there are still several difficulties and concerns about complications, one clinical study reported 72.8% feasibility with favorable safety, and therefore, we anticipate the results of ongoing studies with these new less invasive techniques.
-
Citations
Citations to this article as recorded by- Surgical outcomes are hampered after endoscopic ultrasonography-guided ethanol lavage and/or Taxol injection in cystic lesions of the pancreas
Seong-Ryong Kim, Song Cheol Kim, Ki Byung Song, Kwang-Min Park, Dae Wook Hwang, Jae Hoon Lee, Sang Hyun Shin, Bong Jun Kwak, Young-Joo Lee
Annals of Hepato-Biliary-Pancreatic Surgery.2021; 25(3): 342. CrossRef - Systematic review of endoscopy ultrasound-guided thermal ablation treatment for pancreatic cancer
SabrinaGloria Giulia Testoni, AndrewJames Healey, ChristophF Dietrich, PaoloGiorgio Arcidiacono
Endoscopic Ultrasound.2020; 9(2): 83. CrossRef - Endoscopic ultrasound-guided injective ablative treatment of pancreatic cystic neoplasms
Chen Du, Ning-Li Chai, En-Qiang Linghu, Hui-Kai Li, Xiu-Xue Feng
World Journal of Gastroenterology.2020; 26(23): 3213. CrossRef - Endoscopic ultrasound-guided radiofrequency ablation for pancreatic neuroendocrine tumor
Marc Barthet
Annales d'Endocrinologie.2019; 80(3): 182. CrossRef - Contrast‑enhanced harmonic endoscopic ultrasonography for the differential diagnosis of pancreatic masses: A systematic review and meta‑analysis
Yang Li, Hailin Jin, Dan Liao, Bo Qian, Yeifei Zhang, Min Xu, Shutang Han
Molecular and Clinical Oncology.2019;[Epub] CrossRef - Interventional EUS (with videos)
John T. Maple, Rahul Pannala, Barham K. Abu Dayyeh, Harry R. Aslanian, Brintha K. Enestvedt, Adam Goodman, Sri Komanduri, Michael Manfredi, Udayakumar Navaneethan, Mansour A. Parsi, Zachary L. Smith, Nirav Thosani, Shelby A. Sullivan, Subhas Banerjee
Gastrointestinal Endoscopy.2017; 85(3): 465. CrossRef - Echoendoscopic ablative therapy for solid pancreatic tumors
Woo Hyun Paik, Dong Wan Seo
Journal of Digestive Diseases.2017; 18(3): 135. CrossRef - Endoscopic ultrasound-guided radiofrequency ablation in gastroenterology: New horizons in search
Satyarth Chaudhary, Si-Yu Sun
World Journal of Gastroenterology.2017; 23(27): 4892. CrossRef - Endoscopic Ultrasound-Guided Radiofrequency Ablation of the Pancreatic Tumors: A Promising Tool in Management of Pancreatic Tumors
Kinesh Changela, Rashmee Patil, Sushil Duddempudi, Vinaya Gaduputi
Canadian Journal of Gastroenterology and Hepatology.2016; 2016: 1. CrossRef - UEG Week 2016 Poster Presentations
United European Gastroenterology Journal.2016; 4(5_suppl): A157. CrossRef - Highlights from the 52nd Seminar of the Korean Society of Gastrointestinal Endoscopy
Eun Young Kim, Il Ju Choi, Kwang An Kwon, Ji Kon Ryu, Ki Baik Hahm
Clinical Endoscopy.2015; 48(4): 269. CrossRef
- Surgical outcomes are hampered after endoscopic ultrasonography-guided ethanol lavage and/or Taxol injection in cystic lesions of the pancreas
- 12,466 View
- 113 Download
- 13 Web of Science
- 11 Crossref

- Neoplasia in Chronic Pancreatitis: How to Maximize the Yield of Endoscopic Ultrasound-Guided Fine Needle Aspiration
- Ji Young Bang, Shyam Varadarajulu
- Clin Endosc 2014;47(5):420-424. Published online September 30, 2014
- DOI: https://doi.org/10.5946/ce.2014.47.5.420
-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader ePub
ePub When performing endoscopic ultrasound-guided fine needle aspiration (EUS-FNA), identifying neoplasia in the setting of chronic pancreatitis can be technically challenging. The morphology of an ill-defined mass on sonography, presence of calcifications or intervening collaterals, reverberation from a biliary stent, low yield of tissue procurement, and interpretative errors in cytopathology can result in both false-negative and false-positive results. Although these challenges cannot be completely eliminated, elastography or contrast-enhanced imaging can aid in differentiating an inflammatory mass from a neoplasm. Also, performing more passes of FNA, procuring core biopsy material, performing rapid onsite evaluation, conducting ancillary pathology studies, and even repeating the procedure on a different day can aid in improving the diagnostic performance of EUS-FNA. This review provides a concise update and offers practical tips to improving the diagnostic yield of EUS-FNA when sampling solid pancreatic mass lesions in the setting of chronic pancreatitis.
