INTRODUCTION
The most common reasons for false-negative interpretation at endoscopic ultrasound-guided fine needle aspiration (EUS-FNA) are inadequate sampling and incorrect targeting of lesions. This is encountered more frequently in patients with solid pancreatic masses in the setling of chronic pancreatitis. This review addresses this clinical conrum and offers sugestions to mitigate these limitations.
WHAT IS THE BURDEN OF THE PROBLEM?
The sensitivity of EUS-FNA is only 54% to 74% when sampling solid pancreatic masses in the setting of chronic pancreatitis (Table 1).1,2 The presence of underlying chronic pancreatitis makes the morphological interpretation of neoplasms challenging. While a conglomeration of pancreatitis-induced lobulations may mimic a pancreatic mass (Fig. 1), the presence of acoustic shadowing from a calcified stone may undermine the visibility of a neoplasm (Fig. 2). Also, the coexistence of collateral vasculature in patients with severe chronic pancreatitis makes the process of FNA more challenging (Fig. 3). In one study, the median number of FNA passes required to establish a diagnosis of pancreatic cancer was five in patients with coexisting chronic pancreatitis versus only two in patients without chronic pancreatitis.2
Some of the cytologic features that may mimic malignancy in chronic pancreatitis are occasional atypical cells that include enlarged, single cells with large nuclei; degenerative vacuoles; and occasional mitosis. Also, the marked desmoplasia of pancreatic adenocarcinoma might result in an inadequate, paucicellular specimen. Diagnosing well-differentiated adenocarcinomas can be particularly challenging as they tend to lack the typical hyperchromasia, display only minimal architectural disorder, and have only modestly increased nuclear-to-cytoplasmic ratios.3
The take-home message is that the major practical implication when performing EUS-FNA of solid pancreatic masses in the setting of chronic pancreatitis is that a tumor can be missed or wrongly classified. Also, more FNA passes may be required to establish a definitive diagnosis in these patients.
HOW CAN TECHNOLOGY IMPROVE NEOPLASIA DETECTION IN CHRONIC PANCREATITIS?
Elastography and contrast harmonic imaging are two recent technological developments in endosonography that enable better characterization of pancreatic masses. However, these technologies are not uniformly available worldwide, and the results have not been reproducible in all clinical investigations.
Elastography
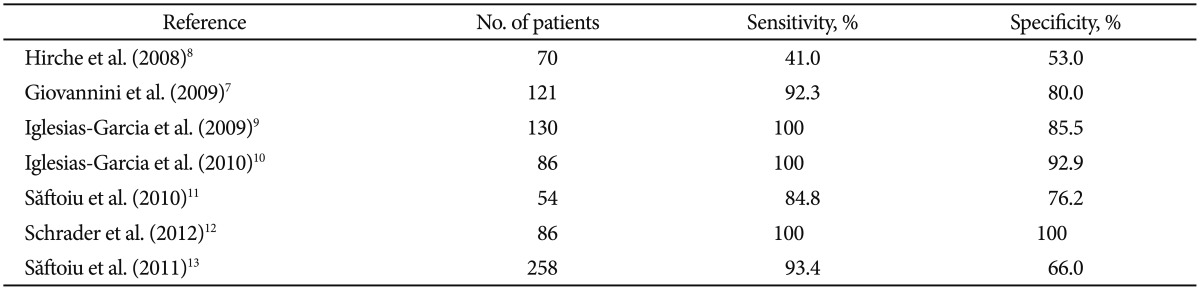
Elasticity imaging has been reported to be useful for the differentiation of benign and malignant tissues, owing to the inherent differences in the hardness of tissues. Malignant tumors are usually stiffer than benign masses, and the strain information induced by small tissue deformations can be computed and displayed in real time. Real-time sonoelastography represents a technical improvement of grayscale ultrasound that allows the estimation of tissue strain during slight compressions induced by the transducer.4 The method works in real time, with the strain information being visually converted into a hue color scale and displayed as a transparent overlay superimposed on the grayscale ultrasound image.5,6 Consequently, soft tissues that are easy to compress are displayed in low hue values (green) and hard tissues that are difficult to strain are displayed in high hue values (blue). A multicenter trial that analyzed 121 focal pancreatic masses reported a ╬║ value of 0.785 for pancreatic masses.7 EUS elastography was proven to have higher sensitivity and specificity than conventional grayscale EUS imaging (92.3% and 80%, respectively), for the differential diagnosis of focal pancreatic masses. Several studies have evaluated the role of elastography in solid pancreatic masses but with disparate results (Table 2).7,8,9,10,11,12,13
Contrast-enhanced EUS
Owing to the recent advances in EUS processors, second-generation intravenous ultrasound contrast agents can be used in combination with low mechanical index techniques to improve the visualization of tissue perfusion and to differentiate benign from malignant focal lesions.14,15 In some centers, contrast-enhanced EUS has become an established indication for the discrimination of focal pancreatic masses, particularly hypoenhancing pancreatic adenocarcinomas versus iso- or hyperenhancing lesions that include mass-forming chronic pancreatitis or neuroendocrine tumors. The most commonly used agent in Europe contains phospholipid-stabilized microbubbles of sulfurhexafluoride (Sono-Vue; Bracco, Milan, Italy), which, on injection, casts an initial early arterial phase (10 to 30 seconds) followed by a late venous phase (30 to 120 seconds).16 Considering hypovascularity as a sign of malignancy yields a diagnostic sensitivity of 92% and a specificity of 100%.17 Furthermore, the vascularity index (i.e., the percentage of Doppler-positive areas relative to the total area of the mass) obtained during the late phase of contrast enhancement is useful for differentiating pancreatic adenocarcinoma from pseudotumoral chronic pancreatitis.18,19 By using this approach for differentiating between inflammatory masses and pancreatic adenocarcinoma, the diagnostic sensitivity improves to 91.1% and specificity to 93.3%.18 A recent meta-analysis including 12 studies and 1,139 patients demonstrated a pooled sensitivity of 94% (95% confidence interval [CI], 0.91 to 0.95) and a specificity of 89% (95% CI, 0.85 to 0.92) for diagnosing pancreatic adenocarcinoma.20 Furthermore, the area under the receiver operating characteristic curve was 0.97. Recently, another study described a new technique, the dynamic quantitative analysis of contrast-enhanced EUS, through the use of time-intensity-curve analysis, and reported a sensitivity and specificity of 95.8% and 92.6%, respectively, in 91 patients with focal pancreatic masses.21
The take-home message is that preliminary data suggest that elastography and, in particular, contrast-enhanced EUS improves the ability to differentiate neoplasms from chronic pancreatitis. However, the management decision by surgeons is based on tissue characteristics and not on sonographic characteristics. No study has shown that, when using contrast-enhanced EUS, performing a biopsy at a particular spot within a mass definitively yields malignant cells. Unless this critical question can be addressed, the real-life utility of contrast-enhanced EUS remains debatable.