-
Citations
Citations to this article as recorded by- How to optimize the diagnostic yield of endoscopic ultrasound-guided fine-needle sampling in solid pancreatic lesions from a technical perspective
Nam Hee Kim, Hong Joo Kim
International Journal of Gastrointestinal Intervention.2023; 12(2): 57. CrossRef - Endoscopic Ultrasound Guided Fine-Needle Aspiration for Solid Lesions in Chronic Pancreatitis: A Systematic Review and Meta-Analysis
Mohamed A. Abdallah, Khalid Ahmed, Wesam Taha, Abdullahi Musa, Erin E. Reardon, Abubaker O. Abdalla, Guru Trikudanathan
Digestive Diseases and Sciences.2022; 67(6): 2552. CrossRef - Pancreatitis crónica para el clínico. Parte 1: etiología y diagnóstico. Documento de posicionamiento interdisciplinar de la Societat Catalana de Digestologia y la Societat Catalana de Pàncrees
Xavier Molero, Juan Ramon Ayuso, Joaquim Balsells, Jaume Boadas, Juli Busquets, Anna Casteràs, Mar Concepción, Míriam Cuatrecasas, Gloria Fernàndez Esparrach, Esther Fort, Francisco Garcia Borobia, Àngels Ginès, Lucas Ilzarbe, Carme Loras, Miquel Masachs,
Gastroenterología y Hepatología.2022; 45(3): 231. CrossRef - Chronic pancreatitis for the clinician. Part 1: Etiology and diagnosis. Interdisciplinary position paper of the Societat Catalana de Digestologia and the Societat Catalana de Pàncrees
Xavier Molero, Juan Ramon Ayuso, Joaquim Balsells, Jaume Boadas, Juli Busquets, Anna Casteràs, Mar Concepción, Míriam Cuatrecasas, Gloria Fernàndez Esparrach, Esther Fort, Francisco Garcia Borobia, Àngels Ginès, Lucas Ilzarbe, Carme Loras, Miquel Masachs,
Gastroenterología y Hepatología (English Edition).2022; 45(3): 231. CrossRef - Comparison of contrast-enhanced versus conventional EUS-guided FNA/fine-needle biopsy in diagnosis of solid pancreatic lesions: a randomized controlled trial
In Rae Cho, Seok-Hoo Jeong, Huapyong Kang, Eui Joo Kim, Yeon Suk Kim, Jae Hee Cho
Gastrointestinal Endoscopy.2021; 94(2): 303. CrossRef - Pitfalls in the MDCT of pancreatic cancer: strategies for minimizing errors
Arya Haj-Mirzaian, Satomi Kawamoto, Atif Zaheer, Ralph H. Hruban, Elliot K. Fishman, Linda C. Chu
Abdominal Radiology.2020; 45(2): 457. CrossRef - Factors affecting cytological results of endoscopic ultrasound guided-fine needle aspiration during learning
Jian-Han Lai, Hsiang-Hung Lin, Ching-Chung Lin
Diagnostic Pathology.2020;[Epub] CrossRef - Resectable pancreatic solid lesions: Time to move from surgical diagnosis?
Alberto Larghi, Mihai Rimbaş, Gianenrico Rizzatti, Giuseppe Quero, Antonio Gasbarrini, Guido Costamagna, Sergio Alfieri
Endoscopic Ultrasound.2020; 9(2): 76. CrossRef - Endoscopic ultrasound-guided sampling of solid pancreatic masses: the fine needle aspiration or fine needle biopsy dilemma. Is the best needle yet to come?
Clara Benedetta Conti, Fabrizio Cereatti, Roberto Grassia
World J Gastrointest Endosc.2019; 11(8): 454. CrossRef - Endoscopic ultrasound-guided sampling of solid pancreatic masses: the fine needle aspiration or fine needle biopsy dilemma. Is the best needle yet to come?
Clara Benedetta Conti, Fabrizio Cereatti, Roberto Grassia
World Journal of Gastrointestinal Endoscopy.2019; 11(8): 454. CrossRef - Predictors of Malignancies in Patients with Inconclusive or Negative Results of Endoscopic Ultrasound-guided Fine-needle Aspiration for Solid Pancreatic Masses
Hyewon Jeong, Chan Sun Park, Ki Bae Kim, Joung-Ho Han, Soon Man Yoon, Hee Bok Chae, Sei Jin Youn, Seon Mee Park
The Korean Journal of Gastroenterology.2018; 71(3): 153. CrossRef - Endoscopic ultrasound elastography for solid pancreatic lesions
Tanyaporn Chantarojanasiri, Pradermchai Kongkam
World Journal of Gastrointestinal Endoscopy.2017; 9(10): 506. CrossRef - Imaging Macrophage Accumulation in a Murine Model of Chronic Pancreatitis with 125I-Iodo-DPA-713 SPECT/CT
Catherine A. Foss, Liansheng Liu, Ronnie C. Mease, Haofan Wang, Pankaj Pasricha, Martin G. Pomper
Journal of Nuclear Medicine.2017; 58(10): 1685. CrossRef - Endoscopic Ultrasound Elastography
Utpal Mondal, Nichole Henkes, Sandeep Patel, Laura Rosenkranz
Pancreas.2016; 45(7): 929. CrossRef - International Digestive Endoscopy Network 2014: Turnpike to the Future
Eun Young Kim, Kwang An Kwon, Il Ju Choi, Ji Kon Ryu, Ki Baik Hahm
Clinical Endoscopy.2014; 47(5): 371. CrossRef
- How to optimize the diagnostic yield of endoscopic ultrasound-guided fine-needle sampling in solid pancreatic lesions from a technical perspective
- 6,426 View
- 69 Download
- 18 Web of Science
- 15 Crossref

- Gastrointestinal Cancers in a Peutz-Jeghers Syndrome Family: A Case Report
- Sang Hee Song, Kun Woo Kim, Won Hee Kim, Chang Il Kwon, Kwang Hyun Ko, Ki Baik Hahm, Pil Won Park, Sung Pyo Hong
- Clin Endosc 2013;46(5):572-575. Published online September 30, 2013
- DOI: https://doi.org/10.5946/ce.2013.46.5.572
-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader ePub
ePub A 17-year-old man was diagnosed as Peutz-Jeghers syndrome (PJS) because of pigmented lip and multiple gastrointestinal polyps. He had anemia and underwent polypectomy on the duodenum and colon. His maternal family members were patients with PJS. His mother used to be screened with endoscopy to remove large polyps. One and half years later, he underwent jejunal segmental resection due to intussusceptions. He underwent endoscopic polypectomy every 2 to 3 years. When he was 23 years old, high-grade dysplasia was found in colonic polyp and his mother underwent partial pancreatectomy due to intraductal papillary mucinous carcinoma. When he was 27 years old, diffuse gastric polyps on the greater curvature of corpus expanded and grew. Therefore, wide endoscopic polypectomy was done. Histological examination revealed focal intramucosal carcinoma and low-grade dysplasia in hamartomatous polyps. We report cases of cancers occurred in first-degree relatives with PJS.