WHAT TECHNIQUES AND STRATEGIES WILL IMPROVE THE DIAGNOSTIC YIELD OF EUS-FNA?
For a cytopathologist, rendering a diagnosis on hypocellular samples is one of the more common causes for delivering a false diagnosis. Therefore, several practical measures must be undertaken to enhance the diagnostic yield of EUS-FNA when sampling solid pancreatic masses in the setting of chronic pancreatitis.
Rapid onsite evaluation
Several studies have shown that the presence of an onsite cytopathologist improves the diagnostic yield, decreases the number of unsatisfactory samples, and minimizes the number of passes required to establish a diagnosis. In addition, two recent meta-analyses on EUS-FNA of pancreatic masses showed that the presence of an onsite cytopathologist was associated with a diagnostic sensitivity of 88% to 95% compared with Ōēż80% in the absence of a cytopathologist.22,23 Therefore, in challenging cases, the presence of an onsite cytopathologist is critical to achieve good clinical outcomes.
Number of FNA passes
The marked desmoplasia of pancreatic adenocarcinoma might result in an inadequate specimen, which is commonly encountered in the setting of chronic pancreatitis. Also, diagnosing well-differentiated cancers can be challenging as they tend to lack the typical hyperchromasia, display minimal architectural disorder, and have only modestly increased nuclear-to-cytoplasmic ratios. Therefore, the number of passes required to establish a diagnosis of malignancy is significantly greater in the presence of coexisting chronic pancreatitis (two passes). This is particularly relevant when rapid onsite evaluation (ROSE) is not possible. These authors and other experts recommend performing a minimum of seven passes by using the "fanning" technique.24,25
FNA technique
Current evidence does not support the routine use of a suction or a stylet during EUS-FNA as they tend to increase the bloodiness of the specimen.26 However, if the FNA pass yields only minimal tissue or a dry tap, then suction may be used, particularly in severe chronic pancreatitis in which the desmoplastic stroma traps the cancer cells, yielding only a scant aspirate. Also, the use of a large-caliber 19 G needle may be considered if the tissue yield is minimal or if core biopsy is desired. A core biopsy yields tissue fragments with an intact histological architecture that is sometimes required, particularly in patients with well-differentiated pancreatic adenocarcinoma, when cytology is inconclusive.
Ancillary studies
In challenging cases, in addition to procuring tissue in cell blocks, the specimen needs to be fixed in alcohol for better delineation of the nuclear morphology. Also, several biomarkers are increasingly available to distinguish reactive ductal epithelium from neoplastic cells. Therefore, additional (sufficient) tissue must be procured in cell blocks to facilitate the performance of ancillary studies. Several biomarkers are increasingly available to distinguish reactive ductal from neoplastic cells.
Repeat EUS-FNA
If the suspicion for malignancy remains very high, the patient is a good surgical candidate, and the lesion appears resectable, then the best option is surgery. As the degree of suspicion for malignancy decreases, the health status of the patient is marginal and the resectability is intermediate; thus, repeat EUS-FNA is probably the best course of action. Three studies have shown that a repeat EUS-FNA yields a correct diagnosis in 61% to 84% of patients.27,28,29 Therefore, when seven or more FNA passes have been performed and the diagnosis is uncertain, performing a repeat EUS-FNA may be the best course of action. In the opinion of these authors, after performing seven FNA passes, one reaches a point of "diminishing return" and persisting with more FNAs is unlikely to be of clinical benefit.
The take-home message is that given the presence of increased collaterals and poor visualization of the mass, it is important to be "efficient" when performing FNA of pancreatic masses, particularly in the setting of chronic pancreatitis. Performing the fanning maneuver during FNA, conducting ROSE, performing adequate number of FNA passes, and procuring additional specimen for ancillary studies are critical steps that must be followed. If none of these prove useful, repeating the procedure at another time is important before subjecting the patient to more invasive evaluations.