-
Citations
Citations to this article as recorded by- Familial and hereditary gastric cancer, an overview
Fátima Carneiro
Best Practice & Research Clinical Gastroenterology.2022; 58-59: 101800. CrossRef - Small bowel intussusception and concurrent sigmoid polyp with malignant transformation in Peutz–Jeghers syndrome
Maidah Algarni, Enas Raml, Nora Trabulsi, Mohammed Nassif
Journal of Surgical Case Reports.2019;[Epub] CrossRef - The first European family with gastric adenocarcinoma and proximal polyposis of the stomach: case report and review of the literature
Rudolf Repak, Darina Kohoutova, Miroslav Podhola, Stanislav Rejchrt, Marek Minarik, Lucie Benesova, Michal Lesko, Jan Bures
Gastrointestinal Endoscopy.2016; 84(4): 718. CrossRef - Gastric Hamartomatous Polyps—Review and Update
Monika Vyas, Xiu Yang, Xuchen Zhang
Clinical Medicine Insights: Gastroenterology.2016; 9: CGast.S38452. CrossRef - Giant rectal polyp prolapse in an adult patient with the Peutz-Jeghers syndrome
Ana Delfina Cano-Contreras, Arturo Meixueiro-Daza, Peter Grube-Pagola, Jose Maria Remes-Troche
BMJ Case Reports.2016; : bcr2016215629. CrossRef - Prevention Strategies for Gastric Cancer: A Global Perspective
Jin Young Park, Lawrence von Karsa, Rolando Herrero
Clinical Endoscopy.2014; 47(6): 478. CrossRef
- Familial and hereditary gastric cancer, an overview
- 6,559 View
- 58 Download
- 6 Crossref

- Technical Advances in Endoscopic Ultrasound (EUS)-Guided Tissue Acquisition for Pancreatic Cancers: How Can We Get the Best Results with EUS-Guided Fine Needle Aspiration?
- Prashant Kedia, Monica Gaidhane, Michel Kahaleh
- Clin Endosc 2013;46(5):552-562. Published online September 30, 2013
- DOI: https://doi.org/10.5946/ce.2013.46.5.552
-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader ePub
ePub Endoscopic ultrasound-guided fine needle aspiration (EUS-FNA) is one of the least invasive and most effective modality in diagnosing pancreatic adenocarcinoma in solid pancreatic lesions, with a higher diagnostic accuracy than cystic tumors. EUS-FNA has been shown to detect tumors less than 3 mm, due to high spatial resolution allowing the detection of very small lesions and vascular invasion, particularly in the pancreatic head and neck, which may not be detected on transverse computed tomography. Furthermore, this minimally invasive procedure is often ideal in the endoscopic procurement of tissue in patients with unresectable tumors. While EUS-FNA has been increasingly used as a diagnostic tool, most studies have collectively looked at all primary pancreatic solid lesions, including lymphomas and pancreatic neuroendocrine neoplasms, whereas very few studies have examined the diagnostic utility of EUS-FNA of pancreatic ductal carcinoma only. As with any novel and advanced endoscopic procedure that may incorporate several practices and approaches, endoscopists have adopted diverse techniques to improve the tissue procurement practice and increase diagnostic accuracy. In this article, we present a review of literature to date and discuss currently practiced EUS-FNA technique, including indications, technical details, equipment, patient selection, and diagnostic accuracy.
-
Citations
Citations to this article as recorded by- The role of endoscopic ultrasound-guided fine-needle aspiration/biopsy in the diagnosis of mediastinal lesions
Jingjing Zhou, Ting Cai, Dongwen Wu, Xiong Chen, Fen Wang
Frontiers in Surgery.2023;[Epub] CrossRef - Diagnostic Yield of Transabdominal Ultrasound-Guided Core Needle Method in Biopsies of Pancreatic Lesions
Bekir Turgut, Süleyman Bakdik, Fatih Öncü, İlknur Küçükosmanoğlu, Meryem İlkay Eren Karanis, Ramazan Saygin Kerimoğlu, Mustafa Saraçoğlu
Ultrasound Quarterly.2023; 39(2): 109. CrossRef - EUS-guided fine-needle biopsy sampling of solid pancreatic tumors with 3 versus 12 to-and-fro movements: a multicenter prospective randomized controlled study
Kosuke Takahashi, Ichiro Yasuda, Nobuhiko Hayashi, Takuji Iwashita, Mitsuru Okuno, Tsuyoshi Mukai, Masatoshi Mabuchi, Seiji Adachi, Shinpei Doi, Johji Imura, Eisuke Ozawa, Hisamitsu Miyaaki, Kazuhiko Nakao
Gastrointestinal Endoscopy.2023; 97(6): 1092. CrossRef - Scope position is a determining factor for diagnostic performance of endoscopic ultrasound-guided fine-needle aspiration for mass lesions in the pancreatic head
Nam Hee Kim, Hong Joo Kim
International Journal of Gastrointestinal Intervention.2023; 12(4): 163. CrossRef - Endoscopic ultrasound-guided tissue acquisition: Needle types, technical issues, and sample handling
Woo Hyun Paik
International Journal of Gastrointestinal Intervention.2022; 11(3): 96. CrossRef - EUS‐guided hydrogel microparticle injection in a cadaveric model
Seong‐Hun Kim, Kai Ding, Avani Rao, Jin He, Manoop S. Bhutani, Joseph M. Herman, Amol Narang, Eun Ji Shin
Journal of Applied Clinical Medical Physics.2021; 22(6): 83. CrossRef - Optimal Techniques for EUS-Guided Fine-Needle Aspiration of Pancreatic Solid Masses at Facilities without On-Site Cytopathology: Results from Two Prospective Randomised Trials
Woo Hyun Paik, Joon Hyuk Choi, Yangsoon Park, Jung Bok Lee, Do Hyun Park
Journal of Clinical Medicine.2021; 10(20): 4662. CrossRef - Factors affecting cytological results of endoscopic ultrasound guided-fine needle aspiration during learning
Jian-Han Lai, Hsiang-Hung Lin, Ching-Chung Lin
Diagnostic Pathology.2020;[Epub] CrossRef - Usefulness of 18F‐FDG‐PET/CT in the diagnosis and prediction of recurrence of pancreatic neuroendocrine neoplasms
Asahi Sato, Toshihiko Masui, Akitada Yogo, Yuichiro Uchida, Kenzo Nakano, Takayuki Anazawa, Kazuyuki Nagai, Kyoichi Takaori, Yuji Nakamoto, Shinji Uemoto
Journal of Hepato-Biliary-Pancreatic Sciences.2020; 27(7): 414. CrossRef - Needle-compatible miniaturized optoelectronic sensor for pancreatic cancer detection
Seung Yup Lee, Julia M. Pakela, Kyounghwan Na, Jiaqi Shi, Barbara J. McKenna, Diane M. Simeone, Euisik Yoon, James M. Scheiman, Mary-Ann Mycek
Science Advances.2020;[Epub] CrossRef - Outcomes of endoscopic ultrasound‐guided biliary drainage: A systematic review and meta‐analysis
A Hedjoudje, A Sportes, S Grabar, A Zhang, S Koch, L Vuitton, F Prat
United European Gastroenterology Journal.2019; 7(1): 60. CrossRef - Which Needle Needs to Be Chosen for Better Outcome of Endoscopic Ultrasound-Guided Tissue Acquisition?
Dong Wook Lee, Eun Young Kim
Gut and Liver.2019; 13(3): 223. CrossRef - Endoscopic Ultrasound-Guided Liver Biopsy Using a Core Needle for Hepatic Solid Mass
Hyung Ku Chon, Hee Chan Yang, Keum Ha Choi, Tae Hyeon Kim
Clinical Endoscopy.2019; 52(4): 340. CrossRef - A comprehensive review of endoscopic ultrasound core biopsy needles
Theodore W. James, Todd H. Baron
Expert Review of Medical Devices.2018; 15(2): 127. CrossRef - Ultrasound-Guided Percutaneous Core Needle Biopsy for the Diagnosis of Pancreatic Disease
Ying Huang, Jingwen Shi, Yun-Yun Chen, Kao Li
Ultrasound in Medicine & Biology.2018; 44(6): 1145. CrossRef - Push vs pull method for endoscopic ultrasound-guided fine needle aspiration of pancreatic head lesions: Propensity score matching analysis
Mitsuru Sugimoto, Tadayuki Takagi, Rei Suzuki, Naoki Konno, Hiroyuki Asama, Yuki Sato, Hiroki Irie, Ko Watanabe, Jun Nakamura, Hitomi Kikuchi, Yuichi Waragai, Mika Takasumi, Minami Hashimoto, Yuko Hashimoto, Takuto Hikichi, Hiromasa Ohira
World Journal of Gastroenterology.2018; 24(27): 3006. CrossRef - High-resolution endoscopic ultrasound imaging and the number of needle passages are significant factors predicting high yield of endoscopic ultrasound-guided fine needle aspiration for pancreatic solid masses without an on-site cytopathologist
Seok Hoo Jeong, Hyun Hwa Yoon, Eui Joo Kim, Yoon Jae Kim, Yeon Suk Kim, Jae Hee Cho
Medicine.2017; 96(2): e5782. CrossRef - Risk factors associated with adverse events during endoscopic ultrasound-guided tissue sampling
Kwang Hyuck Lee, Eun Young Kim, Juhee Cho, Danbee Kang, Seungmin Bang, Hyung Kil Kim, Gwang Ha Kim, Hyun Jong Choi, Joung-Ho Han, Seong Woo Jeon, Ji Kon Ryu, Jeong Seop Moon, Tae Hee Lee, Jin Woong Cho, Tae Hyeon Kim, Young Koog Cheon, Chang-Hwan Park, Jo
PLOS ONE.2017; 12(12): e0189347. CrossRef - Endoscopic ultrasound-guided fine-needle aspiration and cytology for differentiating benign from malignant lymph nodes
Hussein Okasha, Shaimaa Elkholy, Mohamed Sayed, Ahmed Salman, Yahia Elsherif, Emad El-Gemeie
Arab Journal of Gastroenterology.2017; 18(2): 74. CrossRef - Endoscopic ultrasound-guided fine needle core biopsy for the diagnosis of pancreatic malignant lesions: a systematic review and Meta-Analysis
Yongtao Yang, Lianyong Li, Changmin Qu, Shuwen Liang, Bolun Zeng, Zhiwen Luo
Scientific Reports.2016;[Epub] CrossRef - Role of endoscopic ultrasound-guided fine needle aspiration in the diagnosis of mass lesions
Chaoqun Han, Rong Lin, Qin Zhang, Jun Liu, Zhen Ding, Xiaohua Hou
Experimental and Therapeutic Medicine.2016; 12(2): 1085. CrossRef - Pancreatic cancer: diagnosis and treatments
Hong-Yu Li, Zhong-Min Cui, Jiang Chen, Xiao-Zhong Guo, Ying-Yi Li
Tumor Biology.2015; 36(3): 1375. CrossRef - Contrast-Enhanced Harmonic Endoscopic Ultrasound-Guided Fine-Needle Aspiration in the Diagnosis of Solid Pancreatic Lesions: A Retrospective Study
Xiaojia Hou, Zhendong Jin, Can Xu, Minmin Zhang, Jianwei Zhu, Fei Jiang, Zhaoshen Li, Robert Lane Schmidt
PLOS ONE.2015; 10(3): e0121236. CrossRef - Key endoscopic ultrasound features of pancreatic ductal adenocarcinoma smaller than 20 mm
Akira Aso, Eikichi Ihara, Takashi Osoegawa, Kazuhiko Nakamura, Soichi Itaba, Hisato Igarashi, Tetsuhide Ito, Shinichi Aishima, Yoshinao Oda, Masao Tanaka, Ryoichi Takayanagi
Scandinavian Journal of Gastroenterology.2014; 49(3): 332. CrossRef - Endoscopic Ultrasound-Guided Fine Needle Biopsy without Rapid On-Site Cytologic Examination: A Time to Change the Paradigm?
Yeon Suk Kim
Clinical Endoscopy.2014; 47(3): 207. CrossRef - Fine-Needle Biopsy: Should This Be the First Choice in Endoscopic Ultrasound-Guided Tissue Acquisition?
Eun Young Kim
Clinical Endoscopy.2014; 47(5): 425. CrossRef
- The role of endoscopic ultrasound-guided fine-needle aspiration/biopsy in the diagnosis of mediastinal lesions
- 9,054 View
- 92 Download
- 26 Crossref

- Role of Repeated Endoscopic Ultrasound-Guided Fine Needle Aspiration for Inconclusive Initial Cytology Result
- Eun Young Kim
- Clin Endosc 2013;46(5):540-542. Published online September 30, 2013
- DOI: https://doi.org/10.5946/ce.2013.46.5.540
-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader ePub
ePub For tissue diagnosis of suspected pancreatic cancer, endoscopic ultrasound-guided fine needle aspiration (EUS-FNA) is the procedure of choice with high safety and accuracy profiles. However, about 10% of cytologic findings of EUS-FNA are inconclusive. In that situation, careful observation, surgical exploration, or alternative diagnostic tools such as bile duct brushing with endoscopic retrograde cholangiopancreatography or computed tomography-guided biopsy can be considered. However, some concerns and/or risks of these options render repeat EUS-FNA a reasonable choice. Repeated EUS-FNA may impose substantial clinical impact with low risk.
-
Citations
Citations to this article as recorded by- An Unusual Presentation of a Solid Pseudopapillary Tumor of the Pancreas Mimicking Adenocarcinoma
Hyung Ku Chon, Keum Ha Choi, Tae Hyeon Kim
Clinical Endoscopy.2020; 53(5): 615. CrossRef - Repeat Endoscopic Ultrasound-Guided Fine-Needle Aspiration in Patients with Suspected Pancreatic Cancer: Diagnostic Yield and Associated Change in Access to Appropriate Care
Robert A. Mitchell, Dylan Stanger, Constantin Shuster, Jennifer Telford, Eric Lam, Robert Enns
Canadian Journal of Gastroenterology and Hepatology.2016; 2016: 1. CrossRef - Impact of endoscopic ultrasound-guided fine-needle aspiration and multidisciplinary approach in the management of abdominal or mediastinal mass
Giovanna Del Vecchio Blanco, Manuela Coppola, Elena Mannisi, Gerolamo Bevivino, Vincenzo Formica, Ilaria Portarena, Samanta Romeo, Pierpaolo Sileri, Mario Roselli, Francesco Pallone, Omero Alessandro Paoluzi
European Journal of Gastroenterology & Hepatology.2015; 27(9): 1045. CrossRef - Impact of inconclusive endoscopic ultrasound‐guided fine‐needle aspiration results in the management and outcome of patients with solid pancreatic masses
Bo Sun, Xiujiang Yang, Bo Ping, Yiping He, Zhaozhen Zhang
Digestive Endoscopy.2015; 27(1): 130. CrossRef
- An Unusual Presentation of a Solid Pseudopapillary Tumor of the Pancreas Mimicking Adenocarcinoma
- 5,860 View
- 54 Download
- 4 Crossref

- Endoscopic Removal of a Proximally Migrated Metal Stent during Balloon Sweeping after Stent Trimming
- Nam Jun Cho, Tae Hoon Lee, Sang-Heum Park, Han Min Lee, Kyung Hee Hyun, Suck-Ho Lee, Il-Kwun Chung, Sun-Joo Kim
- Clin Endosc 2013;46(4):418-422. Published online July 31, 2013
- DOI: https://doi.org/10.5946/ce.2013.46.4.418
-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader ePub
ePub Placement of a self-expanding metal stent (SEMS) is an effective method for palliation of a malignant biliary obstruction. However, metal stents can cause various complications, including stent migration. Distally migrated metal stents, particularly covered SEMS, can be removed successfully in most cases. Stent trimming using argon plasma coagulation may be helpful in difficult cases despite conventional methods. However, no serious complications related to the trimming or remnant stent removal method have been reported due to the limited number of cases. In particular, proximal migration of a remnant fragmented metal stent after stent trimming followed by balloon sweeping has not been reported. We report an unusual case of proximal migration of a remnant metal stent during balloon sweeping following stent trimming by argon plasma coagulation. The remnant metal stent was successfully removed with rotation technique using a basket and revised endoscopically.
-
Citations
Citations to this article as recorded by- Successful removal of a proximally migrated biliary fully covered self‐expandable metal stent using a sphincterotome
Kazuya Koizumi, Karen Kimura, Ryuhei Jinushi
Digestive Endoscopy.2023;[Epub] CrossRef - Endoscopic Management of a Proximally Migrated Fully Covered SEMS Using the Stent-in-Stent Technique
Arunchai Chang, Varayu Prachayakul
Case Reports in Medicine.2020; 2020: 1. CrossRef - Burning and cutting: a unique technique for management of migrated uncovered metal biliary stents
Shivangi Kothari, Truptesh H. Kothari, Vivek Kaul
VideoGIE.2020; 5(11): 562. CrossRef - Recalcitrant embedded biliary self-expanding metal stents: a novel technique for endoscopic extraction
Marc Bernon, Christo Kloppers, Jessica Lindemann, Jake E.J. Krige, Eduard Jonas
VideoGIE.2019; 4(2): 72. CrossRef - Endoscopic management of internally migrated pancreatic duct stents (with video)
Suryaprakash Bhandari, Atul Sharma, Rajesh Bathini, Amit Maydeo
Indian Journal of Gastroenterology.2016; 35(2): 91. CrossRef
- Successful removal of a proximally migrated biliary fully covered self‐expandable metal stent using a sphincterotome
- 7,197 View
- 82 Download
- 5 Crossref


 KSGE
KSGE


 First
First Prev
Prev



